Abstract
Background
Fevers and leukocytosis after pediatric craniotomy trigger diagnostic evaluation and antimicrobial therapy for possible brain infection. This study determined the incidence and predictors of infection in infants and children undergoing epilepsy neurosurgery.
Methods
We reviewed the postoperative course of 100 consecutive surgeries for pediatric epilepsy, comparing those with and without infections for clinical variables and daily maximum temperatures, blood WBC and differential, and cerebrospinal fluid (CSF) studies.
Results
Infections were the most common adverse events following these surgeries. Four patients (4%) had CSF infections and 12 had non-CSF infections (including one with distinct CSF and bloodstream infections). Most (88%) infections occurred before postoperative day 12 and were associated with larger resections involving ventriculostomies. Fevers (T ≥38.5°C) were observed in the first 12-days postsurgery in 43 % of cases, and were associated with patients undergoing hemispherectomy and multilobar resections. Fevers in the first three days postsurgery identified infections with 73% sensitivity, 69% specificity, and 70% accuracy; two (13%) patients with infections never developed fevers. Peripheral blood WBC >15,000 was found in 49% of patients and 5 cases of infections never had elevated WBC counts. WBC differential, CSF protein, RBC, WBC, and RBC/WBC ratios were poor predictors of infections. Longer hospital stays were associated with infections and hemispherectomy and multilobar resections. Patients with and without infections were equally likely to be seizure free after surgery.
Conclusions
Fevers and elevated blood WBC counts were common after pediatric epilepsy surgery, but CSF infections were uncommon. Positive cultures and other confirmatory microbiologic tests should drive changes in antimicrobial therapy after surgery.
Keywords: Seizure, neurosurgery, ventriculostomy, CSF, infection
INTRODUCTION
Neurosurgery is an increasingly used treatment for infants and young children with seizures unresponsive to anti-epileptic drugs (AEDs). Infants with refractory seizures are at risk for epilepsy-induced encephalopathy and sudden unexpected death [1–2]. Children undergoing epilepsy neurosurgery often have multiple daily seizures starting in the first months of life, usually from structural brain lesions such as cortical dysplasia, tumors, perinatal strokes, tuberous sclerosis complex, Rasmussen encephalitis, and Sturge-Weber syndrome [1]. Surgical treatments for such small children usually involve resection of multiple lobes of the brain and cerebral hemispherectomy, as compared with focal and lobar resections for older patients [3]. Such large resections in very young children increase the risk of perioperative complications including infection [4].
Postoperative fevers are common after cerebral hemispherectomy, occurring in 30 to 82% of cases [5–7]. Postoperative fevers usually result in repeated assessment for infection and may promote protracted empiric broad-spectrum antimicrobial therapy. Identifying factors that predict infection among patients after pediatric epilepsy surgery may improve clinical decision-making and cost effectiveness of diagnostic testing and treatment. This retrospective study from a single institution determined the frequency of CNS and non-CNS infection among children following epilepsy surgery and examined clinical factors and laboratory parameters associated with postoperative infections.
MATERIALS AND METHODS
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by UCLA’s IRB, and since enactment of federal HIPAA rules patients and families have signed research informed consents and authorizations. This study was not a clinical trial, and it is not registered in any public registry.
Study Population
This was a retrospective review of 100 consecutive epilepsy surgery patients undergoing resections (excluding corpus callosotomy) at UCLA’s Pediatric Epilepsy Program from November 2007 to February 2011 (inclusion criteria). A standard perioperative protocol was used to reduce the risk of infections. Antibiotics (cefazolin or, for allergic patients, clindamycin or vancomycin) were administered IV prior to skin incision, and repeated dosages given throughout the procedure as necessary. Foley catheters, arterial and central lines (triple lumen; subclavian, jugular, or groin) were placed using sterile techniques in the operating room after anesthetic induction. Antibiotics were continued for 24-hours postsurgery or until 24-hours after external ventriculostomy drains were removed. With the exception of children already intubated due to acute status epilepticus (SE), patients were extubated prior to arrival to the post-anesthesia care unit [8]. Foley catheters and most peripheral venous and arterial lines were discontinued within 48 hours of surgery. Central venous lines were removed at hospital discharge or changed to peripheral venous lines after two weeks. Blood, urine, and pulmonary samples were sent for bacterial culture for fevers ≥38.5 °C. Day of surgery was defined as day 0.
Ventriculostomy catheters were placed in the operating room for any resection that entered the cerebral ventricles except for anterior temporal lobe resections, and were not changed postsurgery. Cerebrospinal fluid (CSF) was sampled daily from the closed loop ventriculostomy system beginning on the second postoperative day, and sent for cell analysis, culture, and chemistry studies. Ventriculostomy catheters were removed if an overnight test clamp did not result in intracranial pressures greater than 18 cm of water.
Clinical Study Data Collected
The presurgical clinical protocols and evaluation process along with methods describing collection of clinical epilepsy variables, seizure outcome, surgical procedures, repeat operations, and complications have been previously described in detail [1, 9–10]. Additional information gathered for this study included the incidence and location of positive bacterial cultures, daily maximum temperature (Tmax), blood WBC count and differential, and CSF laboratory studies. Fever was defined as temperature of 38.5°C or higher at least once on a given day. An elevated blood WBC count was defined as greater than 15,000 cells/μL.
A case of infection was defined as: (1) microbiological/pathological (gram stain) confirmation of infection (usually from cultures), (2) diarrhea with positive assay for Clostridium difficile toxin, or (3) growth of respiratory pathogen from a bronchoscopy or endotracheal tube specimen with sufficient numbers of polymorphonuclear neurophils (PMN). Positive cultures from urine and tracheal aspirates in the absence of clinical symptoms and laboratory signs of infection (leukocyte esterase or nitrite detected in urinalysis, WBC in tracheal aspirates) were regarded as contaminants.
