Abstract
Background
Communication between patients and clinicians using collaborative goals and treatment plans may overcome barriers to achieving hypertension control in routine diabetes mellitus care. We assessed the interrelation of patient–clinician communication factors to determine their independent associations with hypertension control in diabetes care.
Methods and Results
We identified 566 older adults with diabetes mellitus and hypertension at the DeBakey VA Medical Center in Houston, Tex. Clinical and pharmacy data were collected, and a patient questionnaire was sent to all participants. A total of 212 individuals returned surveys. Logistic regression analyses were performed to assess the effect of patient characteristics, self-management behaviors, and communication factors on hypertension control. Three communication factors had significant associations with hypertension control. Two factors, patients' endorsement of a shared decision-making style (odds ratio 1.61, 95% confidence interval 1.01 to 2.57) and proactive communication with one's clinician about abnormal results of blood pressure self-monitoring (odds ratio 1.89, 95% confidence interval 1.10 to 3.26), had direct, independent associations in multivariate regression. Path analysis was used to investigate the direct and indirect effects of communication factors and hypertension control. Decision-making style (β=0.20, P<0.01) and proactive communication (β=0.50, P<0.0001) again demonstrated direct effects on hypertension control. A third factor, clinicians' use of collaborative communication when setting treatment goals, had a total effect on hypertension control of 0.16 (P<0.05) through its direct effects on decision-making style (β=0.28, P<0.001) and proactive communication (β=0.22, P<0.01).
Conclusions
Three communication factors were found to have significant associations with hypertension control. Patient–clinician communication that facilitates collaborative blood pressure goals and patients' input related to the progress of treatment may improve rates of hypertension control in diabetes care independent of medication adherence.
Keywords: hypertension, diabetes mellitus, physician—patient relations, outcomes assessment, goals
Uncontrolled hypertension is the primary risk factor for the macrovascular complications of diabetes mellitus.1 In the United Kingdom Prospective Diabetes Study, a major cause of cardiovascular deaths among patients with type 2 diabetes mellitus was uncontrolled systolic hypertension.2 Clinical trials have demonstrated reductions in cardiovascular morbidity when blood pressure is lowered below the conservative 140/90 mm Hg target.1,3 Despite the availability of numerous treatments for hypertension in the setting of diabetes mellitus,4,5 rates of hypertension control vary from 30% to 53% even when conservative standards are used.6–8
Editorial p 1355 Clinical Perspective p 1368
Frequently cited reasons for low rates of hypertension control among diabetic individuals include poor access to regular medical care and affordable health insurance.9,10 Factors attributed to clinicians include lack of knowledge or disagreement with treatment guidelines and either a reluctance to intensify treatment or overestimation of the intensity of care provided (ie, clinical inertia).11–13 Furthermore, patients may not adhere to their treatment plans successfully because hypertension is often asymptomatic, the side effects of medications are troublesome,14 and the complexity of treatment regimens for multiple diabetes comorbidities may limit patients' self-efficacy. Out-of-pocket costs, poor social support, and limited health literacy may also interfere with treatment persistence.15,16 In the present study, we propose that the quality of communication between patients and clinicians may help overcome some of these barriers.17–19
The quality of patient–clinician communication has been associated with improved health outcomes for chronic illnesses,20,21 particularly communication that is collaborative (ie, focused on shared understanding of treatment goals and plans) and proactive (ie, encourages patients to recognize and discuss treatments that do not meet goals).22–24 Adherence to treatment is the commonly attributed mechanism by which effective health communication leads to improved health outcomes. The effectiveness of patient-clinician communication through other mechanisms such as enhanced self-efficacy, concordance with regard to treatment goals, and communication about inadequate disease control despite treatment adherence have been less well studied within the context of routine diabetes care but are common elements of protocols in clinical trials.25 The objectives of the present study were to evaluate the independent associations of various communication factors with hypertension control and to characterize the interrelationships of communication and behavioral factors, such as medication adherence, to illustrate the important indirect and total effects of these various measures with hypertension control in older adults with diabetes mellitus.25
Methods
Patient Selection
Study participants were selected among primary care patient panels at the Michael E. DeBakey Veterans Affairs (VA) Medical Center, Houston, Tex, who were receiving ongoing care for diabetes mellitus and hypertension between July 1, 2002, and March 31, 2005. Patients were eligible for inclusion in the study if they were between 50 and 90 years of age, were under treatment for diabetes mellitus and hypertension diagnosed by use of validated criteria,26 and were taking more than 1 medication for hypertension. Exclusion criteria included a diagnosis of dementia or a hemoglobin A1C level >8.5% or serum creatinine level ≥2 mg/dL at the most recent measurement as of April 2005. These criteria were selected to identify middle-aged to older comorbid patients receiving ongoing treatment but without diagnoses for difficult-to-control hypertension. Potential subjects were then stratified into controlled and uncontrolled hypertension categories on the basis of mean blood pressure measurements from all available inpatient and outpatient recordings during the study period. Controlled hypertension was defined as systolic blood pressure (SBP) ≤130 mm Hg and diastolic blood pressure (DBP) ≤80 mm Hg, and uncontrolled hypertension was defined as SBP >140 mm Hg or DBP >90 mm Hg.3 These categories were chosen to identify 2 distinct groups with limited potential for crossover.
