Abstract
Objectives
Assessing the quality of cancer care (QoCC) has become increasingly important to providers, regulators and purchasers of care worldwide. The aim of this study was to develop evidence-based quality indicators (QIs) for colorectal cancer (CRC) to be applied in a population-based setting.
Design
A comprehensive evidence-based literature search was performed to identify the initial list of QIs, which were then selected and developed using a two-step-modified Delphi process involving two multidisciplinary expert panels with expertise in CRC care, quality of care and epidemiology.
Setting
The QIs of the clinical cancer care (QC3) population-based project, which involves all the public and private hospitals and clinics present on the territory of Canton Ticino (South Switzerland).
Participants
Ticino Cancer Registry, The Colorectal Cancer Working Group (CRC-WG) and the external academic Advisory Board (AB).
Main outcome measures
Set of QIs which encompass the whole diagnostic-treatment process of CRC.
Results
Of the 149 QIs that emerged from 181 sources of literature, 104 were selected during the in-person meeting of CRC-WG. During the Delphi process, CRC-WG shortened the list to 89 QI. AB finally validated 27 QIs according to the phase of care: diagnosis (N=6), pathology (N=3), treatment (N=16) and outcome (N=2).
Conclusions
Using the validated Delphi methodology, including a literature review of the evidence and integration of expert opinions from local clinicians and international experts, we were able to develop a list of QIs to assess QoCC for CRC. This will hopefully guarantee feasibility of data retrieval, as well as acceptance and translation of QIs into the daily clinical practice to improve QoCC. Moreover, evidence-based selected QIs allow one to assess immediate changes and improvements in the diagnostic-therapeutic process that could be translated into a short-term benefit for patients with a possible gain both in overall and disease-free survival.
Keywords: QUALITATIVE RESEARCH, Quality in health care < HEALTH SERVICES ADMINISTRATION & MANAGEMENT, Gastrointestinal tumours < ONCOLOGY, Gastrointestinal tumours < GASTROENTEROLOGY
Article summary.
Article focus
Quality of Cancer Care (QoCC) studies on specific quality indicators (QIs) developed worldwide since the late 1990s showed both a continuous improvement of oncological care provided by the clinical structures involved and an increased availability of specialised care in the considered areas.
This study aims to define evidence-based QIs for colorectal cancer (CRC) care, in order to favour a feasible evaluation of the oncological diagnostic-therapeutic process from a population-based cancer registration and data collection point of view.
Key messages
QIs should be defined, developed and tested with scientific evidence-based rigour in a careful and transparent manner, taking into account their degree of relevancy, validity, reliability and feasibility.
The selected CRC QIs can be applied in a population-based setting, implying the inclusion of the elderly, considering age as an extremely important determinant of treatment.
Strengths and limitations of this study
To develop CRC QIs, we used a formal iterative process, the RAND/UCLA Appropriateness Methodology widely diffused and validated within other QoCC research. The selected QIs are representative of the main steps of the diagnostic-therapeutic process.
Owing to the evidence that research studies demonstrated that single-discipline panels select different indicators than do multidisciplinary panels and to maximise the applicability of CRC QI, we constituted two panels of experts, a local Working Group and an external national/international academic Advisory Board (AB), who could offer a multidisciplinary perspective on practice and also guarantee that the selected QIs and their results would be comparable with national and international data.
- Possible limitations of the current work are the following:
- The level of evidence found in the literature. This situation is common to many aspects of healthcare, and it was the reason that the expert panel methodology was developed—specifically, to identify the processes that are most likely to be valid measures of quality when the highest level of evidence is not available.
- The literature selection could have missed some relevant articles. However, members of the Working Group were likely to be very familiar with the literature, and had the opportunity to suggest other indicators based on their experience and literature search; in this way, we believe to have integrated the best research evidence with clinical expertise.
- The feasibility of measuring indicators in terms of data collection and calculation. However, both the Working Group and AB were concerned about the feasibility, validity and reliability of clinical data collection, necessary for the calculation of each single indicator at the population-based level. In fact, in order to warrant an accurate measurement, those indicators reaching more than 70% of the agreement, confirming their scientific and clinical value, but evaluated at least by one of the experts as not feasible and difficult to be collected at the population-based level, were definitely excluded. In this way, we have overcome the feasibility limit.
