Abstract
Traditional antimicrobials are increasingly suffering from the emergence of multidrug resistance among pathogenic microorganisms. To overcome these deficiencies, a range of novel approaches to control microbial infections are under investigation as potential alternative treatments. Multidrug efflux is a key target of these efforts. Efflux mechanisms are broadly recognized as major components of resistance to many classes of chemotherapeutic agents as well as antimicrobials. Efflux occurs due to the activity of membrane transporter proteins widely known as Multidrug Efflux Systems (MES). They are implicated in a variety of physiological roles other than efflux and identifying natural substrates and inhibitors is an active and expanding research discipline. One plausible alternative is the combination of conventional antimicrobial agents/antibiotics with small molecules that block MES known as multidrug efflux pump inhibitors (EPIs). An array of approaches in academic and industrial research settings, varying from high-throughput screening (HTS) ventures to bioassay guided purification and determination, have yielded a number of promising EPIs in a series of pathogenic systems. This synergistic discovery platform has been exploited in translational directions beyond the potentiation of conventional antimicrobial treatments. This venture attempts to highlight different tactical elements of this platform, identifying the need for highly informative and comprehensive EPI-discovery strategies. Advances in assay development genomics, proteomics as well as the accumulation of bioactivity and structural information regarding MES facilitates the basis for a new discovery era. This platform is expanding drastically. A combination of chemogenomics and chemoinformatics approaches will integrate data mining with virtual and physical HTS ventures and populate the chemical-biological interface with a plethora of novel chemotypes. This comprehensive step will expedite the preclinical development of lead EPIs.
Keywords: Multidrug resistance, efflux pump substrates and inhibitors, natural antimicrobials, high-throughput screening
MICROBIAL MULTIDRUG EFFLUX SYSTEMS; OVERVIEW
Multidrug resistance (MDR) has expanded dramatically in a broad range of organisms from bacteria to humans resulting in a global increase in life threatening infections and deaths [1]. There is a high medical need to systematically explore the etiology and principles as well as to devise strategies leading to implementation of effective countermeasures. Efflux mechanisms are broadly recognized as major components of resistance to many classes of chemotherapeutic agents as well as antimicrobials. Efflux occurs due to the activity of membrane transporter proteins widely known as multidrug efflux systems (MES). They are implicated in a variety of physiological processes other than efflux and identifying natural substrates and inhibitors is an active and expanding research discipline [2–4].
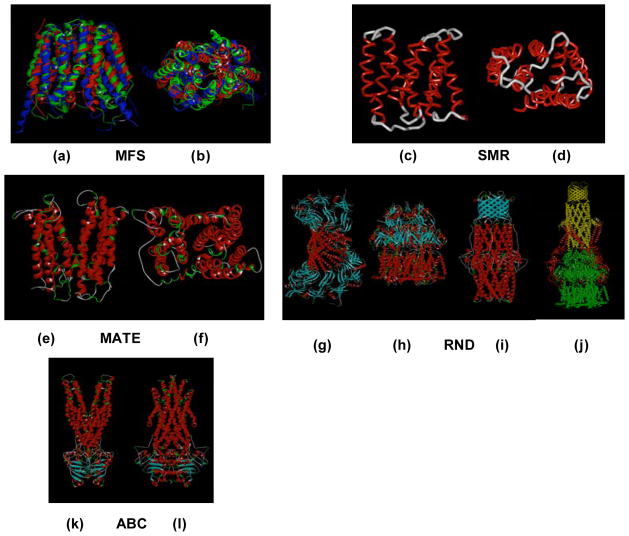
MES perform essential roles in cellular metabolism and activity. They differ in membrane topology, energy coupling mechanisms, and most importantly in substrate specificities [5]. Based on their sequence similarity, they are classified into six super-families including; ATP-binding cassette super family (ABC), major facilitator super family (MFS), resistance-nodulation cell division super family (RND), small multidrug resistance family (SMR), multi-antimicrobial extrusion protein family (MATE), and multidrug endosomal transporter (MET) (Fig. 1) The first five families are found in microorganisms (the MET family appears to be restricted to higher eukaryotes), but representatives of all groups are also expressed in mammalian cells [6]. ABC transporters comprise the largest super family, with seven subfamilies that are designated A to G based on sequence and structural homology [4]. The best-studied families of fungal MES are those from Sacharomyces cerevisiae. The group of S. cerevisiae ABC transporters most closely associated with drug resistance is the pleiotropic drug resistance (PDR) subfamily. There are 28 ABC transporter genes in S. cerevisiae, and 9 of these encode PDR transporters (between them PDR5, PDR10, PDR15, SNQ2, and YOR1 as well as the hexose transporter genes HXT9 and HXT11) that are highly conserved among pathogenic fungal species [7–9]. A challenging clinical scenario involves MES in Pseudomonas aeruginosa [10]. Although sequence analysis of the P. aeruginosa genome has revealed the presence of MES from all five super families, the largest number of predicted pumps, with a total of 12, belong to the RND family [11].
Fig. 1.
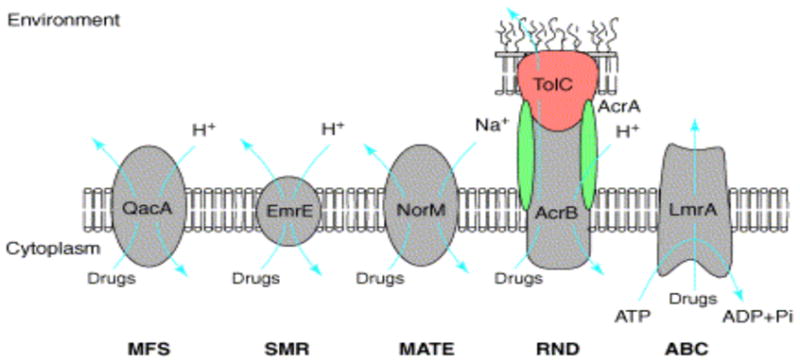
Representative members of the five characterized families of MES [3]. The ABC family including ATP-driven multidrug pumps such as P-glycoprotein and LmrA from Lactococcus lactis. The MFS consists of secondary transporters driven by chemiosmotic energy and includes proton/drug antiporters such as QacA from S. aureus. Both the resistance/nodulation/cell division (RND) and the small multidrug resistance (SMR) families include proton-driven drug efflux pumps such as E. coli AcrB and EmrE, respectively. AcrB functions as a multisubunit complex in association with the outer membrane channel TolC and the membrane fusion protein AcrA. The multidrug and toxic compounds efflux (MATE) family consists of sodium ion-driven drug efflux pumps such as NorM from Vibrio parahaemolyticus.
Crystal structures of MFS and ABC transporters have been resolved (Fig. 2) in a range of organisms (e.g. Multidrug Resistance Protein D (EmrD) [12], Lactose Permease [13] and the Glycerol-3-Phosphate Transporter [14–17]). Recently, advances have been made for the RNDs crystal structures of the components of i) Ac-rAB-TolC from Escherichia coli [18–20] ii) MexAB-OprM from P. aeruginosa - MexB [21], OprM [22], and MexA [23] iii) VceC from the VceCAB (VCE) in Vibrio cholerae, [24] and iv) CusA from CusCBA a member from the heavy-metal efflux (HME) subfamily [25]. Furthermore, the assembled structure of a complete tripartite bacterial RND multidrug efflux pump has been published [26]. There is a special emphasis in the structure of transcriptional regulation systems such as the MexR [27] and MexZ [28], TTgR, which regulates ttgABC, a key system in P. putida DOT-T1E [29, 30], BldR, a member of the multiple antibiotic resistance regulator (MarR) protein family from Sulfolobus solfataricus [31] and SmeT, the repressor of the Stenotrophomonas maltophilia system SmeDEF [32]. Significant progress has also been made in MATE structural insights [33, 34].
Fig. 2.
(a,b) Lateral and axial view of the overlap of the crystal structures of Multidrug Resistance Protein D (red) Lactose Permease (green) and the Glycerol-3-Phosphate Transporter (blue) from MFS family; (c,d) Lateral and axial view of the crystal structure of EmrE multidrug transporter from SMR family (Calpha atoms only);(e,f) Lateral and axial view of the crystal structure of the MATE transporter NorM from Vibrio cholerae. Crystal structures of (g) MexA, (h) MexB, (i) OprM, and (j) a model of the assembly of AcrAB-TolC from RND family; (k,l) Perpendicular views of the crystal structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus.
For the last two decades the pieces of the efflux puzzle have been gradually coming together. A wide variety of MES mutants and MES-associated functional efflux assays have been used to identify both substrates and inhibitors, and efforts at translational studies as well as clinical implementation of lead molecules and therapies based on efflux inhibition have been described. In this report, we assemble a portion of these innovative pieces and we highlight the principals for future discovery strategies aimed at MES.
THE QUEST FOR NATURAL SUBSTRATES - RATIONALE
It has been suggested that amphipathic cations represent the natural MES substrate pool [35]. Specifically, SMR family members expressed by Gram-positive bacteria efflux amphipathic cations exclusively [36, 37, 38] as do most MFS members. For example, the NorA pump of the human pathogen Staphylococcus aureus extrudes cations, quinolones, biocides and dyes [39–41]. The BMR pump of Bacillus subtilis primarily extrudes cations and neutral chloramphenicol [42, 43]. Furthermore, the RND super-family has a broad substrate spectrum that includes, apart from antibiotics, amphipathic cations, biocides, dyes, and steroid hormones [44–49]. This substrate pattern is similarly observed in ABC-transporters expressed by a wide range of organisms [50–51]. Thus, the need to protect a cell from amphipathic cations has evolved in different families of MES across different organisms despite a lack of overall molecular homology or similarity in their mechanism of action. Quite surprisingly, one does not find amphipathic cations in a general list of natural antimicrobials. The known cationic antibiotics of the aminoglycoside group such as streptomycin and kanamycin are hydrophilic substances that get trapped in the cell via specific translocases [52] and are not general MES substrates.