The VA electronic warehouse of computerized patient medical records served as the source of data for identification of eligible patients, and established computer algorithms were used to define the target population and measure key variables.27 We selected 566 potential subjects, 282 in the controlled hypertension group with a mean of 9.2 (range 1 to 34) measurements and 284 in the uncontrolled hypertension group with a mean of 10.3 (range 1 to 43) measurements, from the algorithm-derived target population and mailed a comprehensive questionnaire about their communication and self-care behaviors for hypertension and diabetes control. A single mailing occurred from July to August 2005 and included a $2 patient incentive and an information sheet about the study aims. From this group, 212 (37%) returned completed questionnaires, which constitutes the analytical sample of the present study, including 110 (39%) with uncontrolled and 102 (36%) with controlled hypertension. Compared with the 174 individuals with uncontrolled hypertension who did not return their questionnaires, the 110 study participants with uncontrolled hypertension who did return their questionnaires were significantly more likely to have lower SBP (152.5±10.9 versus 156.4±14.2 mm Hg, P=0.02) and DBP (75.4±10.5 versus 78.8± 11.3 mm Hg, P =0.01) measurements and significantly more likely to have lower hemoglobin A1C levels (6.98±1.2% versus 7.2±1.3%, P=0.034), but they did not differ in any other measured characteristics. No significant differences in clinical or demographic characteristics could be identified between the 102 study participants with controlled hypertension and the 180 individuals with controlled hypertension who did not return questionnaires.
Clinical and Demographic Characteristics
Data were available for the following characteristics during the entire study period: age, race, gender, calculated body mass index (BMI), Deyo comorbidity index,28 number and type of medications prescribed and refilled for hypertension control, all SBP and DBP measurements, and all serum measurements for hemoglobin A1C, glucose, low-density lipoprotein, total cholesterol, and creatinine.
Patient Questionnaire Data
All target participants were mailed a 63-item questionnaire to evaluate patients' self-report of their motivation and intention to control their hypertension and diabetes mellitus,29–31 goals and strategies for hypertension and diabetes control,23 awareness of blood pressure and glucose targets,30 self-monitoring behaviors, readiness to negotiate treatment changes with clinicians, self-reported adherence to blood pressure medications and self-care behaviors,32 and other demographic information. A pilot test of the questionnaire was performed on 57 individuals with hypertension and diabetes mellitus with use of the same subject selection and questionnaire mailing protocol.
A 9-item intention (to treat hypertension) index was developed with items adapted from previously validated measures to assess 3 domains of behavioral intention33: risk perception,30 outcome expectancy,29 and self-efficacy31 (see Table 1 for items and validation). In preliminary investigations, we found that the Deyo comorbidity score was significantly associated with a lower intention index (P=0.002), and lower self-assessment of health literacy was modestly associated with a higher intention index (P=0.04) in a multivariate linear regression analysis.
Table 1. Descriptive Summary of Scales Used in Analyses.
| Variables | Item(s) | Range | Score Interpretation | Mean (SD) | |
|---|---|---|---|---|---|
| Intention to control hypertension index | 1 to 45 | 38.8 (6.3) | Confirmatory Factor Analysis | ||
| Risk perception outcome expectancy |
|
Higher score indicates greater intention to control hypertension | χ2=31.7, P=0.14, RMSEA=0.035 | ||
| Self-efficacy for hypertension control |
|
||||
| Collaborative care for hypertension | 1 to 20 | 10.5 (4.8) | Cronbach's α=0.93 | ||
| Patient activation |
|
0–10=Patient perception of a “less collaborative care” environment | |||
| Goal setting |
|
11–20=Patient perception of a “more collaborative care” environment | |||
| Decision-making style | “When it comes to making decisions about my care, I am most comfortable when”: | 1 to 3 | 2.22 (0.77) | ||
|
3=Shared decision-making preference 2=Intermediate 1= Unitary decision-making preference | ||||
| Proactive communication after BP self-monitoring | “When I check my BP on my own and my BP is high, I contact my doctor or nurse”: | 1 to 3 | 1.62 (0.67) | ||
|
3=Proactive communication with clinicians about self-monitoring results 1= Passive communication with clinicians about self-monitoring results | ||||
RMSEA indicates root mean square error of approximation; HBP, high blood pressure; and BP, blood pressure.