Study Design and Statistical Analysis
Patients with documented infections were compared with those without infections for differences in maximum daily temperatures, blood WBC counts, CSF findings, clinical epilepsy variables, surgery type and postsurgical outcomes. StatView 5 (SAS Institute, Inc. Cary, NC) and STATA (StataCorp, release 9.2) were used for statistical analysis. Univariate comparisons used Student’s t-tests, ANOVA, and Chi-square where appropriate. All tests were two-tailed and because of the multiple comparisons and large cohort size the threshold for significance was set a priori at p<0.01. If more than one variable was significant in univariate analysis we perform logistic regression or two-way ANOVA to examine the relationship between variables and infection.
RESULTS
Cohort and antibiotic usage
This cohort is similar to other studies from this center [1,2]. The mean (year±SD) age at seizure onset was 3.3±4.2, age at surgery was 8.1±5.8, and epilepsy duration was 4.8±4.5 (Table 1). Surgery was performed before 2 years of age in 20%, and 54% had cerebral hemispherectomy (n=43) or multilobar resections (n=11). Lobar/focal resections were in the frontal (n=21), temporal (n=15), and parietal lobes (n=10). Extraoperative intracranial electrodes were used in one patient in this series. Length of hospital stay averaged 9.9±7.2 days (median 8) (Fig. 1A; black bars). Most patients (n=89) were treated with prophylactic cefazolin. Allergic patients were treated with clindamycin (n=8) or vancomycin (n=2). One patient was receiving oral amoxicillin on admission for bronchiolitis. Cefazolin was given at surgery and amoxicillin was continued post-operatively.
Table 1.
Clinical features of pediatric epilepsy surgery with and without postoperative infections (n=100).
| Variable | Infected (n=15) | Non Infected (n=85) | p-value |
|---|---|---|---|
|
| |||
| Age Seizure Onset (years) | 1.1±1.8 | 3.7±4.4 | 0.024 |
|
| |||
| Age at Surgery (years) | 4.9±3.9 | 8.8±6.0 | 0.019 |
|
| |||
| Epilepsy Duration (years) | 3.9±2.9 | 5.0±4.7 | 0.362 |
|
| |||
| Female (n=46) | 53% | 45% | 0.536 |
|
| |||
| Left Resection (n=48) | 33% | 50% | 0.217 |
|
| |||
| History of Infantile Spasms (n=40) | 67% | 35% | 0.022 |
|
| |||
| Daily Seizures (n=77) | 79% | 75% | 0.623 |
|
| |||
| Repeat Craniotomy (n=15) | 13% | 15% | 0.844 |
|
| |||
| In Status Epilepticus (n=4) | 7% | 4% | 0.568 |
|
| |||
| Operation Type | |||
| Hemispherectomy (n=43) | 67% (n=10) | 39% (n=33) | 0.021 |
| Multilobar Resection (n=11) | 20% (n=3) | 9% (n=8) | |
| Lobar/Focal (n=46) | 13% (n=2) | 52% (n=44) | |
|
| |||
| Etiology | |||
| Cortical Dysplasia (n=45) | 33% (n=5) | 47% (n=40) | 0.0027* |
| Ischemia/Atrophy (n=12) | 7% (n=1) | 13% (n=11) | |
| Tuberous Sclerosis Complex (n=11) | 20% (n=3) | 9% (n=8) | |
| Tumor (n=10) | 7% (n=1) | 10% (n=9) | |
| Rasmussen (n=8) | 0% (n=0) | 9% (n=8) | |
| Hemimegalencephaly (n=5) | 20% (n=3) | 2% (n=2) | |
| Hippocampal Sclerosis (n=3) | 0% (n=0) | 4% (n=3) | |
| History of infection (n=2) | 13% (n=2) | 0% (n=0) | |
| Sturge-Weber (n=2) | 0% (n=0) | 2% (n=2) | |
| Non-Diagnostic (n=2) | 0% (n=0) | 2% (n=2) | |
|
| |||
| Seizure Free Last Follow-Up (n=93) | 71% | 75% | 0.797 |
|
| |||
| Other Complication (n=6) | 7% | 6% | 0.906 |
|
| |||
| New CSF Shunts (n=10) | 20% | 8% | 0.161 |
|
| |||
| Duration of Hospital Stay (days) | 17.9±10.2 | 8.5±5.5 | P<0.0001* |
|
| |||
| Private Insurance (n=77) | 87% | 75% | 0.335 |
|
| |||
| Ethnic Category | |||
| White (n=70) | 73% | 69% | 0.929 |
| Hispanic/Latino (n=16) | 13% | 16% | |
| Asian (n=9) | 13% | 8% | |
| African-American (n=2) | 0% | 2% | |
| Middle-East (n=2) | 0% | 2% | |
| Mixed (n=1) | 0% | 1% | |
Underlined values indicate significant contribution to Chi-square.
indicates that both factors remain significant (p<0.01) in logistical regression analysis. Data presented as mean (±SD) or percentages. Repeat craniotomy consisted of 7 patients having second surgery for incomplete cerebral hemispherectomy disconnections, 4 cases of enlargement of prior focal resections, 3 cases of staged resections for tuberous sclerosis complex, and 1 case of staged resection for Rasmussen encephalitis. All prior operations were before accruement for this cohort.
Significant values (p<0.01) indicated in Bold type.
