Abstract
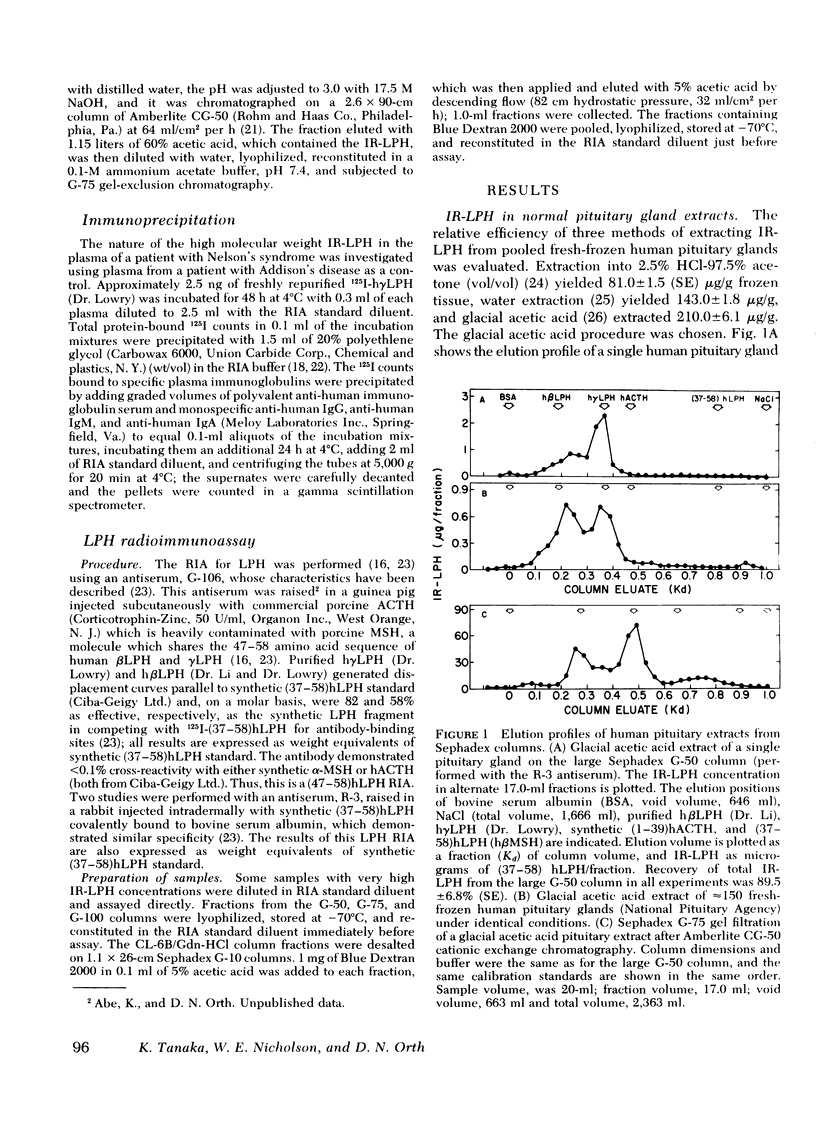
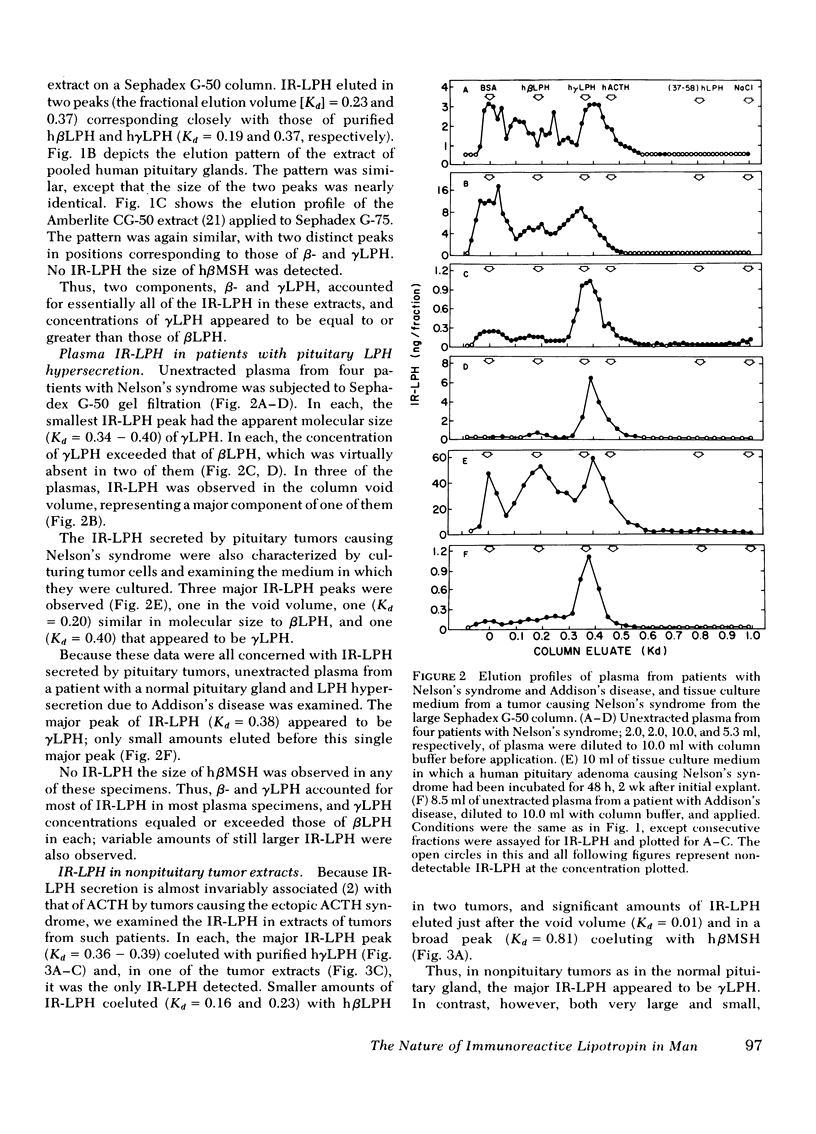
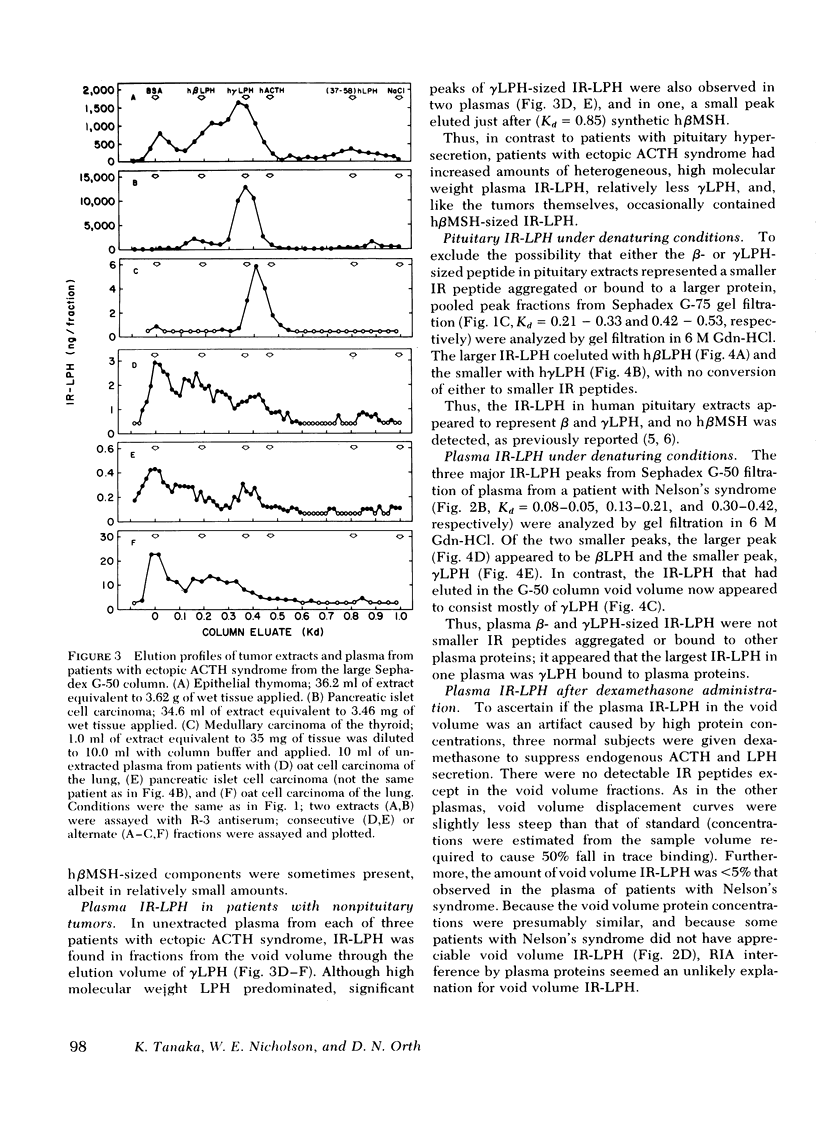
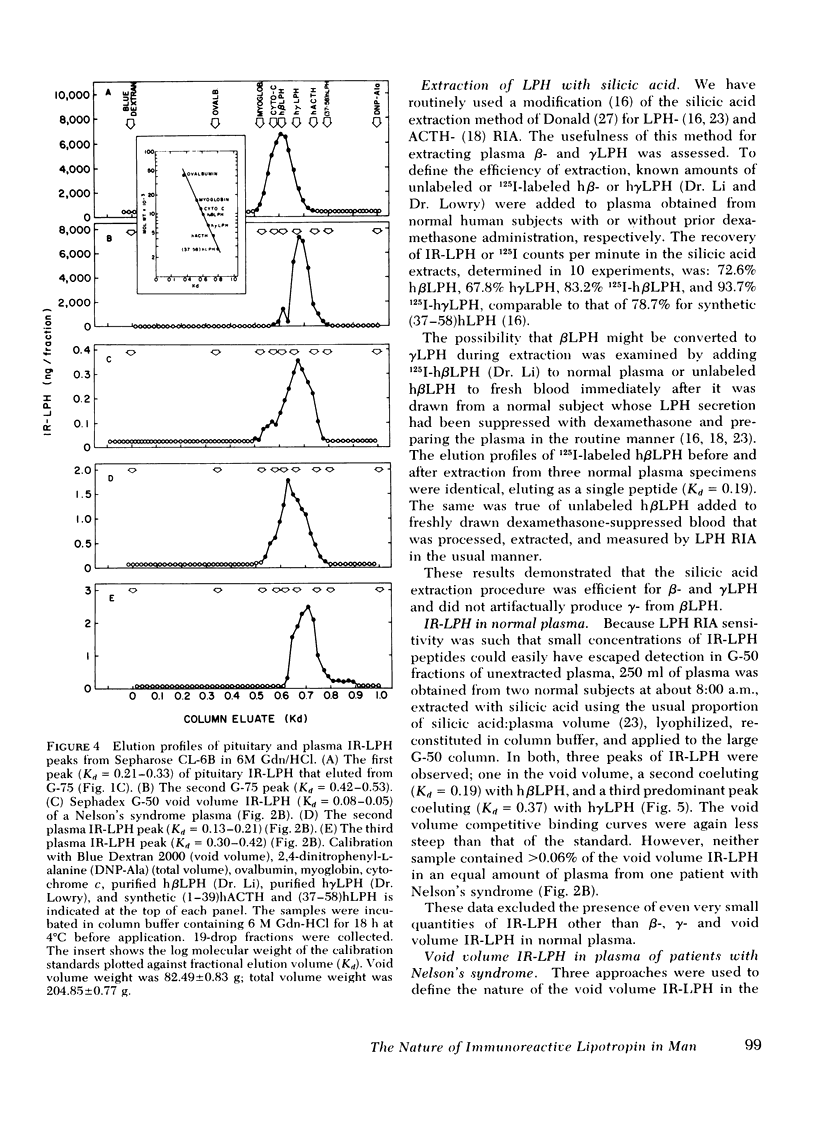
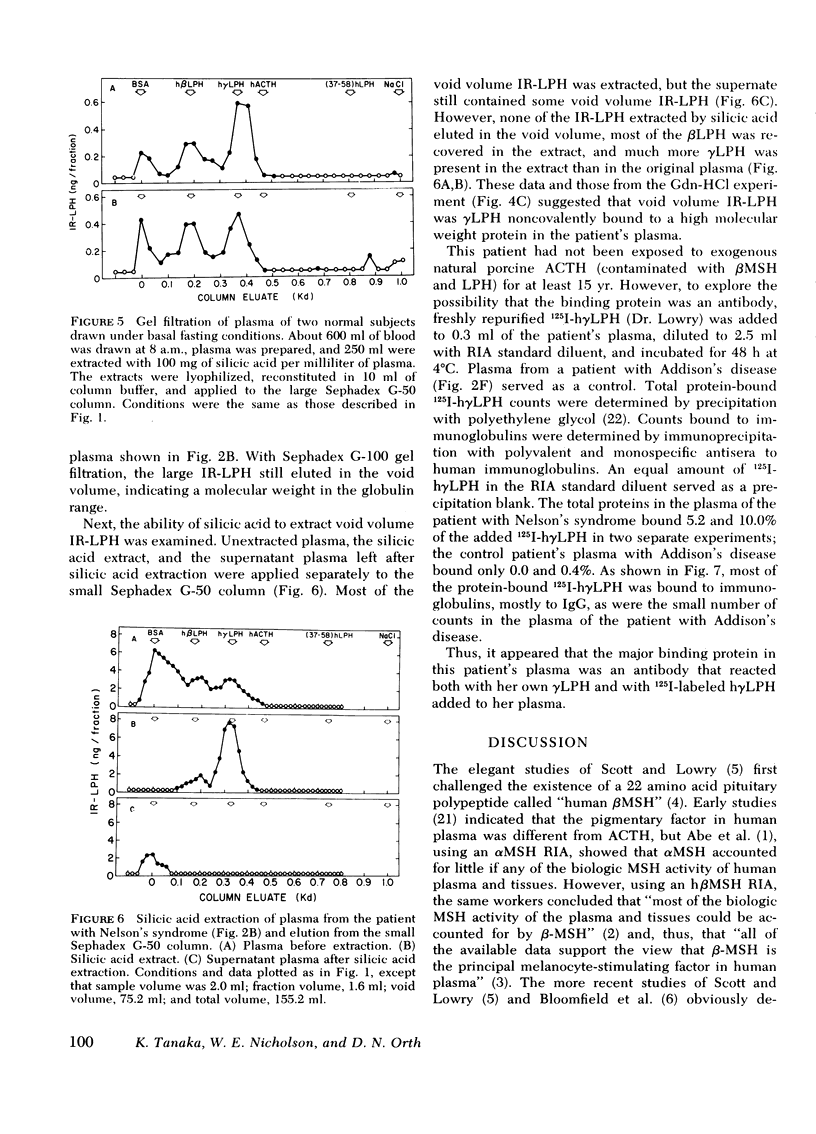
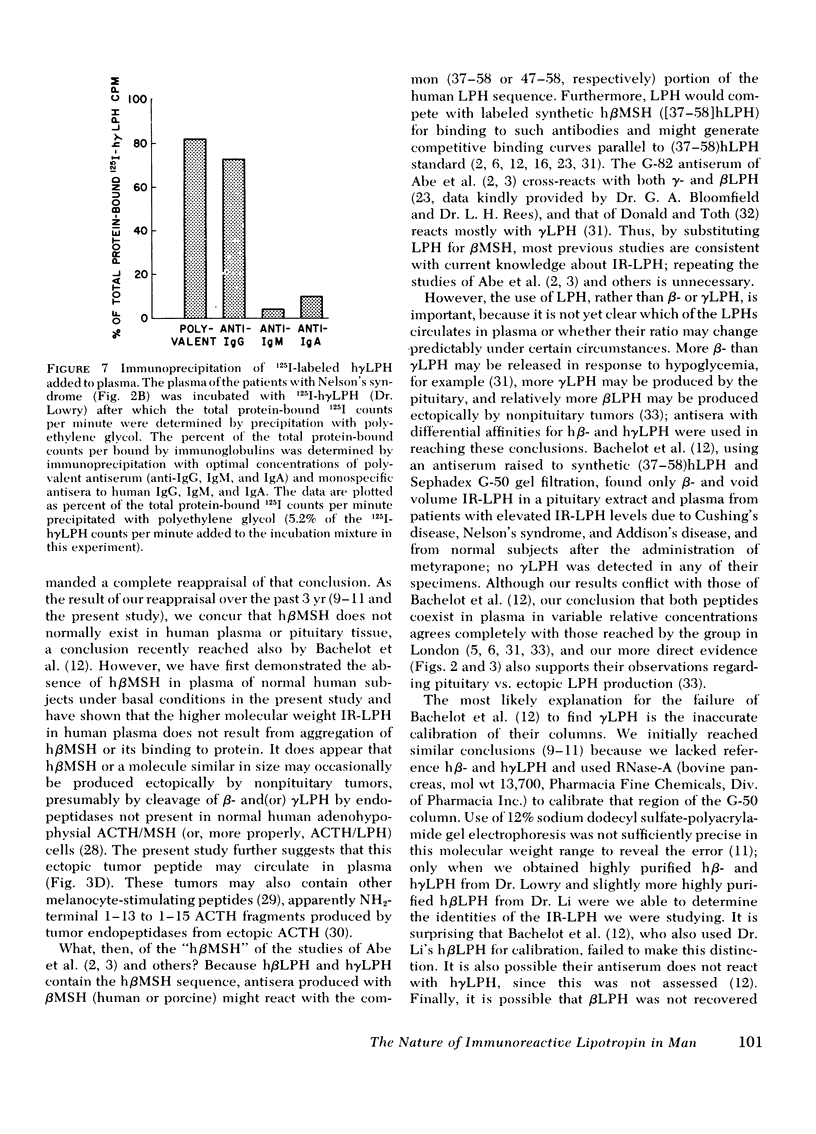
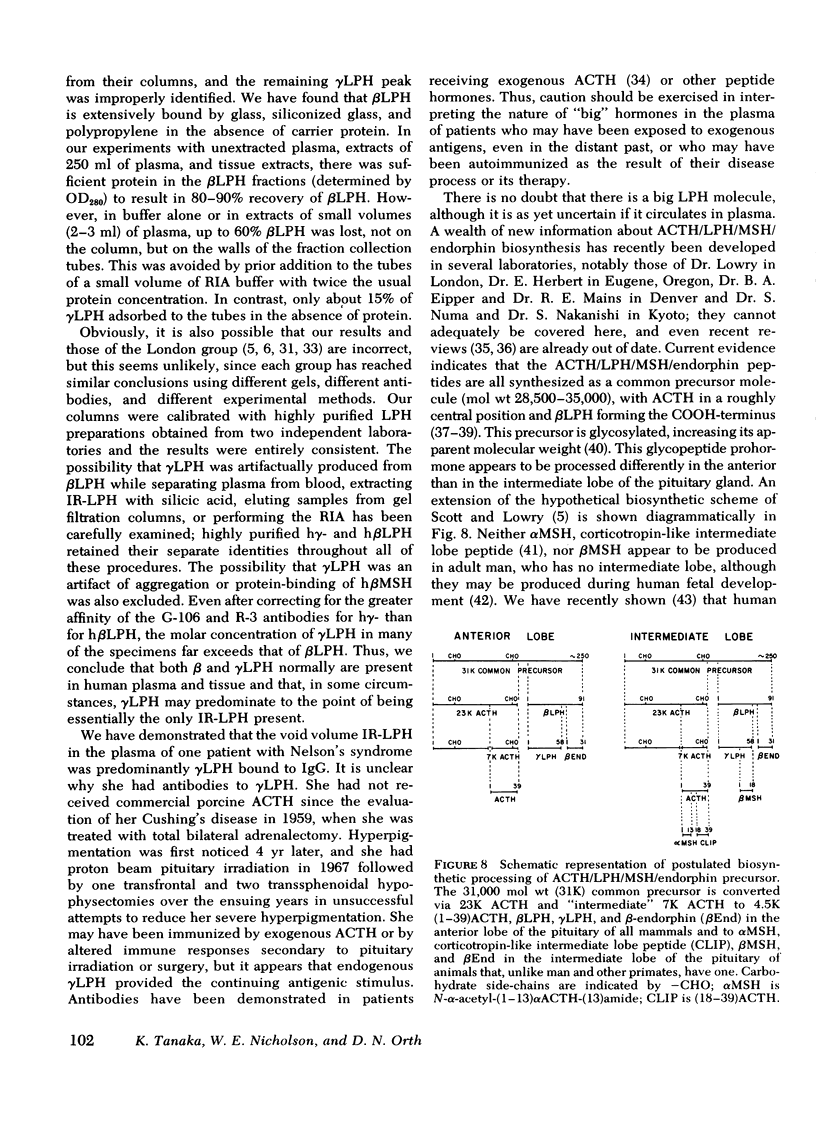
This study was designed to establish definitively the nature of immunoreactive lipotropin (IR-LPH) in human plasma and tissue extracts. Using gel filtration, gel filtration under denaturing conditions, cationic exchange chromatography, immunoprecipitation, and radioimmunoassay, we have studied normal and tumorous human pituitaries, ectopic ACTH- and LPH-secreting tumors, plasma from normal subjects before and after dexamethasone administration, and plasma from patients with primary adrenal insufficiency and pituitary and nonpituitary ACTH- and LPH-secreting tumors. Except in the plasma and tumors of occasional patients with ectopic ACTH syndrome, the smallest IR-LPH appears to be λ-lipotropin (λLPH), which is often the predominant and occasionally the only IR-LPH present. The other major peptide appears to be βLPH, a 91-amino acid molecule that contains λLPH as its 1-58 sequence. Larger immunoreactive materials were observed in some specimens, but the “big” LPH in one plasma was shown to be λLPH bound to IgG.