A series of patient–clinician communication assessment items were included in the patient questionnaire. Among them was the “collaborative care for hypertension” index, which was adapted from 2 subscales of the Patient Assessment of Chronic Illness Care scale34 and consisted of 4 items. The Patient Assessment of Chronic Illness Care is a patient self-report instrument validated to assess the extent to which patients with chronic conditions receive care that aligns with the chronic care model (ie, care that is patient-centered, proactive, and planned and includes collaborative goal setting, problem solving, and follow-up).35 We adapted 2 items from the “patient activation subscale” that best measured the presence of actions that solicit patient input and involvement in decision making.34 The other 2 items were items adapted from the “goal-setting/tailoring subscale” that best measured the acquisition of information for and setting of specific collaborative treatment goals.34 All 4 items were scored along a 5-point scale and summed to create the collaborative care for hypertension index used in the present study. For all descriptive and statistical comparisons, this index was dichotomized to “less” of a collaborative care environment and “more” of a collaborative care environment (see Table 1 for items and scoring). The decision-making style item is another validated measure.36,37 The measure assesses patients' preference for making decisions with their clinicians about treatment choices along a 5-point scale. We used a recent adaptation that scored the measure from least to most shared in orientation (Table 1).23
Several items assessed the use and frequency of self-monitoring practices for diabetes and hypertension control. These included items that asked about home blood pressure monitoring and adherence to a low-salt diet, getting regular exercise, and reducing smoking.32 In addition, 1 item assessed the extent of patients' proactive communication after blood pressure self-monitoring by linking ongoing monitoring of blood pressure with how and when patients notified clinicians of abnormal results (see Table 1 for item and scoring).
Medication Adherence Measures
The quantity and type of medications for hypertension control were actively tracked with the VA computerized database and pharmacy records for all study participants. Two measures were developed to assess patient adherence with prescribed medications for hypertension. A self-report item was adapted for antihypertensive medication use and was included in the patient questionnaire; this item read, “In the past 7 days, on how many days did you take your recommended high blood pressure medications?”32 In addition, a longitudinal medication refill adherence measure was constructed with VA pharmacy data to determine the percentage of weeks during the study in which participants' medications were appropriately refilled; this was achieved by aggregating antihypertensive medication refill data and accounting for gaps in prescription refills.38 Poor refill adherence was defined as >15% of weeks in the total study period for which a gap was present in refilling all prescribed antihypertensive medications. The cutoff for this adherence measure was selected because it constituted the lowest quartile for refill adherence in the present study population. Only the objective poor refill adherence measure is used in the path analyses.
Statistical Analyses
Descriptive statistics were used to characterize the target population and study participants, with categorical variables expressed as percentages and continuous variables expressed as mean±SD. We determined the bivariate and multivariate associations between hypertension control status and all the variables in our conceptual model using logistic regression to test our study hypotheses. Clinical and demographic covariates were also included in the multivariable analysis if an association with hypertension control at a P<0.10 level of significance was present in the bivariate analyses. All multivariate analyses were 2-sided, with a significance threshold of P<0.05. Multivariable analyses were conducted with SAS software, version 8.2 (SAS Institute Inc, Cary, NC).
A structural equation model that used path analysis was conducted to investigate the full range of effects (total, direct, and indirect) of each variable on other study variables and on the outcome of hypertension control.39 Path analysis allows for the simultaneous conduction of multiple linear regression analyses; thus, it permits (1) the specification of complex models with multiple variable influences and (2) the determination of the total effects of independent variables on dependent variables in the model. These include the direct effects typically modeled in pairwise and regression correlations plus the indirect effects mediated through other variables illustrated in the path model. Path analysis does not discover or confirm causality, but it can determine and illustrate whether the data are consistent with the researcher's causal hypothesis. Furthermore, indirect effects are not synonymous with confounding bias. Variables with indirect effects in the causal model do not have significant bivariate associations with hypertension control that may be mitigated by the introduction of confounding factors. All path analyses were conducted with LISREL software, version 8 (Scientific Software International, Lincolnwood, Ill).
The proposed path analytic model's exogenous variables, the variability of each of which was assumed to be determined by causes outside the causal model, are the study variables “intention” and “BMI.” The model's endogenous variables, the variability of each of which was to be explained by either exogenous or endogenous model variables, were the following study variables: collaborative care, poor refill adherence, decision-making style, proactive communication, and hypertension control. Because model variables ranged in nature from being dichotomous to ordinal to continuous, a polychoric correlation matrix was considered appropriate for use in the analysis. Rather than a pairwise approach to case retention, a listwise selection of cases was used for the correlation matrix, which ensured the use of data from a uniform set of cases for each bivariate element in the matrix.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Study participants were stratified by hypertension control status based on all inpatient and outpatient blood pressure measurements taken during the study period. Participants in the uncontrolled hypertension group had a mean SBP of 152.5±10.9 mm Hg compared with 118.5±9.8 mm Hg for those in the controlled hypertension group (P<0.0001). Similar differences were present in mean DBP between participants in the uncontrolled (75.4±10.5 mmHg) versus controlled (63.8±9.0 mmHg) hypertension groups (P<0.0001). The descriptive characteristics of the 212 study participants are shown in Table 2, stratified by hypertension control status. Statistically significant differences were present in SBP and DBP between groups when measurements from (1) all outpatient visits and (2) only primary care provider visits were considered. BMI was the only measured clinical or demographic variable that differed significantly between groups. Despite the complexity of illness in this population (ie, multiple morbidities and polypharmacy), both groups had moderately well-controlled to well-controlled diabetes mellitus and hyperlipidemia.