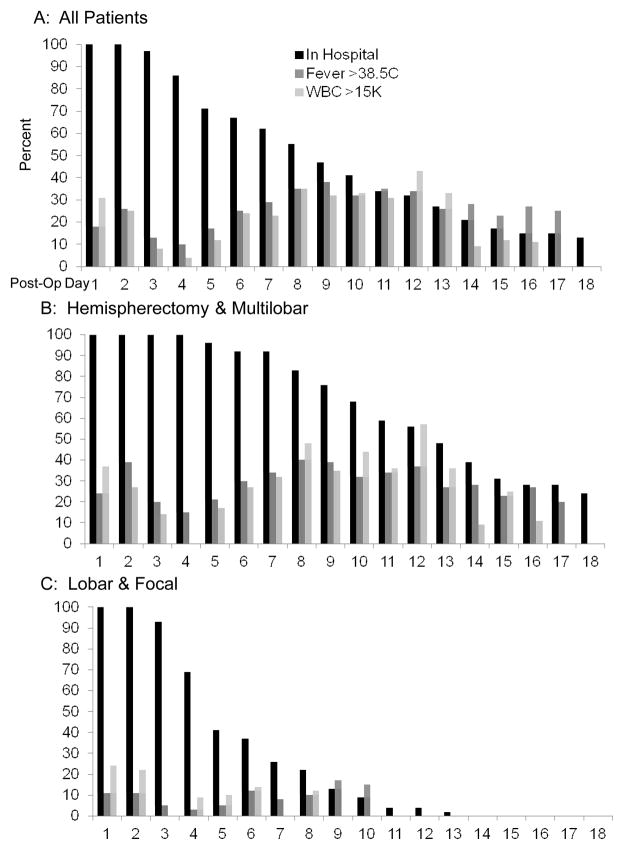
Figure 1.
Percentage of patients in hospital (black bars), with fevers (>38.5°C; medium gray bars), and elevated blood WBC counts (>15,000; light gray bars) for the first 18 days post pediatric epilepsy surgery for all patients (panel A), those undergoing cerebral hemispherectomy and multilobar resections (panel B), and those with lobar and focal resections (panel C).
Infections & Adverse Events
Thirty-one adverse events were identified in 26 children. Of these, infections were the most frequent (n=15 patients; 48% of complications), followed by CSF shunts for new hydrocephalus (n=10; 32%) and other complications (n=6; 19%) consisting of transient partial 3rd nerve palsy (n=2) and individual cases of deep venous thrombosis, scalp hematoma not requiring re-operation, unexpected transient visual field loss, and pseudomeningocele (non-operatively treated). All of the 15 patients with infections had received cefazolin, with 4 (27%) developing infections of the CSF and 12 (80%) non-CSF infections (Table 2). (Patient 3 had an Acinetobacter CSF infection on postoperative day 6, followed 11 days later by catheter associated bloodstream infections with the growth of Candida albicans and Pseudomonas putida in blood cultures). Urine infections were the most common (n=5), followed by CSF (n=4), stool (C. difficile infection) (n=2), pulmonary (n=2), wound (n=1), and blood (n=2). None of the infections were considered life threatening, as no patient became hemodynamically unstable, required respiratory assistance, or showed change in mental status. The average (±SD) number of postoperative days until submission of a specimen that provided microbiological confirmation of infection was 7.5 ± 5.3 days (range 1–22), and most infections (88%) were identified by postoperative day 12.
Table 2.
Surgeries, Organism, and Sites of Treated Infection in Pediatric Epilepsy Surgery Cases
| Surgery | Site | Organism | Age at Seizure Onset (y) | Age at Surg (y) | Etiology | POD when infection identified | Antibiotic, duration (days) | |
|---|---|---|---|---|---|---|---|---|
| 1 | Hemi | CSF | Pseudomonas aeruginosa | 0.8 | 9.3 | Cortical Dysplasia | 5 | Vancomycin, 3 Meropenem, 22 Ceftazidime, 3 Gentamicin,29 Piperacillin/tazobactam, 4 |
| 2 | Hemi | CSF | Enterobacter cloacae | 0.0 | 0.2 | Hemimeg. | 9 | Ceftriaxone, 7 Meropenem, 22 Gentamicin, 28 |
| 3 | Hemi | CSF and blood | Acinetobacter species in CSF, Candida albicans and Pseudomonas putida in blood | 5.8 | 7.8 | Hemimeg. | 6 CSF, 17-blood | Vancomycin, 6 Gentamicin, 7 Tobramycin, 8 Micafungin, 7 Ceftriaxone, 2 Meropenem, 21 Ciprofloxacin, 10 Fluconazole, 2 |
| 4 | Hemi | CSF | CONS | 0.8 | 3.5 | Cortical Dysplasia | 7 | Vancomycin, 16 Gentamicin, 2 Ceftriaxone, 16 Bacitracin, 6 |
| 5 | Hemi | Urine | Pseudomonas aeruginosa | 0.3 | 2.5 | Cortical Dysplasia | 6 | Ceftazidime, 4 |
| 6 | Hemi | Urine | Pseudomonas aeruginosa | Birth | 3.7 | Hemimeg | 22 | Vancomycin, 9 Gentamicin, 5 Cefepime, 2 Ceftriaxone, 7 Linezolid, 2 Silver Sulfadiazine, 7 |
| 7 | Lobar | Urine | Pseudomonas aeruginosa | 0.3 | 2.1 | Tuberous Sclerosis | 7 | Vancomycin, 5 Gentamicin, 4 Ciprofloxacin, 2 Ceftriaxone, 4 Ceftazidime, 5 |
| 8 | Multilobar | Urine | Pseudomonas aeruginosa | 0.1 | 1.4 | Tuberous Sclerosis | 2 | Ceftazidime, 2 Gentamicin, 2 |
| 9 | Lobar | Urine | Enterococcus species | 0.3 | 8.1 | Tuberous Sclerosis | 7 | Vancomycin, 2 Ceftriaxone, 2 |
| 10 | Hemi | Wound | CONS | 0.1 | 2.7 | Cortical Dysplasia | 5 | Vancomycin, 6 Ceftriaxone, 6 |
| 11 | Hemi | Blood | CONS | Birth | 1.0 | Cortical Dysplasia | 11 | Vancomycin, 5 Ceftazidime, 5 |
| 12 | Hemi | Resp | Staphylococcus aureus, Serratia marcescens, Candida tropicalis | 0.1 | 2.8 | Infection | 1 | Gentamicin, 12 Cotrimoxazole, 12 Rifampin, 12 Clindamycin, 3 Fluconazole, 9 Ciprofloxacin 3 |
| 13 | Hemi | Resp | respiratory syncytial virus | 1.0 | 8.8 | Infarction | 5 | Acyclovir 9 Cefdinir, 2 |
| 14 | Multilobar | Stool | Clostridium difficile | 4.0 | 6.5 | Infection | 6 | Vancomycin,7 Acyclovir, 12 Cidofovir, 3 Cefepime, 7 Metronidazole, 8 |
| 15 | Lobar | Stool | Clostridium difficile | 6.0 | 13.8 | NFI | 4 | Metronidazole, 1 |
POD = post-operative day. CONS = coagulase-negative staphylococci. Hemimeg =hemimegalencephaly. Hemi = cerebral hemispherectomy. NF1 = neurofibromatosis type 1.