A series of studies were directed at finding the basis of Gram-negative bacterial resistance to plant antimicrobials. In Rhizobium etli, there is an operon activated by a number of plant phytoalexins. This operon appears to code for an RmrAB MES [53]. Expression of a mutant rmrAB resulted in diminished root colonization, and a 30% increase in susceptibility to the phytoalexins, naringenin and coumaric acid. The soybean antifungal phytoalexin coumestrol induces LfeAB MES expression in Agrobacterium tumefaciens [54]. Mutation in this MES increases the accumulation of coumestrol in the pathogen, and the mutant was out-competed by wild type in colonizing the plant. Interestingly, neither wild type nor mutant was sensitive to coumestrol. In a slightly different approach, a panel of plant antimicrobials was tested using a combination of efflux mutants and EPIs on a set of bacteria that represent the main groups of plant and human pathogens [55]. One of the main observations was the strong potentiation of antimicrobial action in strains with disabled MES. The activity of the majority of plant antimicrobials was considerably greater against the Gram-positive bacteria S. aureus and B. megaterium. Disabling MES in Gram-negative species led to a striking increase in antimicrobial activity. For instance, the activity of the anthraquinone rhein, the principal antimicrobial from rhubarb [56], was potentiated 100–2000-fold depending on the bacterial species. A similar effect was observed with plumbagin [57], resveratrol, gossypol, coumestrol, and berberine. Direct measurement of the uptake of berberine confirmed that MES inactivation significantly increased berberine accumulation into Gram-negative bacteria.
The outer-membrane protein-encoding gene tolC expressed by the bacterial plant pathogen Erwinia chrysanthemi EC16 has been identified and characterized [58]. E. chrysanthemi tolC plays an important role in the survival and colonization of the pathogen in plant tissue conferring resistance to plant antimicrobials. It was established that combination of the signaling molecule in local and systemic plant resistance to salicylic acid and its precursors, t-cinnamic acid and benzoic acid, can activate expression of specific MES-encoding genes in E. chrysanthemi and enhance survival of the bacterium in the presence of model as well as plant-derived antimicrobials [59]. The E. chrysanthemi gene functionally complements the E. coli tolC gene in MES. A tolC mutant of E. chrysanthemi is extremely sensitive to an array of plant-derived chemicals including berberine, rhein, plumbagin, pyrithione, genistein (4,5,7-trihydroxyisoflavone), p-coumaric acid and t-cinnamic acid (phenolic acids), (2-mercaptopyridine-1-oxide) and esculetin (6,7-dihydroxycoumarin). This mutant is unable to grow in planta and its ability to cause plant tissue maceration is severely compromised. Similarly, two host-induced Ralstonia solanacearum genes, acrA and dinF encode for MES that contribute to the overall aggressiveness of this phytopathogen by protecting the bacterium from the toxic effects of host antimicrobial compounds [60].
EPI-DISCOVERY, PROOF OF PRINCIPAL
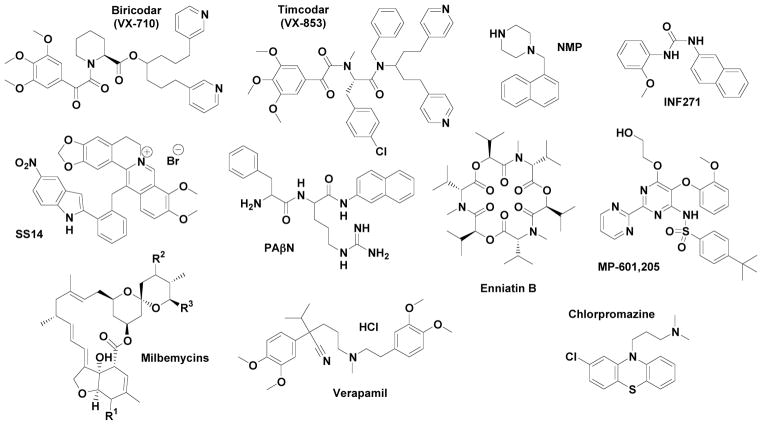
Discovery of EPIs is a promising approach to deal with MES that may improve clinical performance of antibiotics and chemotherapeutic agents [61, 62]. The potential for developing broad acting EPIs is exemplified by reserpine that effectively inhibits both bacterial and mammalian ABC-system P-glycoprotein (P-gp) [63–65], as well as by biricodar (VX-710), timcodar (VX-853) [66, 67] and verapamil [68, 69] (Fig. 3). EPI activity can be verified ab initio by testing the combined action of an EPI compound with a MES substrate added at a sub-inhibitory concentration. The NorA inhibitors 5-nitro-2-phenylindole (INF55) and diphenyl urea INF271 [70] (Fig. 3) were identified by screening a synthetic chemical library using ethidium bromide as a substrate.
Fig. 3.
Structures of representative microbial EPIs
Other approaches involve the discovery of EPIs from natural sources through bioassay-driven purification and structural determination. Several Berberis medicinal plants (Berberis repens, B. aquifolia, and B. fremontii) that produce the plant antimicrobial berberine also synthesize 5′-methoxyhydnocarpin (5′-MHC), an inhibitor of S. aureus NorA [71]. This discovery intensified the search for plant-derived EPIs [72]. Using the berberine efflux assay, investigators discovered a number of EPIs in various plant sources that exhibit activities similar to 5-MHC. Bioassay purification from various plant sources yielded a number of EPIs acting against Gram-positive bacteria with activities similar to 5-MHC, as detected in the berberine efflux assay. The list includes pheophorbide a from Berberis sp., and Mahonia [73, 74], crysoplenol and crysoplenetin from Artemisia annua [75], polyacylated neohesperidosides from Geranium caespitosum [76], chalcones and a stilbene from Dalea versicolor [77, 78]. Additionally, isoflavones such as genistein, orobol, and biochanin also exhibit EPI activity against the NorA pump of Gram-positive bacteria [79].
Similar natural product screening and synthesis campaigns have led to the identification of additional NorA EPIs. The list includes catechin gallates [80], the resin glycosides and tetrasaccharide agents of Ipomoea murucoides [81], polyacylated oligosaccharides from the medicinal Mexican morning glory species [82], N-caffeoylphenalkylamide derivatives [83], citral derived amides [84] kaempferol glycoside from Herissantia tiubae (Malvaceae) [85] and a set of plant-based alkaloids against methicillin-resistant S. aureus [86]. A series of plant phenolic compounds have been functioning as ethidium bromide EPIs in Mycobacterium smegmatis.[87]. A series of synthetic efforts have been concentrated in paroxetine and femoxetine [88], piperine and fluoroquinolone as the basis for designing structural analogues [89, 90], phenothiazines [91] with emphasis on thioridazine and chlorpromazine (Fig. 3) [92]. In conclusion an array of lead chemotypes both natural products and synthetic compounds are available for further analysis.
NORA AS A MODEL FOR SYNERGISTIC DISCOVERY STRATEGIES
This efflux system has been explored beyond the identification of EPIs with emphasis in combinatorial antimicrobial strategies with high probability of preclinical implementation. A hybrid compound (SS14, Fig. 3) created by combining the plant antimicrobial berberine with the synthetic EPI INF55 is an effective antimicrobial against S. aureus including mutant strains that over express NorA [93]. MIC’s for SS14 against S. aureus are 2–16 times lower than berberine and INF55. The hybrid rapidly accumulates in bacterial cells and shows higher efficacy than vancomycin in a Caenorhabditis. elegans model of enterococcal infection [93]. SS14 analogues exhibit similar antimicrobial activities [94, 95] suggesting that significant structural changes can be made to these hybrids without adversely affecting their ability to block MES or their antibacterial activity. Such hybrids should have an advantage over separate compound administration in terms of synchronous or near synchronous delivery to the appropriate bacterial target sites.
Photodynamic therapy (PDT) has recently gained regulatory approval in USA for treating various cancers and age-related macular degeneration [96, 97]. Certain non-toxic photosensitizers (PS) accumulate preferentially in malignant tissues and can be selectively activated by light. A relatively novel application of PDT is in the treatment of localized infections [98]. Clinically used PS for photoinactivation (PDI)/PDT includes phenothiazinium salts, such as toluidine blue O [99] and methylene blue [100]. Phenothiazinium salts, structurally characterized as amphipathic cations, are substrates of microbial MES [101] and specific EPIs have been shown to potentiate antimicrobial photo-inactivation both in Gram-positive and Gram-negative bacteria overexpressing MFS and RND efflux systems [102]. NorA specific EPIs dramatically enhanced the phenothiazinium mediated phototoxicity in S. aureus. Recently, a similar theme emerged when a set of porphyrin-based PSs were identified as substrates of the ABCG2-breast cancer related transporter [103, 104] even though they have an inconclusive recognition pattern in microbial MFS systems [105]. All the reported studies have found that PDT can kill drug-resistant microbes as easily as their native counterparts [106, 107]. European and US-based companies that currently employ PDT for localized infections include Ondine Biopharma, who gained regulatory approval in Canada (and is in process in U.S.) for endodontics, nasal decolonization and gingivitis and Nomir Medical Technologies (Waltham MA) who have developed a near-infrared light inactivation platform providing evidence for its involvement in microbial efflux mechanisms [108].
RND-BASED EPIs
It has been a challenging task to identify RND-based EPIs basically due to the complexicity of MES in Gram-negative bacteria. Phenyl-arginine beta naphthylamide (PAβN, Fig. 3), was identified by assaying an array of synthetic compounds and natural product extracts using P. aeruginosa strains over-expressing each of the three MES (MexAB-OprM, MexCD-OprJ, MexEF-OprN) in the presence of levofloxacin. It has been a valuable tool for drug discovery [109, 110]. This venture has explored i) the development of preclinical candidates including strategies for lead optimization [111], ii) activity in vivo through the use of alternative scaffolds [112], iii) optimization of potency in the pyridopyrimidine series through the application of a pharmacophore model [113] and iv) extensive structural activity relationships testing toxicity, stability, and solubility [114–117]. Mechanistically, PAβN itself is a MES substrate that acts as a competitive inhibitor [117–120]. It seems that PAβN may recognize and bind to the substrate pocket specific for the potentiated antibiotics. Alternatively, due to a close location of binding site, EPI binding may also generate steric hindrance, impairing antibiotic binding at its affinity site. PAβN has been validated against the AcrAB-TolC in K. pneumoniae, E. coli, S. thyphimurium and E. aerogenes [121–124], and in multiple homologous systems including Acinetobacter baumannii [125], Campylobacter jejuni and Campylobacter coli [126, 127].