Table 2. Characteristics of the Study Population (n=212).
| Status of Hypertension Control | |||
|---|---|---|---|
|
|
|||
| Patient Characteristics | Controlled (n=102) | Uncontrolled (n=110) | P |
| Age, mean±SD, y | 66.4±8.4 | 67.4±9.2 | 0.38 |
| Black, n (%) | 16 (16) | 23 (21) | 0.33 |
| Male, n (%) | 100 (98) | 109 (99) | 0.52 |
| Some college education, n (%) | 63 (62) | 61 (55.5) | 0.53 |
| BMI, mean±SD, kg/m2 | 31.3±6.0 | 33.0±6.2 | <0.05 |
| Deyo comorbidity score, mean±SD | 3.06±1.4 | 3.05±1.4 | 0.98 |
| No. of BP medications, mean±SD | 2.30±0.54 | 2.35±0.66 | 0.54 |
| Hemoglobin A1C, mean±SD, % | 6.95±1.16 | 7.0±1.17 | 0.75 |
| Serum creatinine, mean±SD, mg/dL | 1.2±0.6 | 1.2±0.5 | 0.74 |
| LDL cholesterol, mean±SD, mg/dL | 98.9±33.3 | 99.0±32.8 | 0.98 |
| All outpatient SBP, mean±SD, mm Hg | 131.1±11.1 | 146.3±12.4 | <0.0001 |
| All outpatient DBP, mean±SD, mm Hg | 68.5±7.9 | 74.6±8.0 | <0.0001 |
| Primary care provider SBP, mean±SD, mm Hg | 131.9±11.3 | 149.0±12.0 | <0.0001 |
| Primary care provider DBP, mean±SD, mm Hg | 69.1 ±7.9 | 74.7±7.9 | <0.0001 |
BP indicates blood pressure.
Table 3 describes the association of multiple factors with hypertension control in the present study population. These factors are grouped by patient characteristics, self-management behaviors, and communication factors. The results of the bivariate logistic regressions are displayed for selected variables in Table 3. BMI, poor refill adherence, decision-making style, and proactive communication were the only variables significantly associated with hypertension control. Three of these 4 variables remained significant in a multivariable logistic regression (Table 3). Two communication measures remained independently significant even after we accounted for poor refill adherence.
Table 3. Logistic Regression Models for Characteristics Associated With Hypertension Control (n=212).
| OR (95% CI) for Hypertension Control | ||
|---|---|---|
|
|
||
| Variables | Bivariate Analyses | Multivariate Analyses |
| Patient characteristics | ||
| BMI Index | 0.95 (0.91–1.00) | 0.95 (0.89–1.00) |
| Deyo comorbidity score | 1.00 (0.82–1.22) | ⋯ |
| Self-management behaviors | ||
| Intention to control hypertension index | 1.02 (0.98–1.07) | 1.01 (0.96–1.07) |
| Home blood pressure self-monitoring | 1.48 (0.73–3.00) | ⋯ |
| Poor adherence with pharmacy refills | 0.29 (0.15–0.57) | 0.33 (0.15–0.72) |
| Medication adherence (self-report) | 1.50 (0.075–3.03) | ⋯ |
| No. of primary care provider visits | 0.97 (0.92–1.02) | ⋯ |
| Health communication measures | ||
| Collaborative care for hypertension index | 1.44 (0.83–2.49) | 1.15 (0.57–2.29) |
| Decision-making style | 1.55 (1.07–2.25) | 1.61 (1.01–2.57) |
| Proactive communication after self-monitoring | 2.13 (1.29–3.54) | 1.89 (1.10–3.26) |
OR indicates odds ratio; CI, confidence interval.
Path Analysis of Study Variables and Hypertension Control
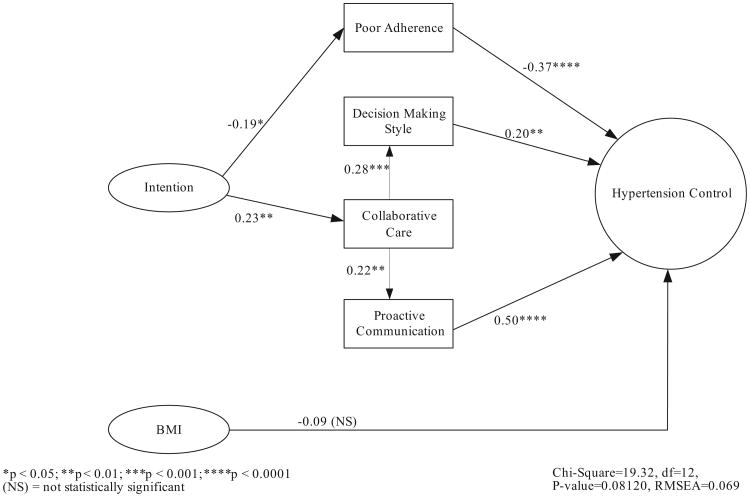
The path model diagram is displayed in Figure 1. It exhibited good fit across a number of model fit indices: χ2 (19.32, df= 12) = nonsignificant (P = 0.08); root mean square error of approximation=0.06; comparative fit index=0.91; and standardized root mean square residu-al=0.072. Except for 1 path coefficient, all individual path model coefficients were statistically significant (P<0.05). The nonsignificant path coefficient was from BMI to hypertension control (γHTNcontrol, bmi=−0.09, P=NS). The total effects of poor adherence, shared decision-making style, and proactive communication on the dependent variable hypertension control were simply the direct effects of those independent variables, as displayed in Figure 1. The complex relationship of collaborative care and hypertension control highlights the illustrative importance of path analysis over and above traditional multivariate regression. Similar to the multivariate regression (Table 3), the direct effect of collaborative care on hypertension control was nonsignificant. However, the significant total effect of collaborative care on hypertension control (total effectHTNcontrol, collaborative care=0.16, P<0.05) included significant direct effects (displayed in Figure 1) on decision-making style and proactive communication and indirect effects on hypertension control. In addition, the total effect of intention on hypertension control (total effectHTNcontrol, intention=0.11, P<0.05) included the significant direct effects (displayed in Figure 1) on collaborative care and poor refill adherence and, again, indirect effects on hypertension control.