Examination of clinical epilepsy variables showed that etiology of seizures was associated with the risk of postoperative infections (Table 1; p=0.0027). Three of five patients with hemimegalencephaly had infections as did both cases with a prior history of brain infection (case 12 had a history of recurrent Group B streptococcal meningitis in infancy and developed respiratory infection postsurgery, and Case 14 had a prior history of herpes simplex virus CNS infection and developed C. difficile stool infection postsurgery). By comparison, very few patients with ischemia/atrophy (1/12), tumors (1/10), and Rasmussen encephalitis (0/8) developed postsurgery infections.
Fevers and Infections
Temperatures of 38.5°C or more (fever) were recorded in 43 patients (43%) by the 18th postoperative day. In general, fever followed a biphasic time distribution. More patients had Tmax ≥38.5°C on postoperative day 2 and 3 than on postoperative day 4, and the fraction of febrile patients increased thereafter until day 9 postsurgery for those remaining in hospital (Fig. 1A; medium gray bars). Of the 37 patients who had at least one day with a Tmax ≥38.5°C in the first 3 days postsurgery, 36 (97%) again had a Tmax ≥ 38.5°C between days 4 to 12. By contrast, only 7 (12%) of 56 patients who were afebrile in the first 3 days postsurgery and remained in hospital and subsequently developed T >38.5 °C between postoperative days 4 and 12. Thus, most patients with early onset of fever continued to have fever beyond the first 3 post-operative days, while most patients remained afebrile throughout their hospital course if they did not develop fever within the first 3 days after neurosurgery.
The risk and the timing of fever onset were associated with the type of neurosurgical procedure. Fevers in the first 12 days were observed in 29 (67%) of patients after cerebral hemispherectomy and 7 (64%) after multilobar resections, but only 7 (15%) after more anatomically limited lobar/focal resections (p<0.0001). Six of the patients with later onset fever after the third postoperative day had undergone cerebral hemispherectomy. One of these developed a CSF infection (Table 2; Case 6).
Of the 15 cases with proven infections, two (13%) never developed a fever in hospital (Table 2; Case 13 and 15). For the remaining 13 cases, the day of the first fever did not correlate with the day of the documentation of infection (r=0.13; p=0.66). Hence, some patients with infections never developed a fever, and most of the fevers that developed after postsurgery day 3 were associated with large cerebral resections.
In univariate analysis, fevers in the first 3 days postsurgery were associated with younger age at surgery, history of infantile spasms, cerebral hemispherectomy, longer hospital stay, and documented infections (Table 3). Those with infections had a higher Tmax on day 1, 2, 3, 7, 8, and 10 postsurgery compared with patients without infections (Fig. 2A). Of the 15 patients with infections, 11 had at least one recorded fever in the first three days postsurgery, including 3 (75%) of the CSF and 8 (73%) of the non-CSF infections. Consequently, fever during the first three postoperative days had a sensitivity of 73%, specificity of 69% (59/85), and an accuracy of 70% (70/100). A logistical regression model was created with the univariate factors noted above as independent variables and fevers in the first three days postsurgery as the dependent variable. This analysis showed that cerebral hemispherectomy was associated with fevers in the first three days (p=0.0077), while none of the other factors including infections were significant (p>0.07).
Table 3.
