Abstract
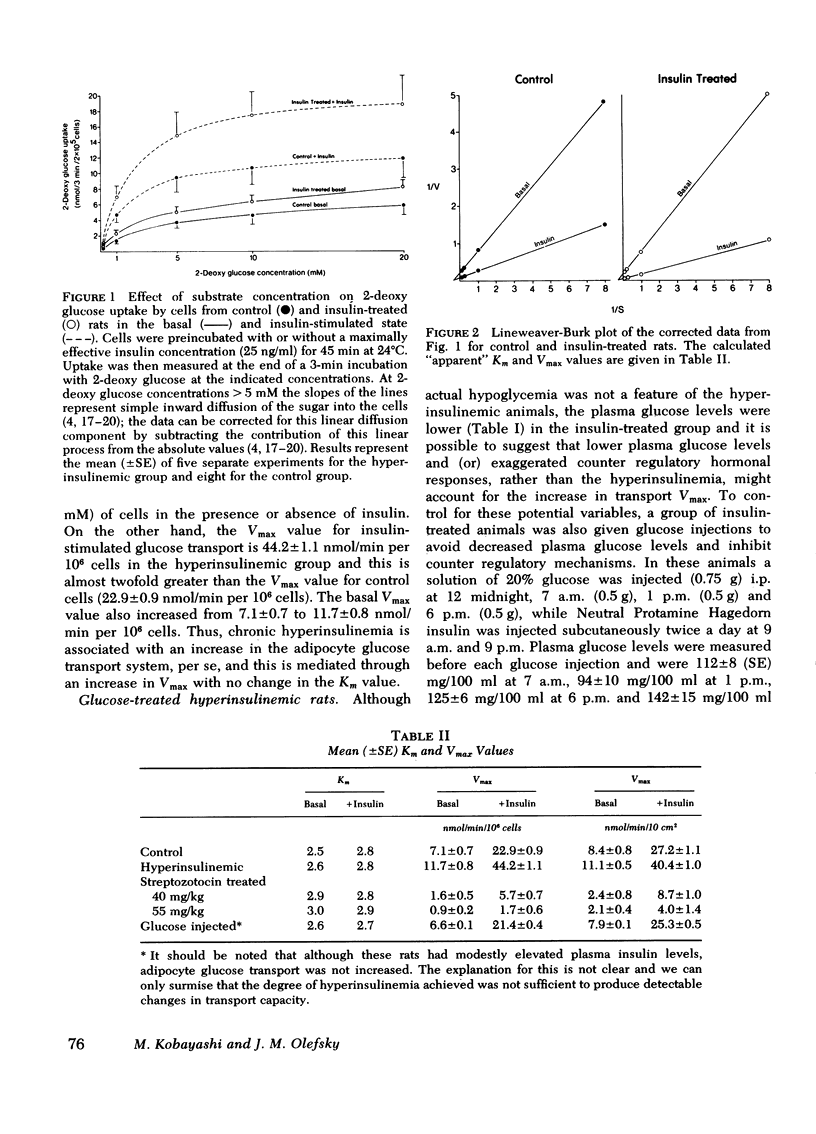
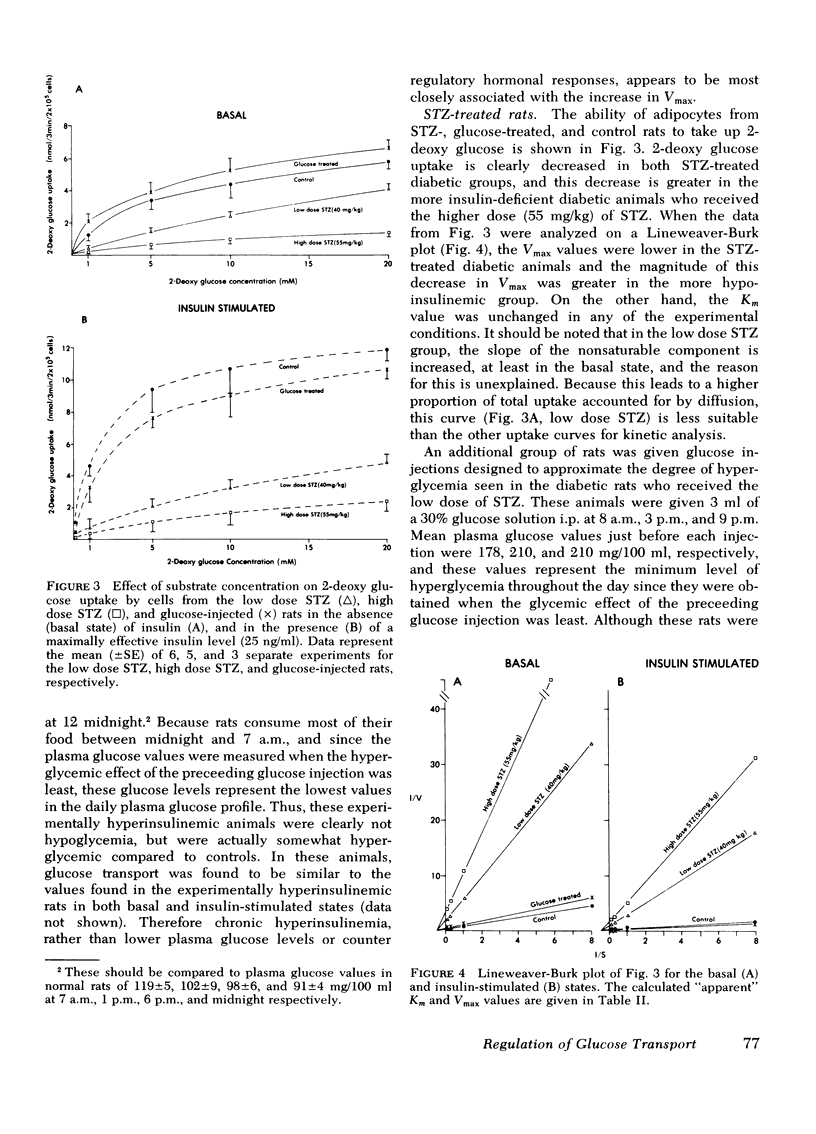
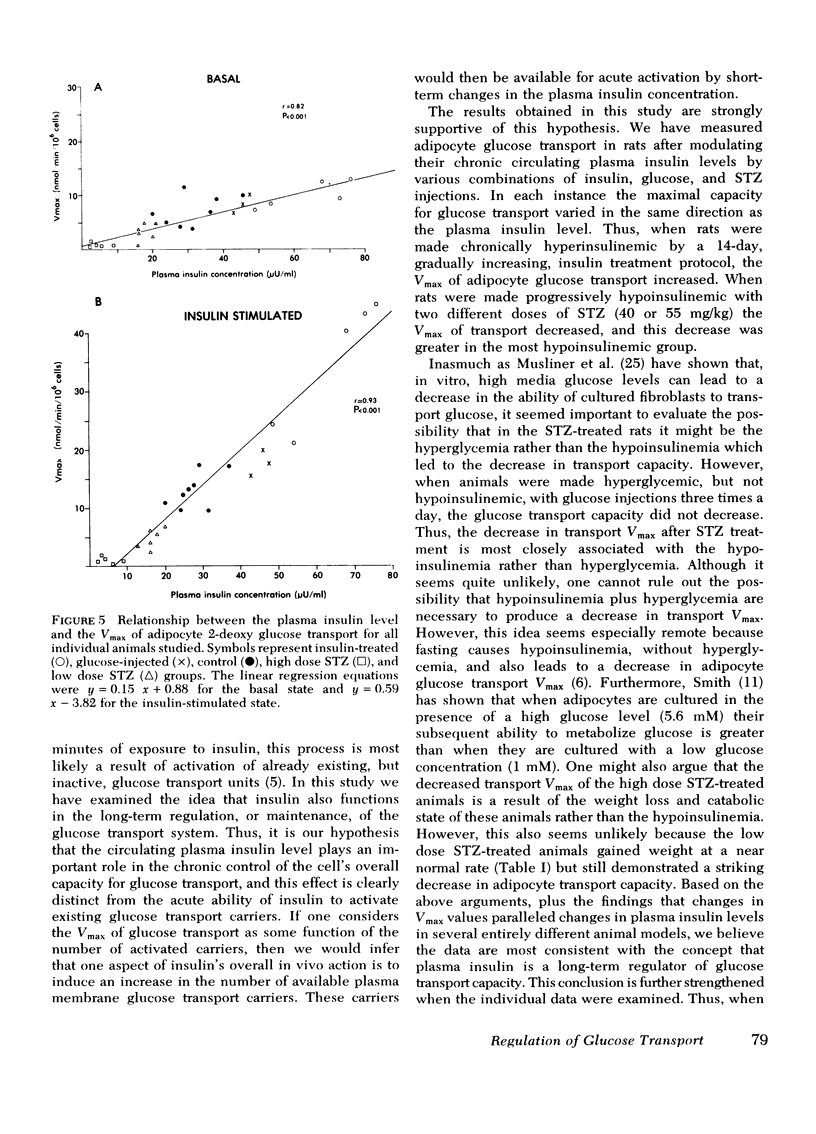
We have tested the idea that the circulating plasma insulin level plays an important role in the long-term regulation, or maintenance, of the cellular glucose transport system, distinct from insulin's ability to acutely accelerate glucose transport. To study this hypothesis, groups of rats were made either hyperinsulinemic or hypoinsulinemic by daily insulin injections, or streptozotocin treatment, respectively. Different levels of hypoinsulinemia were produced by using different doses of streptozotocin (40 and 55 mg/kg). The mean (±SE) 9 a.m. plasma insulin level for each experimental group was: hyperinsulinemic animals, 65±5 μU/ml; controls, 32±3 μU/ml; low dose streptozotocin group, 18±3 μU/ml; and high dose streptozotocin group 5±2 μU/ml. Isolated adipocytes were prepared from each animal and glucose transport was assessed by measuring the initial rates of uptake of the nonmetabolyzable hexose 2-deoxy glucose. The Vmax and Km values for adipocyte glucose transport were calculated from the 2-deoxy glucose uptake data. The results demonstrated that in cells from control animals the Vmax of in vitro adipocyte glucose transport was 7.1±0.7 nmol/min per 106 cells in the basal state and 22.9±0.9 nmol/min per 106 cells in the presence of a maximally effective insulin concentration (25 ng/ml) in the buffer. In cells from the experimentally hyperinsulinemic animals these Vmax values were increased to 11.7±0.8 and 44.2±1.1 nmol/min per 106 cells. Using adipocytes from both groups of streptozotocin-treated (high dose, 55 mg/kg; low dose, 40 mg/kg) insulin-deficient diabetic animals, Vmax values were found to be progressively decreased. Thus, in the low dose group, basal-and insulin-stimulated Vmax values were 1.6±0.5 and 5.7±0.7 nmol/min per 106 cells, as compared to values of 0.9±0.2 and 1.7±0.6 in the high dose group. Thus, when considered as group data a positive relationship was found between circulating plasma insulin levels