Naphthylpiperazines, most notably 1-naphthylmethyl-piperazine (NMP, Fig. 3), are among the most potent unsubstituted arylpiperazines [128], with a dose-dependent ability to increase the intracellular concentration of several antibiotics [129]. NMP seems to be effective in A. baumannii and several Enterobacteriaceae, but not in P. aeruginosa [129, 130]. The list also includes trimethoprim and epinephrine [131], indole derivatives [132] for the AcrAB-TolC, phenothiazines for the BpeAB-OprB MES in Burkholderia pseudomallei. [133], quinoline derivatives in Enterobacter aerogenes isolates [134] and K. pneumoniae [135], and carbonyl cyanide m-chlorophenyl hydrazone (CCCP) and pantoprazole in Helicobacter pylori [136].
FUNGAL EPIs
Yeast EPIs that have been so far identified and characterized belong to five groups: (i) Tacrolimus (FK506) potentiates azoles in strains over expressing the CDR1 and CaMDR genes [137]. (ii) Phenothiazines potentiate ketoconazole in strains overexpressing Pdr5p, Snq2p and Yor1p while also exhibiting intrinsic antimicrobial activity [138]. iii) Propafenones potentiate azoles and terbinafine in strains lacking Cdr1p and Cdr2p [139]. (iv) Isonitrile and enniatines against Fusarium sp Y-53 potentiate cycloheximide or cerulenin in Pdr5p-overexpressing cells [140]. The mode of Pdr5p inhibition by enniatin is competitive against FK506, and its inhibitory activity is more potent with less toxicity than that of FK506. Enniatins exhibit a similar profile as FK506 against mutants of Pdr5p. They were identified after screening a 1.8-million-member designer D-octapeptide combinatorial library for surface-active Pdr5p antagonists. However, enniatins (Fig. 3) did not inhibit Snq2p, a homologue of Pdr5p and they show only modest toxicity against yeast cells [141]. A similar screening process yielded isonitrile 3-(3′-isocyano-cyclopent-2′-enylidene)-propionic acid, a compound whose carboxyl residue is essential for its EPI-activity [140]. (v) N-methylpiperazine (quinazolinone derivative) and milbemycin derivatives (Fig. 3) have been characterized as fungal EPIs that enhance fluconazole efficacy in C. albicans [142, 143]. Attempts to improve the aqueous solubility of screening hits led to the discovery of an analog with improved physical properties and activity against clinically-relevant Candida spp.[144]. The list of fungal EPIs is further expanded with the cyclic peptides unnarmicin A and unnarmicin C that were identified by testing a library of marine microorganisms [145] sulfated sterols from the marine sponge Topsentia sp [146], acridone derivatives [147] and ibuprofen [148].
EPIs & BIOFILMS
Since MES are a key resistance factor in microbial cells, it has been suggested that their expression contributes to biofilm persistence [149–153]. The exact role of MES in biofilm growth and their importance in biofilm-mediated antibiotic resistance are, however, elusive, and studies of Gram-negative bacteria have produced somewhat incongruous results. In E. coli, the putative multidrug resistance pump YhcQ was reported to be involved in antibiotic resistance of biofilms [154], and the AcrAB-TolC pump has been found to be upregulated under stress conditions, such as stationary-phase growth or, as in the natural habitat of this species, exposure to bile acids [155]. Interestingly, exposure to these agents renders E. coli resistant to lipophilic antibiotics, suggesting an upregulation of AcrAB-TolC [155].
In P. aeruginosa, the MexAB-OprM and MexCD-OprJ efflux pumps have been shown to be involved in biofilm-specific mechanisms of resistance [156], especially with regards to the macrolide azithromycin [156]. In contrast, it was found that neither pump was up regulated in a developing biofilm [157]. MES expression may be influenced by factors such as growth rate and the accumulation of metabolites. Most probably, biofilm-mediated resistance is achieved through multiple factors, such as slow growth due to deficient nutrition, reduced penetration due to production of protective extracellular polysaccharides, and efficient efflux [158]. Additionally, it was found that the ndvB gene, coding for a glucosyltransferase, is a genetic determinantof antibiotic resistance in P. aeruginosa [159].
A number of EPIs including thioridazine, NMP, and PAβN reduced biofilm formation, and in combination could abolish biofilm formation completely in MFS (norA) and RND (AcrAB, F, MexAB CD, EF) expressing strains. [160] This synergy hypothesis was successfully applied for the photodynamic inactivation in an Enterococcus faecalis biofilm model using TBO and verapamil [161].
FROM TACTICS TO STRATEGIES
The natural role of MES is very complex as they are important for bacterial metabolism, physiology, and pathogenicity [2, 3]. It has been shown that mutant strains of Salmonella enteric, serovar typhimurium, E. coli, and C. jejuni over expressing MES are resistant to high concentrations of bile salts. In addition, over expression of the MtrCDE efflux system in N. gonorrhoeae enhances bacterial survival [3, 84]. It has been established that RND MES have a role in invasion, adherence and colonization of the host cell. Therefore, the EPI-approach in some cases might also reduce bacterial virulence in vivo. A major obstacle, however, may arise from the fact that MES manipulation may cause unexpected toxicities due to the variety of their physiological roles. In this context, efforts directed at specifically inhibiting efflux pumps operating only in prokaryotes may offer a significantly greater chance of effective therapeutic success. Interestingly, it has been shown that target bacteria respond to clinical challenge with EPIs by developing resistance mutations that decrease the efficacy of the EPI [162, 163] Recently, it has been demonstrated that reserpine can select multidrug resistant S. pneumoniae strains [164]. The threat of cross-resistance to antibiotics elevates the complexity of discovery ventures. To date, the efficacy of EPIs has been demonstrated in the therapy of malignancies in vitro and in vivo; with clinical trials (Table 1). The only documented microbial EPI is currently MP-601,205 (Fig. 3), used for respiratory infections in patients with cystic fibrosis (CF) and ventilator-associated pneumonia. The compound is an aerosol formulation of an approved drug that functions as an EPI.
Table 1.
Panel of Representative EPIs and their Biological Features
| Inhibitor | Limitations | Toxicity | MES | Clinical Trials | Reference |
|---|---|---|---|---|---|
| reserpine | NR | neurotoxicity | MFS, ABC | No | [175] |
| INF55 | NR | NR | MFS | No | [70] |
| INF271 | NR | NR | MFS | No | [70] |
| Biricodar (VX-710) | Pharmacokinetic interaction | NR | MFS, ABC | Yes (Pgp) | [176] |
| Timcodar (VX-853) | NR | NR | MFS, ABC | Site effects | [177] |
| Phenothiazines | Low potency | NR | MFS, ABC | No | [91, 92, 178–180] |
| PaβN | Pharmacokinetic interaction | Potential Toxicity | RND | No | [118] |
| NMP | Pharmacokinetic interaction | NR | RND | No | [129] |
| MP-601,205 | NR | NR | RND | Yes | [118] |
| Milbemycin | NR | NR | ABC | No | [181] |
| Verapamil | Low potency | Hypotension | MFS, ABC | Yes | [182] |
| Enniatins | NR | mitochondrial dysf | ABC | No | [183, 184] |
NR, not reported, INF555-nitro-2-phenylindole, INF271, diphenyl urea
Pgp, P-glycoprotein, PaβN, Phenyl-arginine beta naphthylamide, NMP, 1-naphthylmethyl-piperazine
Natural sources such as specific plants have a distinct role in the effort to identify lead EPIs as well as potential antimicrobial agents. The natural antimicrobial discovery approach, a process ranging from identifying a hit to isolating a pure compound, has increased over the last decade and is thought of as more than promising [165–169]. Still, there are significant technical bottlenecks. There are a limited number of natural product extract libraries and their analysis typically involves exacting isolation of different components of the extract and subsequent time-consuming spectroscopic identification of the separate compounds. HTS campaigns for EPIs using natural or synthetic libraries also suffers from distinct bottlenecks (variety of representative chemotypes in addition to limited number of compounds tested) as well as considerable disadvantages, such as the lack of defined secondary and tertiary evaluation assays.
An innovative HTS strategy based on a functional EPI assay coupled with a comprehensive secondary validation flowchart is capable of overcoming barriers while being highly informative. The University of New Mexico’s Center for Molecular Discovery (UNMCMD) is pioneering the development of cell suspension HTS for EPI discovery utilizing a sensitive multiplex flow cytometry platform. The approach incorporates the Prestwick Chemical Library (PCL, consisting of 1120 FDA approved drugs) along with the Molecular Libraries Small Molecules Repository (MLSMR, http://mlsmr.glpg.com/MLSMR_HomePage/). It paved the way for a series of innovations in chemical genetics including novel flow cytometry efflux assays (Fig. 4 and 5) in a set of yeast transporters including ABC (CDR1, CDR2), MFS (MDR1) and vacuolar V-ATPases [170–173]. Moreover this initiative has generated a number of projects targeting cancer MES including dye-substrate profiling and EPI discovery [170]. UNMCMD has initiated a quest for ABC and MFS substrates and inhibitors both in microbial and mammalian systems. We have proposed a hybrid chemogenomics-chemoinformatics discovery platform employing MES from specific organisms, the MLSMR library and HTS flow cytometry in the hope of developing a transporter-ligand interactome. This approach integrates data from genomic, proteomic and medicinal chemistry databases in concert with physical screening campaigns. It provides the rationale to chemically characterize MES substrates and subsequently accelerate the discovery of potent functional inducers or repressors for transporters as well as lead EPIs. Novel chemical probes will undergo lead optimization and detailed biological characterization. This project attempts to provide “MES signature specific” chemical probes from the MLSMR library to elucidate potential evolutionary relationships and facilitate collaborative ventures in the framework of a broad consortium including European Union Cost Action ATENS, (COST BM0701) and The International Transporter Consortium [174].
Fig. 4.
Duplex format flow cytometric assay for identification of ABCB1, ABCC1 and ABCG2 EPIs
Fig. 5.