Clinical features of pediatric epilepsy surgery with and without fevers in the first three days after surgery
| Fever 1st 3 days (n=37) | No Fever 1st 3 days (n=63) | p-value | |
|---|---|---|---|
|
| |||
| Infections (n=15) | 30% (n=11) | 6% (n=4) | 0.0016 |
|
| |||
| Age Seizure Onset (years) | 2.7±4.3 | 3.7±4.1 | 0.253 |
|
| |||
| Age at Surgery (years) | 6.1±5.2 | 9.4±5.5 | 0.0053 |
|
| |||
| Epilepsy Duration (years) | 3.4±3.3 | 5.7±4.8 | 0.011 |
|
| |||
| Female (n=46) | 32% | 52% | 0.035 |
|
| |||
| Left Resection (n=48) | 62% | 40% | 0.030 |
|
| |||
| History of Infantile Spasms (n=40) | 56% | 30% | 0.0089 |
|
| |||
| Daily Seizures (n=77) | 88% | 68% | 0.088 |
|
| |||
| Repeat Craniotomy (n=15) | 11% | 17% | 0.359 |
|
| |||
| In Status Epilepticus (n=4) | 8% | 2% | 0.108 |
|
| |||
| Operation Type | |||
| Hemispherectomy (n=43) | 62% | 32% | 0.0001* |
| Multilobar Resection (n=11) | 19% | 6% | |
| Lobar/Focal (n=46) | 19% | 62% | |
|
| |||
| Etiology | |||
| Cortical Dysplasia (n=45) | 35% | 52% | P=0.024 |
| Ischemia/Atrophy (n=12) | 16% | 10% | |
| Tuberous Sclerosis Complex (n=11) | 11% | 11% | |
| Tumor (n=10) | 8% | 11% | |
| Rasmussen (n=8) | 11% | 7% | |
| Hemimegalencephaly (n=5) | 13% | 0% | |
| Hippocampal Sclerosis (n=3) | 0% | 5% | |
| History of infection (n=2) | 5% | 0% | |
| Sturge-Weber (n=2) | 0% | 3% | |
| Non-Diagnostic (n=2) | 0% | 3% | |
|
| |||
| Seizure-Free Last Follow-Up (n=93) | 76% | 73% | 0.703 |
|
| |||
| New CSF Shunts (n=10) | 13% | 8% | 0.369 |
|
| |||
| Other Complication (n=6) | 3% | 8% | 0.287 |
|
| |||
| Duration of Hospital Stay (days) | 13.6±7.7 | 7.7±6.0 | p<0.0001 |
|
| |||
| Private Insurance (n=77) | 70% | 81% | 0.220 |
|
| |||
| Ethnic Category | |||
| White (n=70) | 68% | 71% | 0.674 |
| Hispanic/Latino (n=16) | 22% | 13% | |
| Asian (n=9) | 8% | 9% | |
| African-American (n=2) | 3% | 2% | |
| Middle-East (n=2) | 0% | 3% | |
| Mixed (n=1) | 0% | 2% | |
Underlined values indicate significant contribution to Chi-square.
Remains statistically significant (p=0.0077) in logistical regression analysis. Data presented as mean (±SD) or percentages. Significant values (p<0.01) indicated in Bold type.
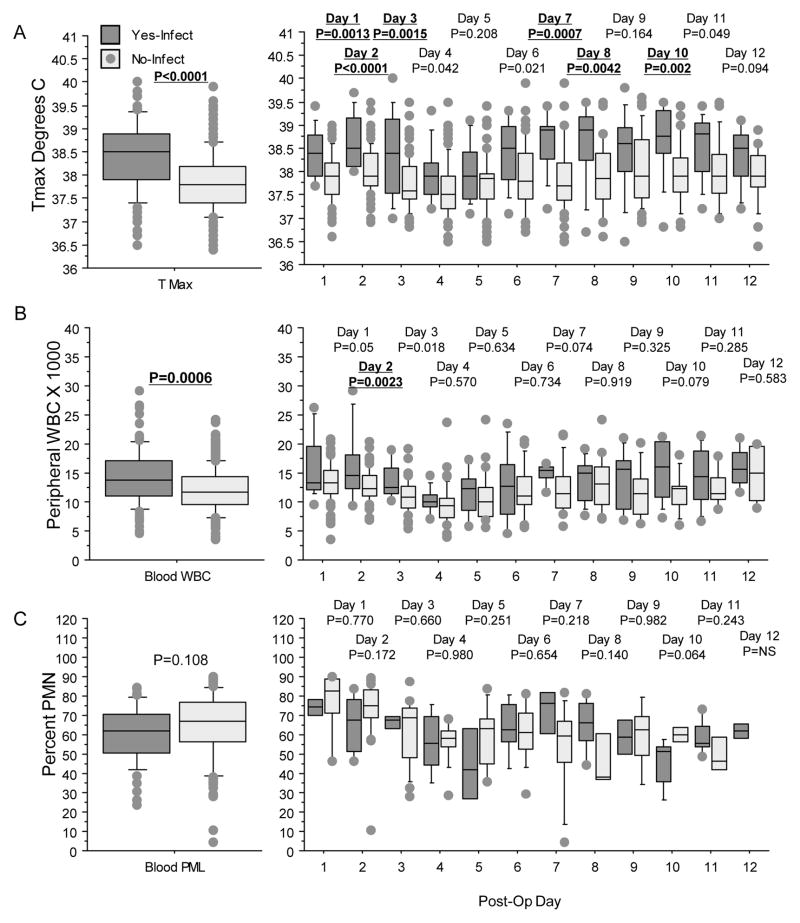
Figure 2.
Box plots displaying maximal daily temperature (A), blood WBC counts (B), and percent blood PMN (C) for those with (dark gray) and without (light gray) infections. Repeated measure ANOVA for all days is shown in the left column and individual days from one to 12 postsurgery shown in the right column. P-values shown above each plot, and significant results (p<0.01) highlighted in bold and underlined. For Tmax (A), repeated measure ANOVA found that those with infections had higher daily temperatures compared with non-infected cases (left graph). This was significant for postsurgery day one, 2,3,7,8, and 10 (right graph). For blood WBC counts (B), repeated measure ANOVA showed that cases with infections had higher counts compared with those without infections (left graph). Only the differences seen on day two postsurgery were significantly different. For blood PMN (C), there were no differences in the repeated measure ANOVA (left graph) and by individual days (right graph).
Peripheral Blood WBC Counts
WBC counts greater than 15,000/μl were noted in 49% patients in the first 12 days postsurgery, and followed a biphasic time distribution similar to that seen with the onset of fever (Fig. 1; light gray bars). Most (18/32) (56%) patients who had at least one WBC count >15,000 in the first three days postsurgery had at least one additional elevated blood white count recorded between postoperative day 4 to 12 if they remained hospitalized. By comparison, 9 (22%) of the 41 patients who had WBC counts less than 15,000 in the first three days postsurgery developed elevated WBC counts from day 4 to 12. Of these 9 cases, 8 involved cerebral hemispherectomy and one had a multilobar resection; one developed CSF infection (Table 2; Case 2). Of the 15 cases with infections, 5 (33%) never demonstrated an elevated blood WBC count while in hospital (Table 2; Cases 3, 5, 8, 13, and 15).