The weak melanocyte-stimulating activity of LPH suggests that ACTH may be the principal pigmentary hormone in man. The fact that λLPH, rather than βLPH, is the predominant form in plasma suggests that the enkephalin-endorphin opiate peptides, which are contained in the “missing” 59-91 sequence from the βLPH precursor of λLPH, may be secreted in parallel with ACTH under both physiological and pathological conditions in man.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Island D. P., Liddle G. W., Fleisher N., Nicholson W. E. Radioimmunologic evidence for alpha-MSH (melanocyte stimulating hormone) in human pituitary and tumor tissues. J Clin Endocrinol Metab. 1967 Jan;27(1):46–52. doi: 10.1210/jcem-27-1-46. [DOI] [PubMed] [Google Scholar]
- Abe K., Nicholson W. E., Liddle G. W., Island D. P., Orth D. N. Radioimmunoassay of beta-MSH in human plasma and tissues. J Clin Invest. 1967 Oct;46(10):1609–1616. doi: 10.1172/JCI105653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe K., Nicholson W. E., Liddle G. W., Orth D. N., Island D. P. Normal and abnormal regulation of beta-msh in man. J Clin Invest. 1969 Aug;48(8):1580–1585. doi: 10.1172/JCI106123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli M., Puett D. Tritium labeling of luteinizing hormone by reductive methylation. Biochim Biophys Acta. 1974 Nov 5;371(1):203–210. doi: 10.1016/0005-2795(74)90169-x. [DOI] [PubMed] [Google Scholar]
- BORNSTEIN J., TREWHELLA P. Adrenocorticotropic activity of blood-plasma extracts. Lancet. 1950 Dec 2;2(6640):678–680. doi: 10.1016/s0140-6736(50)91626-6. [DOI] [PubMed] [Google Scholar]
- Bachelot I., Wolfsen A. R., Odell W. D. Pituitary and plasma lipotropins: demonstration of the artificial nature of betaMSH. J Clin Endocrinol Metab. 1977 May;44(5):939–946. doi: 10.1210/jcem-44-5-939. [DOI] [PubMed] [Google Scholar]
- Bloomfield G. A., Scott A. P., Lowry P. J., Gilkes J. J., Rees L. H. A reappraisal of human beta MSH. Nature. 1974 Dec 6;252(5483):492–493. doi: 10.1038/252492a0. [DOI] [PubMed] [Google Scholar]
- DIXON H. B. Chromatographic isolations of pig and human melanocyte-stimulating hormones. Biochim Biophys Acta. 1960 Jan 1;37:38–42. doi: 10.1016/0006-3002(60)90075-5. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Donald R. A. A rapid method for extracting corticotrophin from plasma. J Endocrinol. 1967 Nov;39(3):451–452. doi: 10.1677/joe.0.0390451. [DOI] [PubMed] [Google Scholar]