Figure 1.
Path analysis diagram of study variables and hypertension control. The direct effects of poor refill adherence, decision-making style, and proactive communication on hypertension control were all statistically significant. The direct effect of BMI on hypertension control was not statistically significant. Intention to control hypertension affects hypertension control through significant direct effects on poor refill adherence and collaborative care, which subsequently has significant indirect effects on hypertension control through decision-making style and proactive communication.
Effect of Collaborative Care and Decision-Making Style on Hypertension Control
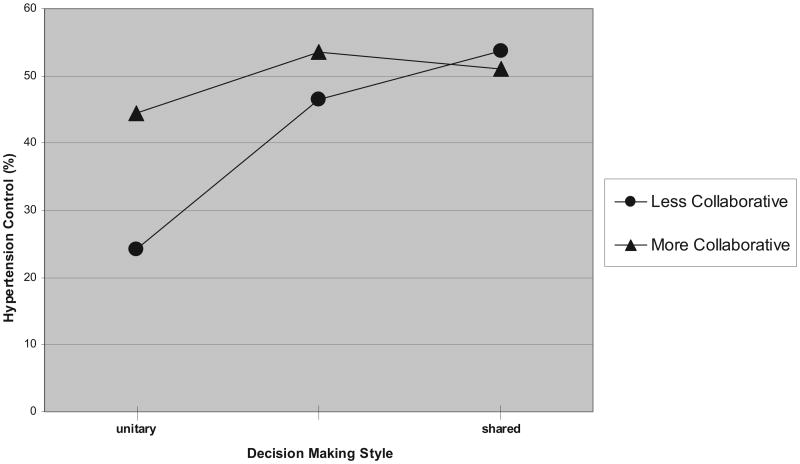
The interrelations of communication factors and hypertension control are further elaborated in Figure 2. Figure 2 illustrates how the indirect effects of the patient's perception of a more versus less collaborative care environment on the rate of hypertension control are mediated by the participant's self-reported preferences for decision-making style. Among participants who preferred a unitary decision-making style, a less collaborative care environment (Figure 2) was associated with a 20% lower rate of hypertension control than for those describing a more collaborative care environment (Figure 2). Patients' responses to the collaborative care index had comparatively less influence on hypertension control when participants endorsed a shared decision-making style. These findings illustrate how the total effect of the collaborative care index on hypertension control is best observed indirectly, when a patient does not report a preference for a shared decision-making style.
Figure 2.
Effect of collaborative care and decision-making style on hypertension control. Less collaborative (•) indicates a score of 0 to 10 on the collaborative care for hypertension index; more collaborative (▲) indicates a score of 11 to 20. For patients who report a less collaborative care environment, a linear relationship exists between hypertension control and decision-making style. The effect of decision-making style on hypertension control is less apparent among patients who report a more collaborative care environment.
Discussion
This study found that collaborative and proactive communication between patients and their clinicians has significant, independent associations with hypertension control in older diabetic patients. The effect of communication was independent of adherence to antihypertensive medications, BMI, number of comorbid conditions, and other clinical factors. In addition, the exclusion criteria and baseline characteristics of the study population demonstrate that broad noncompliance with diabetes treatment was uncommon for both groups. Nevertheless, poor adherence to antihypertensive medications as measured by a pharmacy refill metric was inversely associated with hypertension control.
Of greater interest in the present study is the fact that 3 communication-related measures, in particular, provided confirmatory evidence for the effectiveness of collaborative and proactive patient–clinician communication. Patients' preferences for shared rather than unitary (either doctor-centric or patient-centric) decision-making styles and proactive communication with clinicians after abnormal home blood pressure measurements were associated with hypertension control. The third measure, patients' perception that collaboration was encouraged by their clinician, is likely to contribute to the direct effects of proactive communication. In addition, support for collaborative communication by clinicians had an effect on hypertension control for patients who did not endorse a shared decision-making style. Furthermore, the use of blood pressure self-monitoring alone was not associated with hypertension control unless it contributed to proactive communication with clinicians about abnormal results.