In univariate analysis, WBC counts >15,000 in the first three days postsurgery were not associated with any of the clinical variables used in this study including the development of infections (p=0.183; data not shown). Elevated WBC counts on day 2 postsurgery identified those with subsequent infections with a sensitivity of 40% (6/15), specificity of 79% (48/61), and accuracy of 71% (54/76) (Fig. 2B).
Blood WBC differential counts showed minimal differences comparing those with and without infections (Fig. 2C, 3A–D). Using repeated measure analysis of variance, the percentages of blood PMNs, lymphocytes, monocytes and basocytes were not different in those with and without infections (p>0.067). Univariate analysis found that blood lymphocyte and basocyte counts on day 2 were increased in those with infections compared with patients without infections. However, on the second day postsurgery, blood lymphocytes above 40% showed a sensitivity of 17% (1/7), and no infections were identified among those with basocytes above 1%.
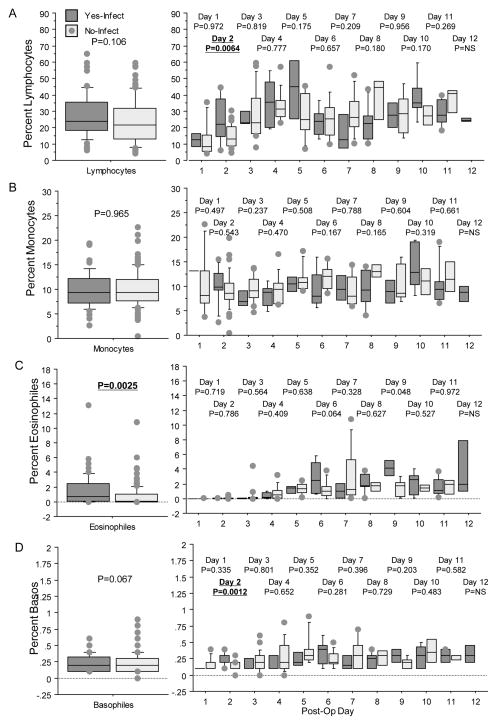
Figure 3.
Box plots displaying percent blood lymphocytes (A), percent blood monocytes (B), percent blood eosinophils (C), and percent blood basocytes (D) for those with (dark gray) and without (light gray) infections. Columns and p-values displayed as in Figure 2. For blood lymphocytes (A), the repeated measure ANOVA showed no differences (left graph) and by individual days there was a significant difference on day 2 with higher values for those with infections (right graph). Other days showed considerable variability in recorded values. For, blood monocytes (B), there were no differences in the repeated ANOVA (left graph) or in daily measures (right graph). For blood eosinophils (C), the repeated measure ANOVA showed higher levels for those with infections (left graph) but not for individual days (right graph). For blood basocytes (D), there was no difference in the repeated ANOVA (left graph), and review by individual days showed a difference on day two postsurgery and considerable variability thereafter.
CSF Studies
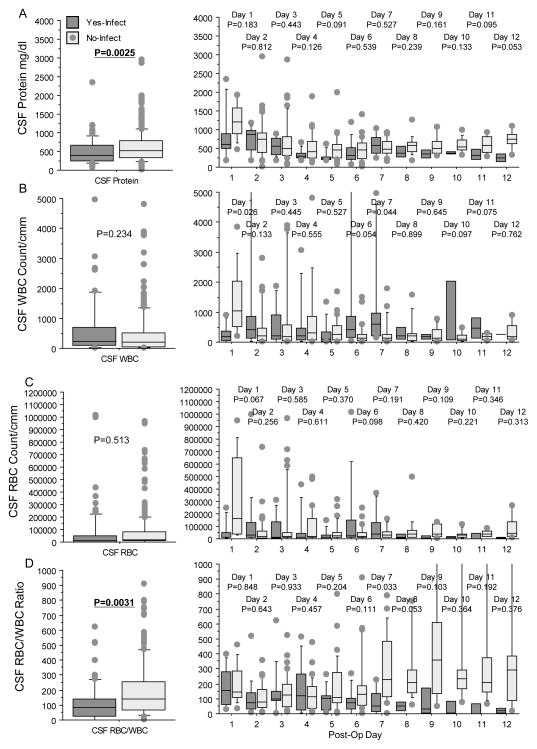
Ventriculostomy catheters were used in 59 (59%) of patients, and were employed in all 43 cases of cerebral hemispherectomy (100%), in seven (64%) multilobar resections, and in eight (17%) lobar/focal cases. The mean (±SD) duration of ventriculostomy was 7.2±3.7 days (median 6 days, range 2–21 days). Ventricular catheters were most often used in younger patients with larger resections, and 14 (24%) CSF and non-CSF infections occurred in those with ventricular catheters, compared with one (2%) infection in patients without CSF catheters (p=0.0034). Compared with patients without infections, those with infections had lower CSF protein levels and CSF RBC/WBC ratios (Fig. 4A, D), while CSF WBC and RBC counts were not different (Fig. 4B, C). However, the CSF protein levels and RBC/WBC ratios were not predictive of CSF infection; they increased after day 7, when three of four CSF infections had already been identified.
Figure 4.
Box plots displaying cerebrospinal (CSF) protein (A), CSF WBC count (B), CSF red blood cell (RBC counts (C), and CSF RBC/WBC ratio (D) for those with (dark gray) and without (light gray) infections. Columns and p-values displayed as in Figures 2 and 3. For CSF protein (A), repeated measure ANOVA showed that those with infections had lower protein levels compared without infections (left graph). Inspection by each day postsurgery showed no difference for any particular day and the difference was a general trend over time. For CSF WBC (B) and RBC counts (C), there were no differences in the repeated measure ANOVA (left graph), and considerable variability each day postsurgery (right graph). For CSF RBC/WBC ratios (D), repeated measure ANOVA showed lower ratios for those with infections compared with no infections (left graph). However, review of individual days showed that this was a general trend, especially after day 7, and there was considerable variability within groups over time.