- Donald R. A., Toth A. A comparison of the -melanocyte-stimulating hormone and corticotropin response to hypoglycemia. J Clin Endocrinol Metab. 1973 May;36(5):925–930. doi: 10.1210/jcem-36-5-925. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E., Guenzi D. High molecular weight forms of adrenocorticotropic hormone are glycoproteins. J Biol Chem. 1976 Jul 10;251(13):4121–4126. [PubMed] [Google Scholar]
- Fleischer N., Abe K., Liddle G. W., Orth D. N., Nicholson W. E. ACTH antibodies in patients receiving depot porcine ACTH to hasten recovery from pituitary-adrenal suppression. J Clin Invest. 1967 Feb;46(2):196–204. doi: 10.1172/JCI105522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz G., Yalow R. S. Ectopic ACTH production in carcinoma of the lung. J Clin Invest. 1974 Apr;53(4):1022–1032. doi: 10.1172/JCI107639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagnoni G., Sabol S. L., Nirenberg M. Synthesis of opiate peptides by a clonal pituitary tumor cell line. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2259–2263. doi: 10.1073/pnas.74.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkes J. J., Bloomfield G. A., Scott A. P., Lowry P. J., Ratcliffe J. G., Landon J., Rees L. H. Development and validation of a radioimmunoassay for peptides related to beta-melanocyte-stimulating hormone in human plasma: the lipotropins. J Clin Endocrinol Metab. 1975 Mar;40(3):450–457. doi: 10.1210/jcem-40-3-450. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hughes J., Smith T. W., Kosterlitz H. W., Fothergill L. A., Morgan B. A., Morris H. R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975 Dec 18;258(5536):577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- ISLAND D. P., SHIMIZU N., NICHOLSON W. E., ABE K., OGATA E., LIDDLE G. W. A METHOD FOR SEPARATING SMALL QUANTITIES OF MSH AND ACTH WITH GOOD RECOVERY OF EACH. J Clin Endocrinol Metab. 1965 Jul;25:975–983. doi: 10.1210/jcem-25-7-975. [DOI] [PubMed] [Google Scholar]
- Lazarus L. H., Ling N., Guillemin R. beta-Lipotropin as a prohormone for the morphinomimetic peptides endorphins and enkephalins. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2156–2159. doi: 10.1073/pnas.73.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. H., Chung D. Isolation and structure of an untriakontapeptide with opiate activity from camel pituitary glands. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1145–1148. doi: 10.1073/pnas.73.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. H., Chung D. Primary structure of human beta-lipotropin. Nature. 1976 Apr 15;260(5552):622–624. doi: 10.1038/260622a0. [DOI] [PubMed] [Google Scholar]
- Ling N., Burgus R., Guillemin R. Isolation, primary structure, and synthesis of alpha-endorphin and gamma-endorphin, two peptides of hypothalamic-hypophysial origin with morphinomimetic activity. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3942–3946. doi: 10.1073/pnas.73.11.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry P. J., Silman R. E., Hope J., Scott A. P. Structure and biosynthesis of peptides related to corticotropins and beta-melanotropins. Ann N Y Acad Sci. 1977 Oct 28;297:49–62. doi: 10.1111/j.1749-6632.1977.tb41845.x. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Fish W. W. Protein polypeptide chain molecular weights by gel chromatography in guanidinium chloride. Methods Enzymol. 1972;26:28–42. doi: 10.1016/s0076-6879(72)26004-9. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Taii S., Numa S. Cell-free translation product containing corticotropin and beta-endorphin encoded by messenger RNA from anterior lobe and intermediate lobe of bovine pituitary. FEBS Lett. 1977 Dec 1;84(1):105–109. doi: 10.1016/0014-5793(77)81067-3. [DOI] [PubMed] [Google Scholar]