and adipocyte glucose transport Vmax, with increased Vmax values in hyperinsulinemic rats and decreased Vmax values in hypoinsulinemic rats. Furthermore, when the individual data were analyzed, highly significant correlation coefficients were found between the height of the plasma insulin level and both the basal (r = 0.82, P < 0.001) and insulin-stimulated (r = 0.93, P < 0.001) Vmax values. The apparent Km for 2-deoxy glucose uptake was the same under all conditions.
In conclusion, assuming that the Vmax of transport is some function of the number of glucose transport carriers per cell, then these results support the hypothesis that in addition to acute acceleration of glucose transport, insulin is also an important long-term regulator of the number of available adipocyte glucose transport carriers.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Czech M. P., Lawrence J. C., Jr, Lynn W. S. Hexose transport in isolated brown fat cells. A model system for investigating insulin action on membrane transport. J Biol Chem. 1974 Sep 10;249(17):5421–5427. [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Di Girolamo M., Mendlinger S., Fertig J. W. A simple method to determine fat cell size and number in four mammalian species. Am J Physiol. 1971 Sep;221(3):850–858. doi: 10.1152/ajplegacy.1971.221.3.850. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Osterlind K., Vinten J., Gammeltoft S. A procedure for measurement of distribution spaces in isolated fat cells. Biochim Biophys Acta. 1972 Nov 24;286(1):1–9. doi: 10.1016/0304-4165(72)90082-7. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Berger M., Ruderman N. B. Glucose metabolism in rat skeletal muscle at rest. Effect of starvation, diabetes, ketone bodies and free fatty acids. Diabetes. 1974 Nov;23(11):881–888. doi: 10.2337/diab.23.11.881. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- KIPINIS D. M. Regulation of glucose uptake by muscle: functional significance of permeability and phosphorylating activity. Ann N Y Acad Sci. 1959 Sep 25;82:354–365. doi: 10.1111/j.1749-6632.1959.tb44916.x. [DOI] [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. V. The penetration and phosphorylation of 2-deoxyglucose in the rat diaphragm. J Biol Chem. 1959 Jan;234(1):171–177. [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Sugar transport in chick embryo fibroblasts. I. A functional change in the plasma membrane associated with the rate of cell growth. J Biol Chem. 1974 Jun 10;249(11):3366–3374. [PubMed] [Google Scholar]
- MORGAN H. E., CADENAS E., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. II. Rate-limiting steps and effects of insulin and anoxia in heart muscle from diabetic rats. J Biol Chem. 1961 Feb;236:262–268. [PubMed] [Google Scholar]
- MORGAN H. E., HENDERSON M. J., REGEN D. M., PARK C. R. Regulation of glucose uptake in heart muscle from normal and alloxan-diabetic rats: the effects of insulin, growth hormone, cortisone, and anoxia. Ann N Y Acad Sci. 1959 Sep 25;82:387–402. doi: 10.1111/j.1749-6632.1959.tb44920.x. [DOI] [PubMed] [Google Scholar]
- Martin F. I., Stocks A. E. Insulin sensitivity and 131-I insulin metabolism in juvenile-type diabetics. Australas Ann Med. 1967 Nov;16(4):289–296. doi: 10.1111/imj.1967.16.4.289. [DOI] [PubMed] [Google Scholar]
- Martin F. I., Stocks A. E. Insulin sensitivity and vascular disease in insulin-dependent diabetics. Br Med J. 1968 Apr 13;2(5597):81–82. doi: 10.1136/bmj.2.5597.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musliner T. A., Chrousos G. P., Amos H. Transport enhancement and reversal: glucose and 3-O-methyl glucose. J Cell Physiol. 1977 May;91(2):155–168. doi: 10.1002/jcp.1040910202. [DOI] [PubMed] [Google Scholar]
- NARAHARA H. T., OZAND P. Studies of tissue permeability. IX. The effect of insulin on the penetration of 3-methylglucose-H3 in frog muscle. J Biol Chem. 1963 Jan;238:40–49. [PubMed] [Google Scholar]