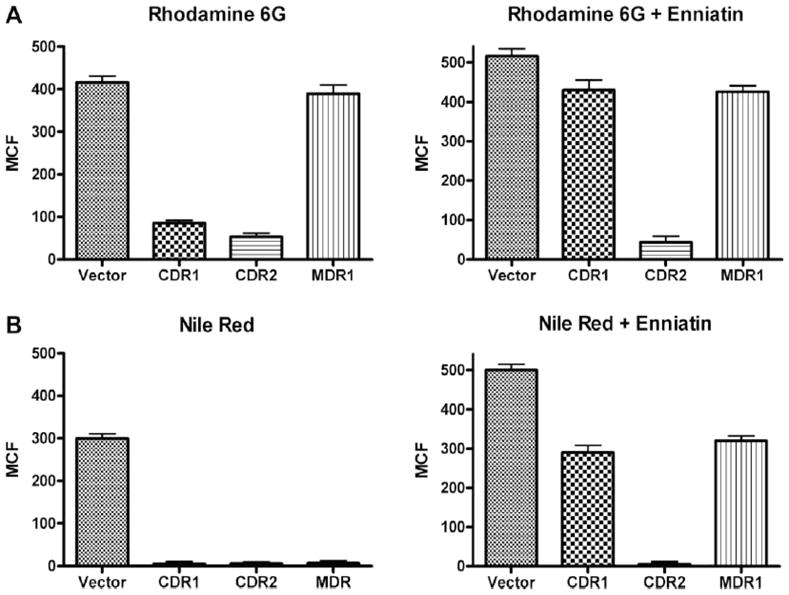
R6G (A) and Nile red (B) content of S. cerevisiae strain AD/pABC3 (vector only) and strains expressing C. albicans Cdr1p (AD/CDR1), Cdr2p (AD/CDR2), or Mdr1p(AD/MDR1) MES genes. Yeast cells were incubated with 15 μM R6G (A) or 7 μM Nile red (B). Enniatin (50 μM) was added to strains preloaded with substrate and incubated for 20 min. Each bar represents the median ± standard deviation (n = 3).
Acknowledgments
Funding for part of the research presented in this review was provided for by Massachusetts Technology Transfer Center (MTTC) to George Tegos NIH (U54, MH084690 Molecular Libraries Probe Production Centers Network, to George Tegos and Larry Sklar) and (R01GM59903, K. Lewis) and (R01AI050875, M. R. Hamblin). The authors will like to thank Melissa Brown & Laura Piddock for permission to use the MES scheme in Fig. 1 and to Martyn Symmons for kindly providing the PDB model of the Ac-rAB-TolC efflux pump.
ABBREVIATIONS
- MES
Multidrug efflux systems
- EPIs
Efflux pump inhibitors
- HTS
high-throughput screening
- ABC
ATP-binding cassette
- MFS
Major facilitator super
- RND
Resistance-nodulation
- SMR
Small multidrug resistance
- MATE
Multi-antimicrobial extrusion
- MET
Multidrug endosomal transporter
- PDR
Pleiotropic drug resistance
- HXT
Hexose transporter
- HME
Heavy-metal efflux
References
- 1.Poole K. Efflux-mediated antimicrobial resistance. J Antimicrob Chemother. 2005;56:20–51. doi: 10.1093/jac/dki171. [DOI] [PubMed] [Google Scholar]
- 2.Piddock L. Multidrug-resistance efflux pumps - not just for resistance. Nat Rev Microbiol. 2006;20:629–36. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 3.Piddock L. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147–56. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 5.Paulsen IT, Chen J, Nelson KE, Saier MHJ. Microbial Multidrug Efflux. Horizon Press; Norfolk: 2002. Comparative genomics of microbial drug efflux systems In: Le wis K, ed; pp. 5–21. [Google Scholar]
- 6.Ren Q, Paulsen IT. Comparative analyses of fundamental differences in membrane transport capabilities in prokaryotes and eukaryotes. PLoS Comput Biol. 2005;3:e27. doi: 10.1371/journal.pcbi.0010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon RD, Lamping E, Holmes AR, et al. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 2009;2:291–321. doi: 10.1128/CMR.00051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paumi CM, Chuk M, Snider J, Stagljar I, Michaelis S. ABC transporters in Saccharomyces cerevisiae and their interactors: new technology advances the biology of the ABCC (MRP) subfamily. Microbiol Mol Biol Rev. 2009;73:577–93. doi: 10.1128/MMBR.00020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman JJ, Mylonakis E. Efflux in fungi: la pièce de résistance. PLoS Pathog. 2009;5:e1000486. doi: 10.1371/journal.ppat.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lister P, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev. 2009;22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stover CK, Pham XQ, Erwin AL, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–64. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 12.Yin Y, He X, Szewczyk P, Nguyen T, Chang G. Structure of the multidrug transporter EmrD from Escherichia coli. Science. 2006;312:741–4. doi: 10.1126/science.1125629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirza O, Guan L, Verner G, Iwata S, Kaback HR. Structural Evidence for Induced Fit and a Mechanism for Sugar/H(+) Symport in LacY. EMBO J. 2006;25:2038. doi: 10.1038/sj.emboj.7601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and Mechanism of the Glycerol-3-Phosphate Transporter from Escherichia Coli. Science. 2003;301:616–20. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 15.Aller SG, Yu J, Ward A, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–22. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zawadzka A, Kim Y, Maltseva N, et al. Characterization of a Bacillus subtilis transporter for petrobactin, an anthrax stealth siderophore. Proc Natl Acad Sci USA. 2009;106:21854–59. doi: 10.1073/pnas.0904793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemieux M. Eukaryotic major facilitator superfamily transporter modeling based on the prokaryotic GlpT crystal structure. Mol Membr Biol. 2007;24:333–41. doi: 10.1080/09687680701496507. [DOI] [PubMed] [Google Scholar]
- 18.Koronakis V, Sharff V, Koronakis E, Luisi B, Hughes C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–19. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- 19.Higgins MK, Bokma E, Koronakis E, Hughes C, Koronakis V. Structure of the periplasmic component of a bacterial drug efflux pump. Proc Natl Acad Sci USA. 2004;101:9994–99. doi: 10.1073/pnas.0400375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eswaran J, Koronakis E, Higgins MK, Hughes C, Koronakis V. Three’s company: component structures bring a closer view of tripartite drug efflux pumps. Curr Opin Struct Biol. 2004;14:741–47. doi: 10.1016/j.sbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Sennhauser G, Bukowska MA, Briand C, Grütter MG. Crystal structure of the multidrug exporter MexB from Pseudomonas aeruginosa. J Mol Biol. 2009;389:134–45. doi: 10.1016/j.jmb.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Phan G, Broutin I, Benabdelhak H, Benas P, Rety S, Picard M, et al. Detailed structural description of Pseudomonas aeruginosa OprM protein in non-symmetrical space groups and dynamical insights into the opening of the periplasmic gate. Structure. 2010;18:507–17. doi: 10.1016/j.str.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Akama H, Matsuura T, Kashiwagi S, et al. Crystal structure of the membrane fusion protein, MexA, of the multidrug transporter in Pseudomonas aeruginosa. J Biol Chem. 2004;279:25939–42. doi: 10.1074/jbc.C400164200. [DOI] [PubMed] [Google Scholar]
- 24.Federici L, Du D, Walas F, et al. The crystal structure of the outer membrane protein VceC from the bacterial pathogen Vibrio cholerae at 1.8 A resolution. J Biol Chem. 2005;280:15307–14. doi: 10.1074/jbc.M500401200. [DOI] [PubMed] [Google Scholar]
- 25.Long F, Su CC, Zimmermann MT, et al. Crystal structures of the CusA efflux pump suggest methionine-mediated metal transport. Nature. 2010;467:484–8. doi: 10.1038/nature09395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Symmons M, Bokma E, Koronakis E, Hughes C, Koronakis V. Proc Natl Acad Sci USA. 2009;106:7173–8. doi: 10.1073/pnas.0900693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilke MS, Heller M, Creagh AL, et al. The crystal structure of MexR from Pseudomonas aeruginosa in complex with its antirepressor ArmR. Proc Natl Acad Sci USA. 2008;105:14832–7. doi: 10.1073/pnas.0805489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alguel Y, Lu D, Quade N, Sauter S, Zhang X. Crystal structure of MexZ, a key repressor responsible for antibiotic resistance in Pseudomonas aeruginosa. J Struct Biol. 2010;172:305–10. doi: 10.1016/j.jsb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Alguel Y, Meng C, Terán W, et al. Crystal structures of multidrug binding protein TtgR in complex with antibiotics and plant antimicrobials. J Mol Biol. 2007;369:829–40. doi: 10.1016/j.jmb.2007.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniels C, Daddaoua A, Lu D, Zhang X, Ramos JL. Domain crosstalk during effector binding to the multidrug binding TTGR regulator. J Biol Chem. 2010;285:21372–81. doi: 10.1074/jbc.M110.113282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Fiore A, Fiorentino G, Vitale RM, et al. Structural analysis of BldR from Sulfolobus solfataricus provides insights into the molecular basis of transcriptional activation in archaea by MarR family proteins. J Mol Biol. 2009;388:559–69. doi: 10.1016/j.jmb.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Hernández A, Maté MJ, Sánchez-Díaz PC, Romero A, Rojo F, Martínez JL. Structural and functional analysis of SmeT, the repressor of the Stenotrophomonas maltophilia multidrug efflux pump SmeDEF. J Biol Chem. 2009;284:14428–38. doi: 10.1074/jbc.M809221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yum S, Xu Y, Piao S, et al. Crystal structure of the periplasmic component of a tripartite macrolide-specific efflux pump. J Mol Biol. 2009;387:1286–97. doi: 10.1016/j.jmb.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 34.He X, Szewczyk P, Karyakin A, et al. Structure of a cation-bound multidrug and toxic compound extrusion transporter. Nature. 2010;467:991–4. doi: 10.1038/nature09408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis K. In search of natural substrates and inhibitors of MDR pumps. J Mol Microbiol Biotechnol. 2001;2:247–54. [PubMed] [Google Scholar]
- 36.Chung YJ, Saier MH., Jr SMR-type multidrug resistance pumps. Curr Opin Drug Discov Devel. 2001;4:237–45. [PubMed] [Google Scholar]