Introduction
Research on quality of cancer care (QoCC) performed during the last decade has demonstrated that the increase in knowledge on treatments with proven efficacy does not directly translate into optimal delivery of such treatments to patients. Moreover, accumulating evidence suggests that underuse and overuse of care may occur for patients with cancer.1 2 In addition to survival analysis, to evaluate and compare quality of care at the population-based level, the assessment of QoCC has become increasingly important to providers, regulators and purchasers of care, owing to the growing demand for services, rising costs, constrained resources and evidence of variation in clinical practice.3
QoCC studies and structured programmes on specific quality indicators (QIs) have been developed worldwide since the late 1990s, showing both a continuous improvement of oncological care provided by the clinical structures involved and an increased availability of specialised care in the considered areas. Most of these studies have been implemented at the regional level on a territory with uniform legislative, health and geographical characteristics, increasing the likelihood of recruitment of involved clinicians.1 4–7
Until now, in Switzerland, no population-based study on QoCC with a prospective design has been implemented. In addition to the yearly renewed international guidelines for each type of cancer, there is still a need to evaluate the real conditions of care in the community. Population-based Cancer Registry data are therefore essential to describe and reflect the real world and routine care as well as to provide regular feedback to healthcare workers and decisionmakers about the management of a disease in daily practice and those treatments that are routinely prescribed and/or effective in all patient groups.8 Moreover, Cancer Registries represent an independent observatory, thus assuring a fair evaluation service and avoiding any conflicts of interest.
We therefore implemented the QIs of the clinical cancer care (QC3) project, focusing on QoCC about the diagnosis-treatment process in the colon-rectum, prostate, uterus, ovary and lung cancers in the territory of Canton Ticino (South Switzerland).
Colorectal cancer (CRC) is an important health issue worldwide. It is the most common malignancy in Europe (excluding non-melanoma skin cancers) and the second most common in terms of cancer-related mortality.9 In Switzerland, CRC is the second and third most frequent tumour in women and men, respectively. About 4000 CRC cases are diagnosed annually, corresponding to a European age-standardised incidence rate equal to 49.4 and 30.6 cases/100 000 inhabitants in men and women, respectively, and representing 11% of all tumours.10–12 CRC is the third leading cancer cause of death in Switzerland, with approximately 1600 deaths/year, corresponding to a European age-standardised mortality rate equal to 18.5 and 10.6 cases/100 000 inhabitants in men and women, respectively. With a 5-year survival probability equal to 60%, Switzerland is the country with the most favourable prognosis in Europe.13 A recent Swiss report with follow-up to 2009 showed an additional 5-year survival increase to 62%.11
The aims of the QC3 project were as follows: (1) to define and confirm evidence-based QoCC indicators for the tumour localisations cited above, in order to favour a feasible evaluation of the oncological diagnostic-therapeutic process from a population-based cancer registration and data collection point of view; (2) to define and implement at the regional level standards of care for each QoCC measure, in terms of minimum and target requirements. In the present report, we will describe the initial part of the QC3 project, meaning the process followed to identify the panel of specific QoCC indicators for CRC, as well as the list of QoCC indicators identified and approved both by a dedicated Working Group of local healthcare providers and by an external independent Advisory Board (AB), in a perspective of data collection feasibility by a population-based cancer registry.
Material and methods
The QC3 project is a prospective, descriptive study on QoCC implemented in a population-based setting; it is performed by the Ticino Cancer Registry over a 3-year time period (2011–2013) on the territory of Canton Ticino (South Switzerland). In this paper, we focus on the initial part of the project: the identification of the CRC QIs which will be used to evaluate QoCC about CRC in our region.
QIs for CRC were developed involving a local expert panel, named the QC3 Colorectal Cancer Working Group (CRC-WG). Elected members, selected on the basis of their expertise and on their daily clinical involvement in CRC care, were contacted to have their interest confirmed in being involved. The final QC3 CRC-WG included two pathologists, four gastroenterologists, two oncologists, three surgeons, two radiologists, two radiation oncologists and one nuclear medicine specialist, making up a total of 16 panellists, all working in the public hospitals or in the private hospitals and clinics of Canton Ticino.