- Olefsky J. M. Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1975 Dec;56(6):1499–1508. doi: 10.1172/JCI108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M. Mechanisms of the ability of insulin to activate the glucose-transport system in rat adipocytes. Biochem J. 1978 Apr 15;172(1):137–145. doi: 10.1042/bj1720137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Effects of age and obesity on insulin binding to isolated adipocytes. Endocrinology. 1975 Jun;96(6):1486–1498. doi: 10.1210/endo-96-6-1486. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Insulin binding in diabetes. Relationships with plasma insulin levels and insulin sensitivity. Diabetes. 1977 Jul;26(7):680–688. doi: 10.2337/diab.26.7.680. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. The effects of spontaneous obesity on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1976 Apr;57(4):842–851. doi: 10.1172/JCI108360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Reaven G. M., Bernstein R., Davis B., Olefsky J. M. Nonketotic diabetes mellitus: insulin deficiency or insulin resistance? Am J Med. 1976 Jan;60(1):80–88. doi: 10.1016/0002-9343(76)90536-2. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Sageman W. S., Swenson R. S. Development of insulin resistance in normal dogs following alloxan-induced insulin deficiency. Diabetologia. 1977 Sep;13(5):459–462. doi: 10.1007/BF01234496. [DOI] [PubMed] [Google Scholar]
- Renner E. D., Plagemann P. G., Bernlohr R. W. Permeation of glucose by simple and facilitated diffusion by Novikoff rat hepatoma cells in suspension culture and its relationship to glucose metabolism. J Biol Chem. 1972 Sep 25;247(18):5765–5776. [PubMed] [Google Scholar]
- Tsuboi K. K., Petricciani J. C. Concentrative accumulation (active transport) of 2-deoxy-D-glucose in primate fibroblasts. Biochem Biophys Res Commun. 1975 Feb 3;62(3):587–593. doi: 10.1016/0006-291x(75)90439-8. [DOI] [PubMed] [Google Scholar]
- Vinten J., Gliemann J., Osterlind K. Exchange of 3-O-methylglucose in isolated fat cells. Concentration dependence and effect of insulin. J Biol Chem. 1976 Feb 10;251(3):794–800. [PubMed] [Google Scholar]
- WICK A. N., DRURY D. R., NAKADA H. I., WOLFE J. B. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957 Feb;224(2):963–969. [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]