- 37.Chung YJ, Krueger C, Metzgar D, Saier MH., Jr Size comparisons among integral membrane transport protein homologues in bacteria, Archaea, and Eucarya. J Bacteriol. 2001;183:1012–21. doi: 10.1128/JB.183.3.1012-1021.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell B, Brown M, Skurray RA. QacA multidrug efflux pump from Staphylococcus aureus: comparative analysis of resistance to diamidines, biguanidines, and guanylhydrazones. Antimicrob Agents Chemother. 1998;42:475–7. doi: 10.1128/aac.42.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaatz GW, Seo SM, O’Brien L, Wahiduzzaman M, Foster TJ. Evidence for the existence of a multidrug efflux transporter distinct from NorA in Staphylococcus aureus. Antimicrob Agents Chemother. 2000;44:1404–6. doi: 10.1128/aac.44.5.1404-1406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huet AA, Raygada J, Mendiratta K, Seo SM, Kaatz GW. Multidrug efflux pump overexpression in Staphylococcus aureus after single and multiple in vitro exposures to biocides and dyes. Microbiology. 2008;154:3144–53. doi: 10.1099/mic.0.2008/021188-0. [DOI] [PubMed] [Google Scholar]
- 41.DeMarco CE, Cushing L, Frempong-Manso E, Seo SM, Jaravaza TA, Kaatz GW. Efflux-related resistance to norfloxacin, dyes, and biocides in bloodstream isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51:3235–9. doi: 10.1128/AAC.00430-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Bambeke F, Balzi E, Tulkens PM. Antibiotic efflux pumps. Biochem Pharmacol. 2000;60:457–70. doi: 10.1016/s0006-2952(00)00291-4. [DOI] [PubMed] [Google Scholar]
- 43.Vazquez-Laslop N, Zheleznova EE, Markham PN, Brennan RG, Neyfakh AA. Recognition of multiple drugs by a single protein: a trivial solution of an old paradox. Biochem Soc Trans. 2000;28:517–20. [PubMed] [Google Scholar]
- 44.Damier-Piolle L, Magnet S, Brémont S, Lambert T, Courvalin P. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob Agents Chemother. 2008;52:557–62. doi: 10.1128/AAC.00732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elkins C, Nikaido H. Substrate specificity of the RND-type multidrug efflux pumps AcrB and AcrD of Escherichia coli is determined predominantly by two large periplasmic loops. J Bacteriol. 2002;184:6490–8. doi: 10.1128/JB.184.23.6490-6498.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao W, Warren M, Black DS, et al. On the mechanism of substrate specificity by resistance nodulation division (RND)-type multidrug resistance pumps: the large periplasmic loops of MexD from Pseudomonas aeruginosa are involved in substrate recognition. Mol Microbiol. 2002;46:889–901. doi: 10.1046/j.1365-2958.2002.03223.x. [DOI] [PubMed] [Google Scholar]
- 47.Santiviago C, Fuentes JA, Bueno SM, et al. The Salmonella enterica sv. Typhimurium smvA, yddG and ompD (porin) genes are required for the efficient efflux of methyl viologen. Mol Microbiol. 2002;46:687–97. doi: 10.1046/j.1365-2958.2002.03204.x. [DOI] [PubMed] [Google Scholar]
- 48.Elkins C, Mullis LB. Mammalian steroid hormones are substrates for the major RND- and MFS-type tripartite multidrug efflux pumps of Escherichia coli. J Bacteriol. 2006;188:1191–5. doi: 10.1128/JB.188.3.1191-1195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajamohan G, Srinivasan VB, Gebreyes WA. Novel role of Acinetobacter baumannii RND efflux transporters in mediating decreased susceptibility to biocides. J Antimicrob Chemother. 2010;65:228–32. doi: 10.1093/jac/dkp427. [DOI] [PubMed] [Google Scholar]
- 50.Lage H. ABC-transporters: implications on drug resistance from microorganisms to human cancers. Int J Antimicrob Agents. 2003;22:188–99. doi: 10.1016/s0924-8579(03)00203-6. [DOI] [PubMed] [Google Scholar]
- 51.Tekaia F, Latgé JP. Aminoglycosides are captured from both periplasm and cytoplasm by the AcrD multidrug efflux transporter of Escherichia coli. Curr Opin Microbiol. 2005;8:385–92. doi: 10.1128/JB.187.6.1923-1929.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aires J, Nikaido H. Aminoglycosides are captured from both periplasm and cytoplasm by the AcrD multidrug efflux transporter of Escherichia coli. J Bacteriol. 2005;187:1923–9. doi: 10.1128/JB.187.6.1923-1929.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Pasayo R, Martinez-Romero E. Multiresistance genes of Rhizobium etli CFN42. Mol Plant Microbe Interact. 2000;13:572–7. doi: 10.1094/MPMI.2000.13.5.572. [DOI] [PubMed] [Google Scholar]
- 54.Palumbo J, Kado CI, Phillips DA. An isoflavonoid-inducible efflux pump in Agrobacterium tumefaciens is involved in competitive colonization of roots. J Bacteriol. 1998;180:3107–13. doi: 10.1128/jb.180.12.3107-3113.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tegos G, Stermitz FR, Lomovskaya O, Lewis K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob Agents Chemother. 2002;46:3133–41. doi: 10.1128/AAC.46.10.3133-3141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agarwal S, Singh SS, Verma S, Kumar S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J Ethnopharmacol. 2000;72:43–6. doi: 10.1016/s0378-8741(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 57.Didry N, Dubreuil L, Pinkas M. Activity of anthraquinonic and naphthoquinonic compounds on oral bacteria. Pharmazie. 1994;49:681–3. [PubMed] [Google Scholar]
- 58.Barabote R, Johnson OL, Zetina E, San Francisco SK, Fralick JA, San Francisco MJ. Erwinia chrysanthemi tolC is involved in resistance to antimicrobial plant chemicals and is essential for phytopathogenesis. J Bacteriol. 2003;185:5772–8. doi: 10.1128/JB.185.19.5772-5778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ravirala R, Barabote RD, Wheeler DM, et al. Efflux pump gene expression in Erwinia chrysanthemi is induced by exposure to phenolic acids. Mol Plant Microbe Interact. 2007;20:313–20. doi: 10.1094/MPMI-20-3-0313. [DOI] [PubMed] [Google Scholar]
- 60.Brown D, Swanson JK, Allen C. Two host-induced Ralstonia solanacearum genes, acrA and dinF, encode multidrug efflux pumps and contribute to bacterial wilt virulence. Appl Environ Microbiol. 2007;73:2777–86. doi: 10.1128/AEM.00984-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lomovskaya O, Warren MS, Lee A, et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob Agents Chemother. 2001;45:105–16. doi: 10.1128/AAC.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lomovskaya O, Watkins W. Inhibition of efflux pumps as a novel approach to combat drug resistance in bacteria. J Mol Microbiol Biotechnol. 2001;3:225–36. [PubMed] [Google Scholar]
- 63.Wang EJ, Casciano CN, Clement RP, Johnson WW. Active transport of fluorescent P-glycoprotein substrates: evaluation as markers and interaction with inhibitors. Biochem Biophys Res Commun. 2001;289:580–5. doi: 10.1006/bbrc.2001.6000. [DOI] [PubMed] [Google Scholar]
- 64.Aeschlimann J, Dresser LD, Kaatz GW, Rybak MJ. Effects of NorA inhibitors on in vitro antibacterial activities and postantibiotic effects of levofloxacin, ciprofloxacin, and norfloxacin in genetically related strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:335–40. doi: 10.1128/aac.43.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beck W, Cirtain M, Glover C, Felsted R, Safa A. Effects of indole alkaloids on multidrug resistance and labeling of P-glycoprotein by a photoaffinity analog of vinblastine. Biochem Biophys Res Com. 1988;153:959–66. doi: 10.1016/s0006-291x(88)81321-4. [DOI] [PubMed] [Google Scholar]
- 66.Mullin S, Mani N, Grossman TH. Inhibition of antibiotic efflux in bacteria by the novel multidrug resistance inhibitors biricodar (VX-710) and timcodar (VX-853) Antimicrob Agents Chemother. 2004;48:4171–6. doi: 10.1128/AAC.48.11.4171-4176.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Germann U, Shlyakhter D, Mason VS, et al. Cellular and biochemical characterization of VX-710 as a chemosensitizer: reversal of P-glycoprotein-mediated multidrug resistance in vitro. Anti-Cancer Drugs. 1997;8:125–40. doi: 10.1097/00001813-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Amaral L, Martins M, Viveiros M. Enhanced killing of intracellular multidrug-resistant Mycobacterium tuberculosis by compounds that affect the activity of efflux pumps. J Antimicrob Chemother. 2007;59:1237–46. doi: 10.1093/jac/dkl500. [DOI] [PubMed] [Google Scholar]
- 69.Poelarends G, Mazurkiewicz P, Konings WN. Multidrug transporters and antibiotic resistance in Lactococcus lactis. Biochim Biophys Acta. 2002;1555:1–7. doi: 10.1016/s0005-2728(02)00246-3. [DOI] [PubMed] [Google Scholar]
- 70.Markham P, Westhaus E, Klyachko K, Johnson ME, Neyfakh AA. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:2404–8. doi: 10.1128/aac.43.10.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stermitz FR, Lorenz P, Tawara JN, Zenewicz LA, Lewis K. Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Natl Acad Sci USA. 2000;97:1433–7. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tegos GP. Substrates and Inhibitors of microbial efflux pumps; Redifine the Role of Plant Antimicrobilas. In: Rai M, Carpinella CM, editors. Naturally occurring bioactive compounds: a new and safe alternative for control of pests and microbial diseases. Cambridge University Press; Cambridge UK: 2006. pp. 45–59. [Google Scholar]
- 73.Stermitz FR, Beeson TD, Mueller PJ, Hsiang J, Lewis K. Staphylococcus aureus MDR efflux pump inhibitors from a Berberis and a Mahonia (sensu strictu) species. Biochem Syst Ecol. 2001;29:793–8. doi: 10.1016/s0305-1978(01)00025-4. [DOI] [PubMed] [Google Scholar]