Treatments and Epilepsy Outcome
The antimicrobial therapy given during hospitalization for the 15 infections varied considerably (Table 2), and was often initiated prior to specific identification of pathogens. Those with infections had longer hospital stays compared with those without infections (Table 1; p<0.0001). As patients undergoing cerebral hemispherectomy and multilobar resections also had longer hospital stays compared with lobar and focal resections (Fig. 1B and 1C), we performed a two-way ANOVA with type of operation and if patients become infected as independent variables and length of hospital stay as the dependent variable. Larger operations (p<0.0001) and becoming infected (p=0.011) were independently associated with longer length of hospital stay without a significant interaction (p=0.47).
There were no cases of acute mortality after surgery. Seizure outcome was available for 93 patients, with a mean (±SD) follow-up of 1.6±0.9 years. Of these, 74% were seizure free and there were no differences in surgical outcome for those with and without infections, fever and elevated blood WBC counts (Tables 1 and 3).
DISCUSSION
In comparison to the rate of confirmed infections (15% of infants and children), fevers and elevated blood peripheral WBC counts were much more common (43% and 49%) in the first twelve days after epilepsy surgery in this pediatric cohort. The risk of CSF infection in this cohort was 4%, comparable to that observed in other series of pediatric epilepsy patients undergoing neurosurgery (1.3–5.7%) [6, 11–12].
The overall rate of infection in this study was comparable to that observed in one randomized controlled trial using cefazolin and gentamicin prophylaxis for all craniotomies (9.8%)[13], but higher than observed in trials using vancomycin or fusidic acid (1.8–2.4%)[14–15]. This difference in the rate of all infections may be due to the younger age of the children in our study population and their unique surgical indications. The development of infections was associated with two baseline characteristics: larger resections (cerebral hemispherectomy and multilobar resections) and use of ventriculostomy catheters (24% among those having CSF drainage catheters placed during surgery vs. 2% among those without catheters). Most of the infections in those with the presence of ventricular catheters did not involve the CSF, and likely reflect the risks associated with the more protracted hospitalizations and longer prophylactic antibiotic therapy that occur with larger neurosurgical resections in younger children.
Prophylactic antibiotic use is associated with reduced post-craniotomy infection rates in adults, including a 52% reduction in incision infections [4, 16–17]. One meta-analysis of data from six randomized controlled studies indicated that prophylactic antibiotic treatment was associated with a pooled odds ratio of 0.43, indicating a reduced risk of meningitis (p =0.03) [4]. In our cohort, prophylactic cefazolin provided gram-positive and limited gram-negative coverage. Three of the four CSF infections were due to organisms that are uniformly resistant to this antibiotic. It is reasonable to consider other prophylactic antibiotic regimens, but the cost-effectiveness and risks of administering additional or broader spectrum antibiotics in children undergoing elective craniotomies for epilepsy are not known. A randomized controlled trial would be needed to assess the risks and benefits of such a change.
Postoperative fevers were common, and understandably evoked concern. The development of fevers in the first 3 days postsurgery was associated with younger age at surgery, history of infantile spasms, and cerebral hemispherectomy. Fevers within the first 12 days were observed more frequently in patients with larger resections. Furthermore, there was a bimodal distribution of fevers >38.5°C in the first 3 days and again from day 6 to 14 postsurgery that was weakly associated with the development of infection: fevers in the first 3 postoperative days only predicted infection with 73% sensitivity and 69% specificity. Similarly, WBC >15,000 was common postsurgery and followed a biphasic time distribution. WBC counts on day 2 postsurgery were higher in patients with infections compared to patients without infections but WBC on post-operative day 2 predicted an infection with a sensitivity of 40% and specificity of 79%. No other parameters (WBC differential, CSF differential, CSF protein levels, and CSF RBC/WBC ratios) were useful in differentiating between infection and aseptic meningitis. Instead, cultures of blood, urine, and ventricular CSF allowed recognition of infection in most cases. Diarrhea and respiratory tract symptoms led to recognition of Clostridium difficile and respiratory virus infection in other cases. Parameters of inflammation such as serum C- reactive protein or procalcitonin, or surface expression of CD64 expression on phagocytes were not measured in this study, but may be worthy of examination, based on their potential utility in differentiating infection from other causes of inflammation.[18–19].
There are several limitations of this study. This was a retrospective analysis and blood laboratory tests were not performed on a daily basis for every patient. Blood laboratory tests were obtained from 94% to 60% of our cohort in the first three days postsurgery, that declined to about 50% from day 4 to 12 postsurgery in all patients. Future prospective studies may need to include daily WBC counts to fully assess their utility. Daily CSF studies were not performed in those without ventriculostomy catheters. However, most patients had these studies, especially in the first five to seven days postsurgery. Furthermore, this study presumed no infections occurred among those with negative cultures and other microbiological studies. Finally, our results are most germane to pediatric patients undergoing craniotomy for epilepsy surgery procedures. It is unclear if similar results would be found for other types of large pediatric neurosurgical procedures.
Despite these limitations, this study provides useful information on the incidence and types of infections after large cranial surgical procedures in infants and children, and shows that larger resections and the ongoing presence of CSF drainage catheters increase the risk of identifying infections following neurosurgery for epilepsy in pediatric patients. These data demonstrate that current prophylactic clinical protocols were associated with a low rate of CSF infection despite the frequent observation of fevers and elevated blood WBC counts. They highlight the low utility of repeatedly performing peripheral blood white blood cell counts and CSF cytology (RBC and WBC counts). Instead, cultures and other selected microbiological tests should be emphasized when infection is suspected in this setting. Future studies should focus on identification of more accurate predictors of infection and examine the risks and benefits of expanded antimicrobial therapy for pediatric patients at highest risk of post-operative infection.