Strategies to improve hypertension control have typically focused on clinical inertia (ie, poor adherence to and awareness of guidelines and reluctance to intensify treatment or overestimation of the intensity of care) and patient-specific factors (eg, access to and costs of routine care, inadequate social support, polypharmacy, and comorbidity).7,9–11,16 Most clinical trials target these clinician and/or patient factors with the expectation that the intervention will enhance adherence to medications or treatment guidelines as a mechanism for improving hypertension control.40,41 In contrast, the results of the present study provide empirical evidence that supports the effectiveness of explicitly setting collaborative hypertension goals and empowering patients to discuss abnormal blood pressure levels. Furthermore, the effectiveness of these communication factors was independent of antihypertensive medication adherence.
The present results are consistent with the few previous studies that have identified correlations between outcomes of diabetes care and the degree to which physician communication was informative, collaborative, and facilitated patient involvement in care,20,42–44 as well as the communicative abilities of patients to discuss treatment goals and plans.23,45 The findings of Heisler et al23 suggest that patient–clinician agreement on treatment goals (ie, concordance) may be associated with diabetes outcomes via mechanisms that are not entirely explained by adherence. In that study, patients and clinicians were independently asked to describe their treatment goals and plans. Greater concordance with regard to treatment goals and plans was correlated with increased patient self-efficacy and self-management with regard to diabetes care, which in turn has predicted improved diabetes outcomes.46 The present findings suggest that clinicians' collaborative communication or patients' preferences for shared decision making may facilitate patient–clinician treatment concordance, and the use of proactive communication with clinicians after abnormal home measurements may provide cues for treatment adjustment when goals are not achieved.
The findings by Kravitz et al47,48 offer additional insights into the mechanism by which collaborative communication and proactive communication cues lead to hypertension control. The results of their work suggest that patients' requests for clinical services (eg, a medication prescription or diagnostic test) were powerful predictors of “request fulfillment,” especially when patients had a trusting relationship with their clinician.47 In the present study, report of a collaborative patient–clinician relationship was strongly associated with proactive communication after self-monitoring, which had a subsequent association with hypertension control. On the basis of the mechanism of patients' requests and request fulfillment described by Kravitz et al, one potential explanation for the present results is that a cooperative relationship, characterized in part by physician support of patient participation and goal setting, enhances proactive communication by the patient and treatment adjustments by clinicians when prompted.
Study Limitations
The present study has limitations. Most importantly, the low response rate to our survey and the overwhelmingly male, older, VA-based population limit the generalizability of our study results. The findings of the present study are preliminary and novel, and they warrant further testing and validation in other populations. Second, the study design limited the ability to make causal inferences of the associations between predictor variables and hypertension control. A prospective study would provide additional insight into how changes in health communication impact clinician behavior and patient self-management, as well as into the causal relation of health communication and adherence to treatment. Third, all communication measures were obtained by patient self-report. However, the primary outcome of the study, hypertension control, was obtained objectively and was based on multiple values from several sites over many months. In addition, demographic and clinical values were obtained from electronic patient records, including the pharmacy refill data.
Conclusions
The objective of the present study was to determine the role of health communication in hypertension control in older adults with diabetes mellitus. After we controlled for clinical factors known to affect hypertension care, 3 communication factors were found to have independent associations with hypertension control. These findings suggest that the rates of hypertension control in older diabetic adults may be improved if patients and clinicians collaboratively set specific hypertension goals that define treatment success and encourage patient-directed communication with clinicians after patients experience abnormal home blood pressure measurements. Additional studies are needed to verify and extend these preliminary and novel findings.
Clinical Perspective.
Uncontrolled hypertension is the primary risk factor for the macrovascular complications of diabetes mellitus. Clinical trials have demonstrated reductions in cardiovascular morbidity when high blood pressure is controlled. Despite the availability of numerous treatments, hypertension remains uncontrolled in more than half of all diabetic individuals receiving treatment. Communication that facilitates patient–clinician collaboration when setting goals and treatment plans may overcome barriers to hypertension control in routine diabetes care. We assessed particular characteristics of patient–clinician communication to determine their associations with hypertension control in diabetes care independent of patient characteristics, medication adherence, and self-management behaviors. Three communication factors had significant associations with hypertension control. Two factors, patients' preference for shared decision making with their clinician and proactive communication with their clinician about abnormal results after blood pressure self-monitoring, had direct independent associations with hypertension control. A third factor, collaborative communication by clinicians when setting treatment goals, had an indirect effect on hypertension control. The impact of this factor was most apparent when a patient did not endorse a shared decision-making style. This study provides preliminary evidence that patient–clinician communication can facilitate collaborative blood pressure goals and proactive recognition by patients of inadequate treatment. Collaborative communication during clinical encounters initiated by patients or clinicians may improve rates of hypertension control in diabetes care independent of medication adherence.