- 74.Stermitz FR, Tawara-Matsuda J, Lorenz P, Mueller P, Zenewicz L, Lewis K. 5-0-Methoxyhydnocarpin-D and pheophorbide A: Berberis species components that potentiate berberine growth inhibition of resistant Staphylococcus aureus. J Nat Prod. 2000;63:1146–9. doi: 10.1021/np990639k. [DOI] [PubMed] [Google Scholar]
- 75.Stermitz F, Scriven LN, Tegos G, Lewis K. Two flavonols from Artemisa annua which potentiate the activity of berberine and norfloxacin against a resistant strain of Staphylococcus aureus. Planta Med. 2002;68:1140–1. doi: 10.1055/s-2002-36347. [DOI] [PubMed] [Google Scholar]
- 76.Stermitz FR, Halligan KM, Morel C, Tegos GP, Lewis K. Polyacylated neohesperidosides From Geranium caespitosum: bacterial multidrug resistance pump inhibitors. Bioorg Med Chem Lett. 2003;13:1915–8. doi: 10.1016/s0960-894x(03)00316-0. [DOI] [PubMed] [Google Scholar]
- 77.Belofsky G, Percivil D, Lewis K, Tegos G, Ekart J. Phenolic Metabolites of Dalea versicolor that Enhance Antibiotic Activity Against Multi-Drug Resistant Bacteria. J Nat Prod. 2004;67:481–4. doi: 10.1021/np030409c. [DOI] [PubMed] [Google Scholar]
- 78.Belofsky G, Carreno R, Lewis K, Ball A, Casadei G, Tegos GP. Metabolites of the “smoke tree”, Dalea spinosa, potentiate antibiotic activity against multidrug-resistant Staphylococcus aureus. J Nat Prod. 2006;69:261–4. doi: 10.1021/np058057s. [DOI] [PubMed] [Google Scholar]
- 79.Morel C, Stermitz FR, Tegos G, Lewis K. Isoflavones as potentiators of antibacterial activity. J Agric Food Chem. 2003;51:5677–9. doi: 10.1021/jf0302714. [DOI] [PubMed] [Google Scholar]
- 80.Gibbons S, Moser E, Kaatz GW. Catechin gallates inhibit multidrug resistance (MDR) in Staphylococcus aureus. Planta Med. 2004;70:1240–2. doi: 10.1055/s-2004-835860. [DOI] [PubMed] [Google Scholar]
- 81.Chérigo L, Pereda-Miranda R, Fragoso-Serrano M, Jacobo-Herrera N, Kaatz GW, Gibbons S. Inhibitors of bacterial multidrug efflux pumps from the resin glycosides of Ipomoea murucoides. J Nat Prod. 2008;71:1037–45. doi: 10.1021/np800148w. [DOI] [PubMed] [Google Scholar]
- 82.Pereda-Miranda R, Kaatz GW, Gibbons S. Polyacylated oligosaccharides from medicinal Mexican morning glory species as antibacterials and inhibitors of multidrug resistance in Staphylococcus aureus. J Nat Prod. 2006;69:406–9. doi: 10.1021/np050227d. [DOI] [PubMed] [Google Scholar]
- 83.Michalet S, Cartier G, David B, et al. N-caffeoylphenalkylamide derivatives as bacterial efflux pump inhibitors. Bioorg Med Chem Lett. 2007;17:1755–8. doi: 10.1016/j.bmcl.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 84.Thota N, Koul S, Reddy MV, et al. Citral derived amides as potent bacterial NorA efflux pump inhibitors. Bioorg Med Chem. 2008;16:6535–43. doi: 10.1016/j.bmc.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 85.Falcão-Silva V, Silva DA, de Souza MF, Siqueira-Junior JP. Modulation of drug resistance in Staphylococcus aureus by a kaempferol glycoside from Herissantia tiubae (Malvaceae) Phytother Res. 2009;10:1367–70. doi: 10.1002/ptr.2695. [DOI] [PubMed] [Google Scholar]
- 86.Mohtar M, Johari SA, Li AR, et al. Inhibitory and resistance-modifying potential of plant-based alkaloids against methicillin-resistant Staphylococcus aureus (MRSA) Curr Microbiol. 2009;59:181–6. doi: 10.1007/s00284-009-9416-9. [DOI] [PubMed] [Google Scholar]
- 87.Lechner D, Gibbons S, Bucar F. Plant phenolic compounds as ethidium bromide efflux inhibitors in Mycobacterium smegmatis. J Antimicrob Chemother. 2007;62:345–8. doi: 10.1093/jac/dkn178. [DOI] [PubMed] [Google Scholar]
- 88.Wei P, Kaatz GW, Kerns RJ. Structural differences between paroxetine and femoxetine responsible for differential inhibition of Staphylococcus aureus efflux pumps. Bioorg Med Chem Lett. 2004;14:3093–7. doi: 10.1016/j.bmcl.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 89.Sangwan P, Koul JL, Koul S, et al. Piperine analogs as potent Staphylococcus aureus NorA efflux pump inhibitors. Bioorg Med Chem. 2008;16:9847–57. doi: 10.1016/j.bmc.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 90.German N, Wei P, Kaatz GW, Kerns RJ. Synthesis and evaluation of fluoroquinolone derivatives as substrate-based inhibitors of bacterial efflux pumps. Eur J Med Chem. 2008;43:2453–63. doi: 10.1016/j.ejmech.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 91.Sabatini S, Kaatz GW, Rossolini GM, Brandini D, Fravolini A. >From phenothiazine to 3-phenyl-1,4-benzothiazine derivatives as inhibitors of the Staphylococcus aureus NorA multidrug efflux pump. J Med Chem. 2008;51:4321–30. doi: 10.1021/jm701623q. [DOI] [PubMed] [Google Scholar]
- 92.Rodrigues L, Wagner D, Viveiros M, et al. Thioridazine and chlorpromazine inhibition of ethidium bromide efflux in Mycobacterium avium and Mycobacterium smegmatis. J Antimicrob Chemother. 2008;61:1076–82. doi: 10.1093/jac/dkn070. [DOI] [PubMed] [Google Scholar]
- 93.Ball A, Casadei G, Samosorn S, et al. Conjugating berberine to a multidrug efflux pump inhibitor creates an effective antimicrobial. ACS Chem Biol. 2006;1:594–600. doi: 10.1021/cb600238x. [DOI] [PubMed] [Google Scholar]
- 94.Samosorn S, Tanwirat B, Muhamad N, et al. Antibacterial activity of berberine-NorA pump inhibitor hybrids with a methylene ether linking group. Bioorg Med Chem. 2009;17:3866–72. doi: 10.1016/j.bmc.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tomkiewicz D, Casadei G, Larkins-Ford J, et al. Berberine-INF55 (5-nitro-2-phenylindole) hybrid antimicrobials: effects of varying the relative orientation of the berberine and INF55 components. Antimicrob Agents Chemother. 2010;54:3219–24. doi: 10.1128/AAC.01715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dolmans D, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–7. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 97.Bressler N, Bressler SB. Photodynamic therapy with verteporfin (Visudyne): impact on ophthalmology and visual sciences. Investig Ophthalmol Vis Sci. 2000;41:624–8. [PubMed] [Google Scholar]
- 98.Hamblin M, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–50. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson MA. Study of the uptake of toluidine blue O by Porphyromonas gingivalis and the mechanism of lethal photosensitization. Photochem Photobiol. 1998;68:370–6. [PubMed] [Google Scholar]
- 100.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J Antimicrob Chemother. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 101.Tegos G, Hamblin MR. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob Agents Chemother. 2006;50:196–203. doi: 10.1128/AAC.50.1.196-203.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tegos G, Masago K, Aziz F, Higginbotham A, Stermitz FR, Hamblin MR. Inhibitors of bacterial multidrug efflux pumps potentiate antimicrobial photoinactivation. Antimicrob Agents Chemother. 2008;52:3202–9. doi: 10.1128/AAC.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Robey R, Fetsch PA, Polgar O, Dean M, Bates SE. The livestock photosensitizer, phytoporphyrin (phylloerythrin), is a substrate of the ATP-binding cassette transporter ABCG2. Res Vet Sci. 2006;81:345–9. doi: 10.1016/j.rvsc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 104.Robey R, Steadman K, Polgar O, Bates SE. ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy. Cancer Biol Ther. 2005;4:187–94. [PubMed] [Google Scholar]
- 105.Grinholc M, Zawacka-Pankau J, Gwizdek-Wiśniewska A, Bielawski KP. Evaluation of the role of the pharmacological inhibition of Staphylococcus aureus multidrug resistance pumps and the variable levels of the uptake of the sensitizer in the strain-dependent response of Staphylococcus aureus to PPArg(2)-based photodynamic inactivation. Photochem Photobiol. 2010;86:1118–26. doi: 10.1111/j.1751-1097.2010.00772.x. [DOI] [PubMed] [Google Scholar]
- 106.Wilson M, Yianni C. Killing of methicillin-resistant Staphylococcus aureus by low-power laser light. J Med Microbiol. 1995;42:62–6. doi: 10.1099/00222615-42-1-62. [DOI] [PubMed] [Google Scholar]
- 107.Soncin M, Fabris C, Busetti A, et al. Approaches to selectivity in the Zn(II)-phthalocyanine-photosensitized inactivation of wild-type and antibiotic-resistant Staphylococcus aureus. Photochem Photobiol Sci. 2002;1:815–9. doi: 10.1039/b206554a. [DOI] [PubMed] [Google Scholar]
- 108.Bornstein E, Gridley S, Wengender P, Robbins A. Photodamage to multidrug-resistant gram-positive and gram-negative bacteria by 870 nm/930 nm light potentiates erythromycin, tetracycline and ciprofloxacin. Photochem Photobiol. 2010;86:617–27. doi: 10.1111/j.1751-1097.2010.00725.x. [DOI] [PubMed] [Google Scholar]
- 109.Barrett J. MC-207110 Daiichi Seiyaku/Microcide Pharmaceuticals. Curr Opin Investig Drugs. 2001;2:212–5. [PubMed] [Google Scholar]
- 110.Zechini B, Versace I. Inhibitors of Multidrug Resistant Efflux Systems in Bacteria. Recent Patents on Anti-Infective Drug Discovery. 2009;4:37–50. doi: 10.2174/157489109787236256. [DOI] [PubMed] [Google Scholar]
- 111.Nakayama K, Ishida Y, Ohtsuka M, et al. MexAB-OprM-specific efflux pump inhibitors in Pseudomonas aeruginosa. Part 1: discovery and early strategies for lead optimization. Bioorg Med Chem Lett. 2003;13:4201–4. doi: 10.1016/j.bmcl.2003.07.024. [DOI] [PubMed] [Google Scholar]