Published studies and references were identified through a comprehensive search on PubMed/MEDLINE, using initially specific strings/expressions, such as the following: “quality of care OR quality indicators AND colorectal cancer”, “diagnosis OR diagnostic AND quality indicators AND colorectal cancer”, “pathology OR pathological AND quality indicators AND colorectal cancer”, “surgery OR surgical AND quality indicators AND colorectal cancer”, “radiation oncology OR radiotherapy AND quality indicators AND colorectal cancer”, “chemotherapy AND quality indicators AND colorectal cancer”, “surveillance OR follow-up OR outcome AND quality indicators AND colorectal cancer”, “preoperative care OR perioperative care OR intraoperative care OR postoperative care AND colorectal cancer”, “population-based AND quality indicators AND colorectal cancer”. For each of the identified candidate QIs, we performed a systematic literature review to identify the highest level of evidence supporting the validity of that QI for articles published from 1990 onwards. The reference list of included articles were also examined to identify any additional article that had not been identified in the MEDLINE search. We included all the peer-reviewed articles, excluding case reports, letters, abstracts or editorials. If evidence at the highest level were limited or absent, then lower levels of evidence were evaluated. For example, if data were not available from randomised controlled trials, cohort or case-control studies, case series and expert opinion or clinical guidelines were reviewed. A selection of already approved QIs provided by the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), the National Initiative on Cancer Care Quality (NICCQ), the Quality Oncology Practice Initiative (QOPI) and the Florida Initiative for Quality Cancer Care (FIQCC) were included in the evaluation list, with the aim to transfer them from the clinical setting to the population-based setting.1 2 4 7 14–20
The initial QIs list emerged from 181 sources of literature, and it was proposed to the CRC-WG in the context of an in-person meeting held at the very beginning of the process. The list was then left to the QC3 CRC-WG's evaluation for a period of 2 weeks. The participants were asked to provide a whole opinion with written comments about those QIs considered pertinent for the assessment of CRC care quality, to suggest additional QIs not already included in the list and to delete those QIs considered to be not suitable. In order to make the selection and evaluation easier, the QIs were subdivided into chapters recalling the Donabedian's21 and the National Initiative for Cancer Care Quality schemes: diagnosis and staging, pathology, treatment, follow-up, outcome.2
Delphi round 1
The QIs selection was performed by using a two-step-modified Delphi process.22 The initial list of QIs, reanalysed by the QC3 CRC-WG, was formatted as a questionnaire, where for each indicator was specified the numerator, the denominator and the sources of evidence from which it was extracted. The questionnaire was distributed by regular mail to the QC3 CRC-WG, so to maintain it anonymously, along with a stamped, addressed return envelope and an attached letter with the deadline date of 2 weeks from the receipt and instructions for voting. Respondents were asked to rate each QI adopting the RAND Appropriateness Methodology (scales 1–9, 1=extremely inappropriate; 9=extremely appropriate), according to the selection criteria of relevance, scientific soundness (validity, reliability, comparability) and feasibility (precise definition and specification, data feasibility, reliability of data collection).23–25 Each QI was judged as validated if it reached a strong consensus for acceptance (≥70% of the QC3 CRC-WG rated the QI with a vote ≥7), discarded if it reached a strong consensus for exclusion (≥70% of the QC3 CRC-WG rated the QI with a vote ≤3) and as a standby if there was an unclear consensus (4≤ votes ≤6), which implies an eventual in-person meeting.