Acknowledgments
The authors would like to thank Megan Zimmer for her assistance with development of the survey tool, recruitment of participants, and data collection. In addition, the authors thank Carol Ashton, MD, MPH, and Mark Kunik, MD, MPH, for their valuable insights during the development of this study, and David Hyman, MD, for his review of an early draft of this manuscript.
Sources of Funding: This article is the result of work supported with resources and the use of facilities at the Houston Center for Quality of Care & Utilization Studies, Michael E. DeBakey VA Medical Center. Drs Naik, Kallen, and Street receive support from the Houston Center for Education and Research on Therapeutics. Dr Naik is also supported by a National Institute on Aging K23 grant (5K23AG027144).
Footnotes
Disclosures: None.
References
- 1.Sowers JR. Treatment of hypertension in patients with diabetes. Arch Intern Med. 2004;164:1850–1857. doi: 10.1001/archinte.164.17.1850. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Vijan S, Hayward RA. Treatment of hypertension in type 2 diabetes mellitus: blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med. 2003;138:593–602. doi: 10.7326/0003-4819-138-7-200304010-00018. [DOI] [PubMed] [Google Scholar]
- 5.Fuller J, Stevens LK, Chaturvedi N, Holloway JF. Antihypertensive therapy for preventing cardiovascular complications in people with diabetes mellitus. Cochrane Database Syst Rev. 2000:CD002188. doi: 10.1002/14651858.CD002188. [DOI] [PubMed] [Google Scholar]
- 6.Kerr EA, Gerzoff RB, Krein SL, Selby JV, Piette JD, Curb JD, Herman WH, Marrero DG, Narayan KM, Safford MM, Thompson T, Mangione CM. Diabetes care quality in the Veterans Affairs Health Care System and commercial managed care: the TRIAD study. Ann Intern Med. 2004;141:272–281. doi: 10.7326/0003-4819-141-4-200408170-00007. [DOI] [PubMed] [Google Scholar]
- 7.Berlowitz DR, Ash AS, Hickey EC, Glickman M, Friedman R, Kader B. Hypertension management in patients with diabetes: the need for more aggressive therapy. Diabetes Care. 2003;26:355–359. doi: 10.2337/diacare.26.2.355. [DOI] [PubMed] [Google Scholar]
- 8.Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565–574. doi: 10.7326/0003-4819-136-8-200204160-00005. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Vasan RS. Epidemiology of uncontrolled hypertension in the United States. Circulation. 2005;112:1651–1662. doi: 10.1161/CIRCULATIONAHA.104.490599. [DOI] [PubMed] [Google Scholar]
- 10.Borzecki AM, Oliveria SA, Berlowitz DR. Barriers to hypertension control. Am Heart J. 2005;149:785–794. doi: 10.1016/j.ahj.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 11.Hyman DJ, Pavlik VN. Self-reported hypertension treatment practices among primary care physicians: blood pressure thresholds, drug choices, and the role of guidelines and evidence-based medicine. Arch Intern Med. 2000;160:2281–2286. doi: 10.1001/archinte.160.15.2281. [DOI] [PubMed] [Google Scholar]
- 12.Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, Miller CD, Ziemer DC, Barnes CS. Clinical inertia. Ann Intern Med. 2001;135:825–834. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- 13.Fine LJ, Cutler JA. Hypertension and the treating physician: understanding and reducing therapeutic inertia. Hypertension. 2006;47:319–320. doi: 10.1161/01.HYP.0000200692.23410.c9. [DOI] [PubMed] [Google Scholar]
- 14.Glasgow RE, Anderson RM. In diabetes care, moving from compliance to adherence is not enough: something entirely different is needed. Diabetes Care. 1999;22:2090–2092. doi: 10.2337/diacare.22.12.2090. [DOI] [PubMed] [Google Scholar]
- 15.Haynes RB, McKibbon KA, Kanani R. Systematic review of randomised trials of interventions to assist patients to follow prescriptions for medications. Lancet. 1996;348:383–386. doi: 10.1016/s0140-6736(96)01073-2. [DOI] [PubMed] [Google Scholar]
- 16.Bosworth HB, Olsen MK, Oddone EZ. Improving blood pressure control by tailored feedback to patients and clinicians. Am Heart J. 2005;149:795–803. doi: 10.1016/j.ahj.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 17.Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651–661. doi: 10.1016/s0277-9536(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 18.Marinker M, Blenkinsopp A, Bond C. From Compliance to Concordance: Achieving Shared Goals in Medicine Taking. London, United Kingdom: Royal Pharmaceutical Society of Great Britain; 1997. [Google Scholar]
- 19.Von Korff M, Glasgow RE, Sharpe M. Organising care for chronic illness. BMJ. 2002;325:92–94. doi: 10.1136/bmj.325.7355.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152:1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan SH, Greenfield S, Ware JE., Jr Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27:S110–S127. doi: 10.1097/00005650-198903001-00010. [DOI] [PubMed] [Google Scholar]