- 112.Nakayama K, Ishida Y, Ohtsuka M, et al. MexAB-OprM specific efflux pump inhibitors in Pseudomonas aeruginosa Part 2: achieving activity in vivo through the use of alternative scaffolds. Bioorg Med Chem Lett. 2003;13:4205–8. doi: 10.1016/j.bmcl.2003.07.027. [DOI] [PubMed] [Google Scholar]
- 113.Nakayama K, Kawato H, Watanabe J, et al. MexAB-OprM specific efflux pump inhibitors in Pseudomonas aeruginosa. Part 3: Optimization of potency in the pyridopyrimidine series through the application of a pharmacophore model. Bioorg Med Chem Lett. 2004;14:475–9. doi: 10.1016/j.bmcl.2003.10.060. [DOI] [PubMed] [Google Scholar]
- 114.Yoshida K, Nakayama K, Ohtsuka M, et al. MexAB-OprM specific efflux pump inhibitors in Pseudomonas aeruginosa. Part 7: highly soluble and in vivo active quaternary ammonium analogue D13-9001, a potential preclinical candidate. Bioorg Med Chem Lett. 2007;15:7087–97. doi: 10.1016/j.bmc.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 115.Nakayama K, Kuru N, Ohtsuka M, et al. MexAB-OprM specific efflux pump inhibitors in Pseudomonas aeruginosa. Part 4: Addressing the problem of poor stability due to photoisomerization of an acrylic acid moiety. Bioorg Med Chem Lett. 2004;14:2493–7. doi: 10.1016/j.bmcl.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 116.Renau T, Léger R, Filonova L, et al. Conformationally-restricted analogues of efflux pump inhibitors that potentiate the activity of levofloxacin in Pseudomonas aeruginosa. Bioorg Med Chem Lett. 2003;13:2755–8. doi: 10.1016/s0960-894x(03)00556-0. [DOI] [PubMed] [Google Scholar]
- 117.Yoshida K, Nakayama K, Yokomizo Y, et al. MexAB-OprM specific efflux pump inhibitors in Pseudomonas aeruginosa. Part 6: exploration of aromatic substituents. Bioorg Med Chem. 2006;14:8506–18. doi: 10.1016/j.bmc.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 118.Lomovskaya O, Bostian KA. Practical applications and feasibility of efflux pump inhibitors in the clinic - A vision for applied use. Biochem Pharmacol. 2006;71:910–18. doi: 10.1016/j.bcp.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 119.Pagès J, Masi M, Barbe J. Inhibitors of efflux pumps in Gram negative bacteria. Trends Mol Med. 2005;11:382–9. doi: 10.1016/j.molmed.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 120.Mahamoud A, Chevalier J, Libert-Franco S, Kern WV, Pages JM. Antibiotic efflux pumps in Gram negative bacteria: the inhibitory response strategy. J Antimicrob Chemother. 2007;59:1223–9. doi: 10.1093/jac/dkl493. [DOI] [PubMed] [Google Scholar]
- 121.Mallèa M, Chevalier J, Eyraud A, Pagès JM. Inhibitors of antibiotic efflux pumps in resistant Enterobacter aerogenes strains. Biochem Biophys Res Commun. 2002;293:1370–3. doi: 10.1016/S0006-291X(02)00404-7. [DOI] [PubMed] [Google Scholar]
- 122.Mazzariol A, Tokue Y, Kanegawa TM, Cornaglia G, Nikaido H. High level fluoroquinolone-resistant clinical isolates of Escherichia coli overproduce multidrug efflux protein. Antimicrob Agents Chemother. 2000;44:3441–3. doi: 10.1128/aac.44.12.3441-3443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hasdemir U, Chevalier J, Nordmann P, Pages JM. Detection and prevalence of active drug efflux mechanism in various multidrugresistant Klebsiella pneumoniae strains from Turkey. J Clin Microbiol. 2004;42:2701–6. doi: 10.1128/JCM.42.6.2701-2706.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baucheron S, Imberechts H, Chaslus-Dancla E, Cloeckaert A. The AcrB multidrug transporters plasy a major role in high-level fluorquinolone resistance in Salmonella enterica serovar thyphimurium phage typer DT 204. Microb Drug Resist. 2002;8:281–9. doi: 10.1089/10766290260469543. [DOI] [PubMed] [Google Scholar]
- 125.Pannek S, Higgins PG, Steinke P, et al. Multidrug efflux inhibition in Acinetobacter baumannii: comparison between 1-(1-naphthylmethyl)-piperazine and phenyl-arginine-beta-naphthylamide. J Antimicrob Chemother. 2006;57:970–4. doi: 10.1093/jac/dkl081. [DOI] [PubMed] [Google Scholar]
- 126.Hannula M, Hänninen ML. Effect of putative efflux pump inhibitors and inducers on the antimicrobial susceptibility of Campylobacter jejuni and Campylobacter coli. J Med Microbiol. 2008;57:851–5. doi: 10.1099/jmm.0.47823-0. [DOI] [PubMed] [Google Scholar]
- 127.Lin J, Martinez A. Effect of efflux pump inhibitors on bile resistance and in vivo colonization of Campylobacter jejuni. J Antimicrob Chemother. 2006;58:966–72. doi: 10.1093/jac/dkl374. [DOI] [PubMed] [Google Scholar]
- 128.Thorarensen A, Presley-Bodnar AL, Marotti KR, et al. 3-Arylpiperidines as potentiators of existing antibacterial agents. Bioorg Med Chem Lett. 2002;11:1903–6. doi: 10.1016/s0960-894x(01)00330-4. [DOI] [PubMed] [Google Scholar]
- 129.Kern W, Steinke P, Schumacher A, Schuster S, von Baum H, Bohnert JA. Effect of 1-(1-naphthylmethyl)-piperazine, a novel putative efflux pump inhibitor, on antimicrobial drug susceptibility in clinical isolates of Escherichia coli. J Antimicrob Chemother. 2006;57:339–43. doi: 10.1093/jac/dki445. [DOI] [PubMed] [Google Scholar]
- 130.Coban A, Tanriverdi, Cayci Y, Erturan Z, Durupinar B. Effects of efflux pump inhibitors phenyl-arginine-beta-naphthylamide and 1-(1-naphthylmethyl)-piperazine on the antimicrobial susceptibility of Pseudomonas aeruginosa isolates from cystic fibrosis patients. J Chemother. 2009;21:592–4. doi: 10.1179/joc.2009.21.5.592. [DOI] [PubMed] [Google Scholar]
- 131.Piddock L, Garvey MI, Rahman MM, Gibbons S. Natural and synthetic compounds such as trimethoprim behave as inhibitors of efflux in Gram-negative bacteria. J Antimicrob Chemother. 2010;65:1215–23. doi: 10.1093/jac/dkq079. [DOI] [PubMed] [Google Scholar]
- 132.Tang J, Wang H. Indole derivatives as efflux pump inhibitors for TolC protein in a clinical drug-resistant Escherichia coli isolated from a pig farm. Int J Antimicrob Agents. 2006;5:497–8. doi: 10.1016/j.ijantimicag.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 133.Chan Y, Ong YM, Chua KL. Synergistic interaction between phenothiazines and antimicrobial agents against Burkholderia pseudomallei. Antimicrob Agents Chemother. 2007;51:623–30. doi: 10.1128/AAC.01033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mahamoud A, Chevalier J, Davin-Regli A, Barbe J, Pagès JM. Quinoline derivatives as promising inhibitors of antibiotic efflux pump in multidrug resistant Enterobacter aerogenes isolates. Curr Drug Targets. 2006;7:843–7. doi: 10.2174/138945006777709557. [DOI] [PubMed] [Google Scholar]
- 135.Chevalier J, Bredin J, Mahamoud A, Malléa M, Barbe J, Pagès JM. Inhibitors of antibiotic efflux in resistant Enterobacter aerogenes and Klebsiella pneumoniae strains. Antimicrob Agents Chemother. 2004;48:1043–6. doi: 10.1128/AAC.48.3.1043-1046.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang Z, Liu ZQ, Zheng PY, Tang FA, Yang PC. Influence of efflux pump inhibitors on the multidrug resistance of Helicobacter pylori. World J Gastroenterol. 2010;16:1279–84. doi: 10.3748/wjg.v16.i10.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maesaki S, Marichal P, Hossain MA, Sanglard D, Vanden Bossche H, Kohno S. Synergic effects of tactolimus and azole antifungal agents against azole-resistant Candida albican strains. J Antimicrob Chemother. 1996;42:747–53. doi: 10.1093/jac/42.6.747. [DOI] [PubMed] [Google Scholar]
- 138.Kolaczkowski M, Kolaczkowska A, Motohashi N, Michalak K. New high-throughput screening assay to reveal similarities and differences in inhibitory sensitivities of multidrug ATP-binding cassette transporters. Antimicrob Agents Chemother. 2009;54:1516–27. doi: 10.1128/AAC.00956-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schuetzer-Muehlbauer M, Willinger B, Egner R, Ecker G, Kuchler K. Reversal of antifungal resistance mediated by ABC efflux pumps from Candida albicans functionally expressed in yeast. Int J Antimicrob Agents. 2003;3:291–300. doi: 10.1016/s0924-8579(03)00213-9. [DOI] [PubMed] [Google Scholar]
- 140.Yamamoto S, Hiraga K, Abiko A, Hamanaka N, Oda K. A new function of isonitrile as an inhibitor of the Pdr5p multidrug ABC transporter in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 2005;330:622–8. doi: 10.1016/j.bbrc.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 141.Niimi K, Harding DR, Parshot R, et al. Chemosensitization of fluconazole resistance in Saccharomyces cerevisiae and pathogenic fungi by a D-octapeptide derivative. Antimicrob Agents Chemother. 2004;48:1256–71. doi: 10.1128/AAC.48.4.1256-1271.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lemoine R, Glinka TW, Watkins WJ, et al. Quinazolinone-based fungal efflux pump inhibitors. Part 1: Discovery of an (N-methylpiperazine)-containing derivative with activity in clinically relevant Candida spp. Bioorg Med Chem Lett. 2004;14:5127–31. doi: 10.1016/j.bmcl.2004.07.070. [DOI] [PubMed] [Google Scholar]
- 143.Watkins WJ, Lemoine RC, Chong L, et al. Quinazolinone fungal efflux pump inhibitors. Part 2: In vitro structure-activity relationships of (N-methyl-piperazinyl)-containing derivatives. Bioorg Med Chem Lett. 2004;14:5133–7. doi: 10.1016/j.bmcl.2004.07.071. [DOI] [PubMed] [Google Scholar]