- Orth D. N., Nicholson W. E. High molecular weight forms of human ACTH are glycoproteins. J Clin Endocrinol Metab. 1977 Jan;44(1):214–217. doi: 10.1210/jcem-44-1-214. [DOI] [PubMed] [Google Scholar]
- Orth D. N., Nicholson W. E., Mitchell W. M., Island D. P., Liddle G. W. Biologic and immunologic characterization and physical separation of ACTH and ACTH fragments in the ectopic ACTH syndrome. J Clin Invest. 1973 Jul;52(7):1756–1769. doi: 10.1172/JCI107357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAYNE R. W., RABEN M. S., ASTWOOD E. B. Extraction and purification of corticotropin. J Biol Chem. 1950 Dec;187(2):719–731. [PubMed] [Google Scholar]
- Phifer R. F., Orth D. N., Spicer S. S. Specific demonstration of the human hypophyseal adrenocortico-melanotropic (ACTH-MSH) cell. J Clin Endocrinol Metab. 1974 Oct;39(4):684–692. doi: 10.1210/jcem-39-4-684. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: identification of beta-lipotropin peptides and their arrangement relative to corticotropin in the precursor synthesized in a cell-free system. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5300–5304. doi: 10.1073/pnas.74.12.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. P., Lowry P. J. Adrenocorticotrophic and melanocyte-stimulating peptides in the human pituitary. Biochem J. 1974 Jun;139(3):593–602. doi: 10.1042/bj1390593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. P., Ratcliffe J. G., Rees L. H., Landon J., Bennett H. P., Lowry P. J., McMartin C. Pituitary peptide. Nat New Biol. 1973 Jul 18;244(133):65–67. doi: 10.1038/newbio244065a0. [DOI] [PubMed] [Google Scholar]
- Shapiro M., Nicholson W. E., Orth D. N., Mitchell W. M., Liddle G. W. Differences between ectopic MSH and pituitary MSH. J Clin Endocrinol Metab. 1971 Sep;33(3):377–381. doi: 10.1210/jcem-33-3-377. [DOI] [PubMed] [Google Scholar]
- Silman R. E., Chard T., Lowry P. J., Smith I., Young I. Human foetal pituitary peptides and parturition. Nature. 1976 Apr 22;260(5553):716–718. doi: 10.1038/260716a0. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Nicholson W. E., Orth D. N. Molecular size and plasma levels of immunoreactive beta-MSH under physiological and pathological conditions in man. Front Horm Res. 1977;4:208–214. doi: 10.1159/000400368. [DOI] [PubMed] [Google Scholar]