Delphi round 2
The Delphi Round 2 questionnaire was performed with the same modalities as in the first round and enclosed the frequency distribution of the round 1 votes, allowing the panellists to eventually alter their responses, in the light of colleagues’ assessments.23
AB review
The list of selected QIs derived from the two Delphi rounds was then submitted to an independent external national/international academic multidisciplinary AB, in order to get an additional evaluation on the suitability of QIs as ‘quality’ indexes according to the criteria shown in the previous paragraph. The intent was to achieve at least one health professional for each specialty. The AB included one pathologist, one gastroenterologist, two oncologists, two surgeons, one radiologist, one radiation oncologist, one nuclear medicine specialist and one epidemiologist, making up a total of 10 experts in CRC care (see Acknowledgements); all the panellists were involved daily in CRC care and they had been contacted with the same modalities of the QC3 CRC-WG. The selected QIs as well as the corresponding literature sources were distributed to the AB as an electronic form where their opinion about QIs were expressed both as megatrends (ie, response yes/no to the suitability of each QI) and as eventual additional comments.26 We considered every single QI as finally approved by the AB if it achieved ≥70% of the agreement (ie, ≥70% of respondents should have answered ‘yes’). Besides the vote (‘yes’ vs ‘no’), the panellists had the chance to comment on a single QI from a population-based cancer registration and data collection point of view. Therefore, those QIs reaching more than 70% of the agreement, confirming their scientific and clinical value, but evaluated by at least one of the experts ‘not completely feasible and difficult to be collected at the population-based level’, were definitely excluded.
Results
The QI selection process began in January 2011 and ended in December 2011.
Participation of CRC-WG members throughout the process was high: 15 (100%) participated in the in-person meeting, 12 (80%) completed both the Delphi rounds 1 and 2. The Delphi round 1 questionnaire respondent time was in the range of 18–60 days, while for the round 2, the delay time was in the range of 8–55 days; these delays and the time for recruitment of the AB influence the long time spent on this part of the project.
Figure 1 summarises the entire process used to select QI for CRC care. The literature search produced 181 citations dealing with CRC QoCC, also including the already validated QIs provided by ASCO, NCCN, NICCQ, QOPI and FIQCC.1 2 4 7 14–20 From this search, we initially selected a total of 149 QIs, which were proposed to the CRC-WG in the context of the initial in-person meeting. The following discussion and revision reduced the list to 104 QIs before the modified Delphi process started; these QIs were divided into the following areas: diagnosis and staging, pathology, treatment, follow-up and outcome. After the whole Delphi process, the list was shortened to 89 QIs, distributed as follows: diagnosis and staging (N=16), pathology (N=20), treatment (N=38), follow-up (N=10) and outcome (N=5). The QIs finally underwent the AB's evaluation; this last step, according to the procedure described in the methods, shortened the final list to 27 QIs: diagnosis (N=6), pathology (N=3); treatment (N=16), follow-up (N=0) and outcome (N=2). Table 1 reports detailed information for each QI: (1) QI description; (2) criteria for patients’ inclusion in the numerator and denominator; (3) list of the necessary medical documentation that should be collected by the Cancer Registry to extract the needed and relevant information to build the specific QI, such as the report of the endoscopy, the pathology report of the biopsy and/or surgical resection, the preoperative radiological reports (eg, CT and MRI), the surgery report, the tumour board documentation, the oncological report, the radiotherapy report and database/documentation of the regional Office of Population Registry Rosters for the assessment of patients’ vital status (for outcome QIs); (4) QI rationale and (5) related references.
Figure 1.