Acknowledgments
Funding: This work was supported by grants from the National Institutes of Health [R01 NS38992] to GWM and the Short Term Training Program (STTP) at David Geffen School of Medicine at UCLA to JP.
References
- 1.Hemb M, Velasco TR, Parnes MS, et al. Improved outcomes in pediatric epilepsy surgery: the UCLA experience, 1986–2008. Neurology. 2010;74:1768–75. doi: 10.1212/WNL.0b013e3181e0f17a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peacock WJ, Wehby-Grant MC, Shields WD, et al. Hemispherectomy for intractable seizures in children: a report of 58 cases. Childs Nerv Syst. 1996;12:376–84. doi: 10.1007/BF00395089. [DOI] [PubMed] [Google Scholar]
- 3.Jonas R, Asarnow RF, LoPresti C, et al. Surgery for symptomatic infant-onset epileptic encephalopathy with and without infantile spasms. Neurology. 2005;64:746–50. doi: 10.1212/01.WNL.0000151970.29205.70. [DOI] [PubMed] [Google Scholar]
- 4.Barker FG., 2nd Efficacy of prophylactic antibiotics against meningitis after craniotomy: a meta-analysis. Neurosurgery. 2007;60:887–94. doi: 10.1227/01.NEU.0000255425.31797.23. discussion -94. [DOI] [PubMed] [Google Scholar]
- 5.de Almeida AN, Marino R, Jr, Aguiar PH, Teixeira MJ. Postoperative fever after hemispherectomy: the role of non-infectious factors. Seizure. 2006;15:340–3. doi: 10.1016/j.seizure.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Kossoff EH, Vining EP, Pyzik PL, et al. The postoperative course and management of 106 hemidecortications. Pediatr Neurosurg. 2002;37:298–303. doi: 10.1159/000066309. [DOI] [PubMed] [Google Scholar]
- 7.Sood S, Asano E, Chugani HT. Role of external ventriculostomy in the management of fever after hemispherectomy. J Neurosurg Pediatr. 2008;2:427–9. doi: 10.3171/PED.2008.2.12.427. [DOI] [PubMed] [Google Scholar]
- 8.Koh S, Mathern GW, Glasser G, et al. Status epilepticus and frequent seizures: incidence and clinical characteristics in pediatric epilepsy surgery patients. Epilepsia. 2005;46:1950–4. doi: 10.1111/j.1528-1167.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- 9.Cook SW, Nguyen ST, Hu B, et al. Cerebral hemispherectomy in pediatric patients with epilepsy: comparison of three techniques by pathological substrate in 115 patients. J Neurosurg. 2004;100:125–41. doi: 10.3171/ped.2004.100.2.0125. [DOI] [PubMed] [Google Scholar]
- 10.Mathern GW, Giza CC, Yudovin S, et al. Postoperative seizure control and antiepileptic drug use in pediatric epilepsy surgery patients: the UCLA experience, 1986–1997. Epilepsia. 1999;40:1740–9. doi: 10.1111/j.1528-1157.1999.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Hwang YS, Shin JJ, Kim TH, Shin HS, Park SK. Surgical complications of epilepsy surgery procedures : experience of 179 procedures in a single institute. J Korean Neurosurg Soc. 2008;44:234–9. doi: 10.3340/jkns.2008.44.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinbok P, Gan PY, Connolly MB, et al. Epilepsy surgery in the first 3 years of life: a Canadian survey. Epilepsia. 2009;50:1442–9. doi: 10.1111/j.1528-1167.2008.01992.x. [DOI] [PubMed] [Google Scholar]
- 13.Young RF, Lawner PM. Perioperative antibiotic prophylaxis for prevention of postoperative neurosurgical infections. A randomized clinical trial. J Neurosurg. 1987;66:701–5. doi: 10.3171/jns.1987.66.5.0701. [DOI] [PubMed] [Google Scholar]
- 14.Blomstedt GC, Kytta J. Results of a randomized trial of vancomycin prophylaxis in craniotomy. J Neurosurg. 1988;69:216–20. doi: 10.3171/jns.1988.69.2.0216. [DOI] [PubMed] [Google Scholar]
- 15.Mindermann T, Zimmerli W, Gratzl O. Randomized placebo-controlled trial of single-dose antibiotic prophylaxis with fusidic acid in neurosurgery. Acta Neurochir (Wien) 1993;121:9–11. doi: 10.1007/BF01405175. [DOI] [PubMed] [Google Scholar]
- 16.Korinek AM, Baugnon T, Golmard JL, van Effenterre R, Coriat P, Puybasset L. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery. 2006;59:126–33. doi: 10.1227/01.NEU.0000220477.47323.92. discussion -33. [DOI] [PubMed] [Google Scholar]
- 17.Korinek AM, Golmard JL, Elcheick A, et al. Risk factors for neurosurgical site infections after craniotomy: a critical reappraisal of antibiotic prophylaxis on 4,578 patients. Br J Neurosurg. 2005;19:155–62. doi: 10.1080/02688690500145639. [DOI] [PubMed] [Google Scholar]
- 18.Groselj-Grenc M, Ihan A, Pavcnik-Arnol M, Kopitar AN, Gmeiner-Stopar T, Derganc M. Neutrophil and monocyte CD64 indexes, lipopolysaccharide-binding protein, procalcitonin and C-reactive protein in sepsis of critically ill neonates and children. Intensive Care Med. 2009;35:1950–8. doi: 10.1007/s00134-009-1637-7. [DOI] [PubMed] [Google Scholar]
- 19.Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006;34:1996–2003. doi: 10.1097/01.CCM.0000226413.54364.36. [DOI] [PubMed] [Google Scholar]