- 22.Glasgow RE, Goldstein MG, Ockene JK, Pronk NP. Translating what we have learned into practice: principles and hypotheses for interventions addressing multiple behaviors in primary care. Am J Prev Med. 2004;27:88–101. doi: 10.1016/j.amepre.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Heisler M, Vijan S, Anderson RM, Ubel PA, Bernstein SJ, Hofer TP. When do patients and their physicians agree on diabetes treatment goals and strategies, and what difference does it make? J Gen Intern Med. 2003;18:893–902. doi: 10.1046/j.1525-1497.2003.21132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naik AD, Schulman-Green D, McCorkle R, Bradley EH, Bogardus ST. Will older persons and their clinicians use a shared decision making instrument? J Gen Intern Med. 2005;20:640–643. doi: 10.1111/j.1525-1497.2005.0114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naik AD, Issac TT, Street RL, Jr, Kunik ME. Understanding the quality chasm for hypertension control in diabetes: a structured review of “comaneuvers” used in clinical trials. J Am Board Fam Med. 2007;20:469–478. doi: 10.3122/jabfm.2007.05.070026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selby JV, Peng T, Karter AJ, Alexander M, Sidney S, Lian J, Arnold A, Pettitt D. High rates of co-occurrence of hypertension, elevated low-density lipoprotein cholesterol, and diabetes mellitus in a large managed care population. Am J Manag Care. 2004;10:163–170. [PubMed] [Google Scholar]
- 27.Johnson ML, Pietz K, Battleman DS, Beyth RJ. Prevalence of comorbid hypertension and dyslipidemia and associated cardiovascular disease. Am J Manag Care. 2004;10:926–932. [PubMed] [Google Scholar]
- 28.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 29.Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, Rowland CR. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication, using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveria SA, Chen RS, McCarthy BD, Davis CC, Hill MN. Hypertension knowledge, awareness, and attitudes in a hypertensive population. J Gen Intern Med. 2005;20:219–225. doi: 10.1111/j.1525-1497.2005.30353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moss-Morris R, Weinman J, Petrie K, Horne R, Cameron LDB. The Revised Illness Perception Questionnaire (IPQ-R) Psychol Health. 2002;17:1–16. [Google Scholar]
- 32.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23:943–950. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 33.Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: WH Freeman; 1997. [Google Scholar]
- 34.Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the Patient Assessment of Chronic Illness Care. Med Care. 2005;43:436–444. doi: 10.1097/01.mlr.0000160375.47920.8c. [DOI] [PubMed] [Google Scholar]
- 35.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff. 2001;20:64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 36.Deber RB, Kraetschmer N, Irvine J. What role do patients wish to play in treatment decision making? Arch Intern Med. 1996;156:1414–1420. [PubMed] [Google Scholar]
- 37.Strull WM, Lo B, Charles G. Do patients want to participate in medical decision making? JAMA. 1984;252:2990–2994. [PubMed] [Google Scholar]
- 38.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 39.Wright S. The method of path coefficients. Ann Math Stat. 1934;5:161–215. [Google Scholar]
- 40.Boulware LE, Daumit GL, Frick KD, Minkovitz CS, Lawrence RS, Powe NR. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med. 2001;21:221–232. doi: 10.1016/s0749-3797(01)00356-7. [DOI] [PubMed] [Google Scholar]
- 41.Renders CM, Valk GD, Griffin SJ, Wagner EH, Eijk Van JT, Assendelft WJ. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care. 2001;24:1821–1833. doi: 10.2337/diacare.24.10.1821. [DOI] [PubMed] [Google Scholar]
- 42.Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med. 2002;17:243–252. doi: 10.1046/j.1525-1497.2002.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenfield S, Kaplan SH, Ware JE, Jr, Yano EM, Frank HJ. Patients' participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med. 1988;3:448–457. doi: 10.1007/BF02595921. [DOI] [PubMed] [Google Scholar]
- 44.Street RL, Jr, Piziak VK, Carpentier WS, Herzog J, Hejl J, Skinner G, McLellan L. Provider-patient communication and metabolic control. Diabetes Care. 1993;16:714–721. doi: 10.2337/diacare.16.5.714. [DOI] [PubMed] [Google Scholar]
- 45.Lorig K. Action planning: a call to action. J Am Board Fam Med. 2006;19:324–325. doi: 10.3122/jabfm.19.3.324. [DOI] [PubMed] [Google Scholar]
- 46.Harris MA, Wysocki T, Sadler M, Wilkinson K, Harvey LM, Buckloh LM, Mauras N, White NH. Validation of a structured interview for the assessment of diabetes self-management. Diabetes Care. 2000;23:1301–1304. doi: 10.2337/diacare.23.9.1301. [DOI] [PubMed] [Google Scholar]
- 47.Kravitz RL, Bell RA, Azari R, Krupat E, Kelly-Reif S, Thom D. Request fulfillment in office practice: antecedents and relationship to outcomes. Med Care. 2002;40:38–51. doi: 10.1097/00005650-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Krsavitz RL, Bell RA, Azari R, Kelly-Reif S, Krupat E, Thom DH. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673–1681. doi: 10.1001/archinte.163.14.1673. [DOI] [PubMed] [Google Scholar]