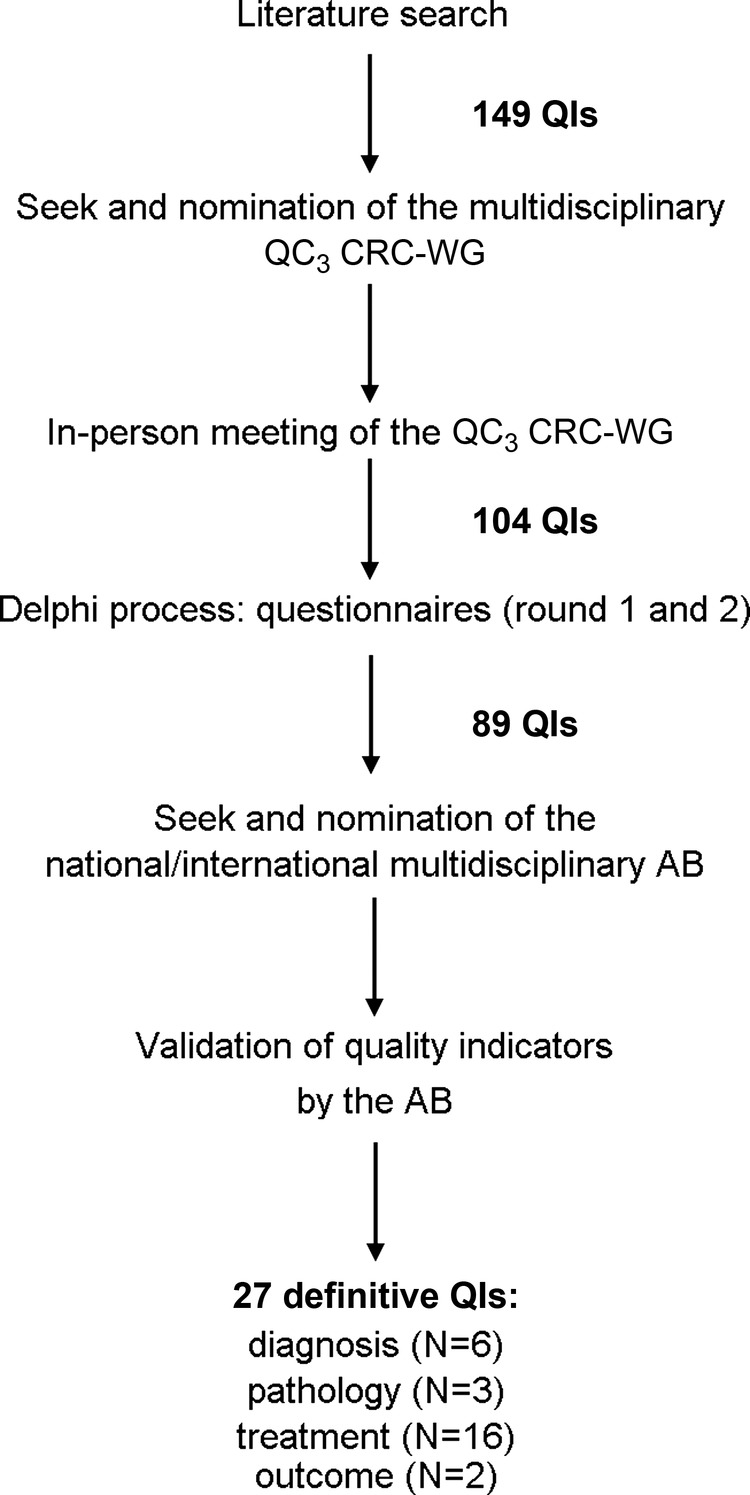
Process used to select quality indicators for colorectal cancer care. AB, Advisory Board; QC3 CRC-WG, QC3 Colorectal Cancer Working Group; QI, Quality Indicators.
Table 1.
Quality indicators of colorectal cancer care according to diagnostic-therapeutic process (diagnosis, pathology, treatment—surgery, chemotherapy and radiotherapy—and outcome) and tumour site
| Clinical domain | Site | Quality indicator | Numerator | Denominator | Medical documentation | Rationale | Reference | |
|---|---|---|---|---|---|---|---|---|
| Diagnosis (n=6) | C&R | Proportion of patients with colorectal cancer and diagnosis based on symptoms vs screening vs accidental finding | Number of patients with colorectal cancer whose diagnosis is based on symptoms, defined as appearance or persistence of clinical events and signs, such as rectal bleeding, occult blood in stool, weight loss with no apparent cause, general abdominal discomfort, bowel obstruction, change in bowel habits, constant tiredness, anaemia |
Number of patients with colorectal cancer | Request form of endoscopic examination, endoscopy and surgical pathology reports, reports/discharge letters coming from all hospital units/departments (ie, surgery, medicine, radiation oncology, medical oncology) | Assessment of the patient's take charge | 18 34–38 | |
| Number of patients with colorectal cancer whose diagnosis is based on screening, defined as regular examination, such as faecal occult blood test (FOBT) or colonoscopy in asymptomatic patients |
||||||||
| Number of patients with colorectal cancer whose diagnosis is an accidental finding following examinations or therapies for other diseases (eg, hospital admission for other causes…) |
||||||||
| C&R | Proportion of patients with colorectal cancer, evaluated by preoperative colonoscopy | Number of patients with colorectal cancer who have been evaluated by a preoperative colonoscopy | Number of patients with colorectal cancer undergoing surgery | Endoscopy report, request form of pathology examination, pathology report of endoscopy | Planning of further diagnostic procedures and treatments, comprehensiveness of diagnostic and staging evaluation |
7 16 18 40–41 | ||
| R | Proportion of patients with rectal cancer and description of the tumour localisation (distance ab ano) in the endoscopic/pathological documentation | Number of patients with rectal cancer who have the description of the tumour localisation, in terms of distance ab ano, in the endoscopic/pathological documentation |
Number of patients with rectal cancer undergoing endoscopy | Endoscopy report, request form of pathology examination, pathology report of endoscopy | Planning of further diagnostic procedures and treatments, comprehensiveness of diagnostic and staging evaluation | 1 19 56 57 | ||
| C&R | Proportion of patients with colorectal cancer and requests for an initial CT and/or an MRI examination completed by clinical information according to the ACR guidelines | Number of patients with colorectal cancer for which the request of an initial CT and/or an MRI examination is completed by clinical information according to the ACR guidelines |