- 144.Watkins W, Chong L, Cho A, et al. Quinazolinone fungal efflux pump inhibitors. Part 3: (N-methyl)piperazine variants and pharmacokinetic optimization. Bioorg Med Chem Lett. 2007;17:2802–6. doi: 10.1016/j.bmcl.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 145.Tanabe K, Lamping E, Adachi K, et al. Inhibition of fungal ABC transporters by unnarmicin A and unnarmicin C, novel cyclic peptides from marine bacterium. Biochem Biophys Res Commun. 2007;364:990–5. doi: 10.1016/j.bbrc.2007.10.110. [DOI] [PubMed] [Google Scholar]
- 146.Digirolamo J, Li XC, Jacob MR, Clark AM, Ferreira D. Reversal of fluconazole resistance by sulfated sterols from the marine sponge Topsentia sp. J Nat Prod. 2009;72:1524–8. doi: 10.1021/np900177m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Singh P, Kaur J, Yadav B, Komath SS. Targeting efflux pumps-In vitro investigations with acridone derivatives and identification of a lead molecule for MDR modulation. Bioorg Med Chem. 2010;18:4212–3. doi: 10.1016/j.bmc.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 148.Pina-Vaz C, Rodrigues AG, Costa-de-Oliveira S, Ricardo E, Mårdh PA. Potent synergic effect between ibuprofen and azoles on Candida resulting from blockade of efflux pumps as determined by FUN-1 staining and flow cytometry. J Antimicrob Chemother. 2005;56:678–85. doi: 10.1093/jac/dki264. [DOI] [PubMed] [Google Scholar]
- 149.Pamp S, Gjermansen M, Johansen HK, Tolker-Nielsen T. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol Microbiol. 2008;68:223–40. doi: 10.1111/j.1365-2958.2008.06152.x. [DOI] [PubMed] [Google Scholar]
- 150.Zhang L, Mah TF. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J Bacteriol. 2008;190:4447–52. doi: 10.1128/JB.01655-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mukherjee P, Chandra J, Kuhn DM, Ghannoum MA. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun. 2003;71:4333–40. doi: 10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ramage G, Bachmann S, Patterson TF, Wickes BL, López-Ribot JL. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother. 2002;49:973–80. doi: 10.1093/jac/dkf049. [DOI] [PubMed] [Google Scholar]
- 153.Imuta N, Nishi J, Tokuda K, Fujiyama R, Manago K, Iwashita, et al. Infect Immun. 2008;76:1247–56. doi: 10.1128/IAI.00758-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lynch S, Dixon L, Benoit M, Brodie E, Keyhan M, Hu P, et al. The Escherichia coli efflux pump TolC promotes aggregation of enteroaggregative E. coli 042. Antimicrob Agents Chemother. 2007;51:3650–8. doi: 10.1128/AAC.00601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rosenberg E, Bertenthal D, Nilles ML, Bertrand KP, Nikaido H. Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol Microbiol. 2003;48:1609–19. doi: 10.1046/j.1365-2958.2003.03531.x. [DOI] [PubMed] [Google Scholar]
- 156.Gillis R, White K, Choi K, Wagner V, Schweizer H, Iglewski B. Molecular basis of azithromycin-resistant Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2005;49:3858–67. doi: 10.1128/AAC.49.9.3858-3867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.De Kievit T, Parkins M, Gillis R, Srikumar R, Ceri H, Poole K, et al. Multidrug efflux pumps: expression patterns and contribution to antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2001;45:1761–70. doi: 10.1128/AAC.45.6.1761-1770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.O’Toole G, Stewart P. Biofilms strike back. Nat Biotechnol. 2005;23:1378–9. doi: 10.1038/nbt1105-1378. [DOI] [PubMed] [Google Scholar]
- 159.Mah T, Pitts B, Pellock B, Walker GC, Stewart PS, O’Toole GA. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature. 2003;426:306–10. doi: 10.1038/nature02122. [DOI] [PubMed] [Google Scholar]
- 160.Kvist M, Hancock V, Klemm P. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl Environ Microbiol. 2008;74:7376–82. doi: 10.1128/AEM.01310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kishen A, Upadya M, Tegos GP, Hamblin MR. Efflux pump inhibitor potentiates antimicrobial photodynamic inactivation of Enterococcus faecalis biofilm. Photochem Photobiol. 2010;86:1343–9. doi: 10.1111/j.1751-1097.2010.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Klyachko K, Schuldiner S, Neyfakh AA. Mutations affecting substrate specificity of the Bacillus subtilis multidrug transporter. BMR J Bacteriol. 1997;179:2189–93. doi: 10.1128/jb.179.7.2189-2193.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ahmed M, Borsch C, Neyfakh A, Schuldner S. Mutants of Bacillus subtilis multidrug transporter Bmr with altered sensitivity to the antihypertensive alkaloid reserpine. J Biol Chem. 1993;268:11086–9. [PubMed] [Google Scholar]
- 164.Garvey M, Piddock LJ. The efflux pump inhibitor reserpine selects multidrug-resistant Streptococcus pneumoniae strains that overexpress the ABC transporters PatA and PatB. Antimicrob Agents Chemother. 2008;52:1677–85. doi: 10.1128/AAC.01644-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gibbons S. Phytochemicals for bacterial resistance--strengths, weaknesses and opportunities. Planta Med. 2008;74:594–602. doi: 10.1055/s-2008-1074518. [DOI] [PubMed] [Google Scholar]
- 166.Ji H, Li XJ, Zhang HY. Natural products and drug discovery. EMBO reports. 2009;10:194–200. doi: 10.1038/embor.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Demain A, Sanchez S. Microbial drug discovery: 80 years of progress. J Antibiot (Tokyo) 2009;62:5–16. doi: 10.1038/ja.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lewis K, Ausubel FM. Prospects for plant-derived antibacterials. Nat Biotechnol. 2006;24:1504–7. doi: 10.1038/nbt1206-1504. [DOI] [PubMed] [Google Scholar]
- 169.Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nat Rev Microbiol. 2008;6:17–27. doi: 10.1038/nrmicro1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Winter S, Lovato DM, Khawaja HM, Edwards BS, Steele ID, Young SM, et al. High-throughput screening for daunorubicin-mediated drug resistance identifies mometasone furoate as a novel ABCB1-reversal agent. J Biomol Screen. 2008;13:185–93. doi: 10.1177/1087057108314610. [DOI] [PubMed] [Google Scholar]
- 171.Johnson R, Allen C, Melman SD, et al. Identification of inhibitors of vacuolar proton-translocating ATPase pumps in yeast by high-throughput screening flow cytometry. Anal Biochem. 2010;398:203–11. doi: 10.1016/j.ab.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ivnitski-Steele I, Larson RS, Lovato DM, et al. High-throughput flow cytometry to detect selective inhibitors of ABCB1, ABCC1, and ABCG2 transporters. Assay Drug Dev Technol. 2008;2:263–76. doi: 10.1089/adt.2007.107. [DOI] [PubMed] [Google Scholar]
- 173.Ivnitski-Steele I, Holmes AR, Lamping E, Monk BC, Cannon RD, Sklar LA. Identification of Nile red as a fluorescent substrate of the Candida albicans ATP-binding cassette transporters Cdr1p and Cdr2p and the major facilitator superfamily transporter. Anal Biochem. 2009;394:87–91. doi: 10.1016/j.ab.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Giacomini KM, Huang SM, Tweedie DJ, et al. The International Transporter Consortium. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–36. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Villarán R, Tomás-Camardiel M, de Pablos RM, et al. Endogenous dopamine enhances the neurotoxicity of 3-nitropropionic acid in the striatum through the increase of mitochondrial respiratory inhibition and free radicals production. J Neurotoxicology. 2008;2:244–58. doi: 10.1016/j.neuro.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 176.Gandhi L, Harding MW, Neubauer M, et al. A phase II study of the safety and efficacy of the multidrug resistance inhibitor VX-710 combined with doxorubicin and vincristine in patients with recurrent small cell lung cancer. Cancer. 2007;109:924–32. doi: 10.1002/cncr.22492. [DOI] [PubMed] [Google Scholar]
- 177.Pusztai L, Wagner P, Ibrahim N, et al. Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer. 2005;104:682–91. doi: 10.1002/cncr.21227. [DOI] [PubMed] [Google Scholar]
- 178.Bailey A, Paulsen IT, Piddock LJ. RamA confers multidrug resistance in Salmonella enterica via increased expression of acrB, which is inhibited by chlorpromazine. Antimicrob Agents Chemother. 2008;10:3604–11. doi: 10.1128/AAC.00661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Marquez B. Bacterial efflux systems and efflux pumps inhibitors. Biochimie. 2005;87:1137–47. doi: 10.1016/j.biochi.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 180.Couto I, Costa SS, Viveiros M, Martins M, Amaral L. Efflux-mediated response of Staphylococcus aureus exposed to ethidium bromide. J Antimicrob Chemother. 2008;62:504–13. doi: 10.1093/jac/dkn217. [DOI] [PubMed] [Google Scholar]
- 181.Ryder N. Antifungal agents. IDrugs. 1999;12:1253–5. [PubMed] [Google Scholar]
- 182.Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41:1967–72. [PubMed] [Google Scholar]
- 183.Tonshin A, Teplova VV, Andersson MA, Salkinoja-Salonen MS. The Fusarium mycotoxins enniatins and beauvericin cause mitochondrial dysfunction by affecting the mitochondrial volume regulation, oxidative phosphorylation and ion homeostasis. Toxicology. 2010;276:49–57. doi: 10.1016/j.tox.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 184.Lee M, Galazzo JL, Staley AL, et al. Microbial fermentation-derived inhibitors of efflux-pump-mediated drug resistance. Farmaco. 2001;56:81–5. doi: 10.1016/s0014-827x(01)01002-3. [DOI] [PubMed] [Google Scholar]