Number of patients with colorectal cancer undergoing initial CT and/or MRI examination | Radiology (CT and/or MRI examination) report | Providing the necessary information for a comprehensive radiological examination, assessment of the quality of the flux of clinical information | 7 58 | ||
| R | Proportion of patients with low rectal* cancer undergoing pelvic MRI of staging | Number of patients with low rectal* cancer who have undergone a pelvic MRI of staging | Number of patients with low rectal* cancer | Radiology (MRI examination) report, discharge letters coming from all hospital units/departments (ie, surgery, medicine, medical oncology, radiation oncology) |
Planning of further diagnostic procedures and treatments, comprehensiveness of diagnostic and staging evaluation | 19 59–61 | ||
| R | Proportion of patients with rectal cancer and a preoperative MRI reporting the description of the radial margin status (mm) | Number of patients with rectal cancer who have undergone a preoperative MRI reporting the description of the radial margin status (mm) |
Number of patients with rectal cancer undergoing preoperative MRI | Radiology (MRI examination) report | Planning of further diagnostic procedures and treatments, comprehensiveness of diagnostic and staging evaluation | 62 | ||
| Pathology (n=3) | R | Proportion of patients with rectal cancer for which the request for the pathological examination includes the information of neo-adjuvant RT±ChT | Number of patients with rectal cancer for which the request for the pathological examination includes the information of neo-adjuvant RT±ChT | Number of patients with rectal cancer undergoing neo-adjuvant RT±ChT and surgery† | Request form of pathology examination, surgical pathology report | Providing the necessary information for a comprehensive pathological examination, assessment of the quality of the flux of clinical information |
Proposed by the QC3 CRC-WG |
|
| C&R | Proportion of patients with colorectal cancer and a sufficient number of tumour samples (≥3) | Number of patients with colorectal cancer for which 3 or more tumour samples were processed for the pathological analysis |
Number of patients with colorectal cancer undergoing surgery† | Surgical pathology report | Comprehensiveness of pathology examination | Proposed by the QC3 CRC-WG |
||
| C&R | Proportion of patients with colorectal cancer and a surgical pathology report including the following characteristics:▸ Surgical intervention description ▸ Sample length ▸ Tumour localisation according to WHO ▸ Tumour size ▸ Histological type according to WHO ▸ Histological grade ▸ Resection margins ▸ Lymph-vascular invasion ▸ Perineural invasion ▸ Tumour deposits (discontinuous extramural extension) ▸ Pathological staging (AJCC pTNM) ▸ Number of retrieved lymph nodes ▸ Treatment effect ▸ Macroscopic integrity of the mesorectum (for the rectum only) (this quality indicator should be provided for each characteristic) |
Number of patients with colorectal cancer whose pathological report includes the following characteristics: ▸ Surgical intervention description ▸ Sample length ▸ Tumour localisation according to WHO ▸ Tumour size ▸ Histological type according to WHO ▸ Histological grade ▸ Resection margins ▸ Lymph-vascular invasion ▸ Perineural invasion ▸ Tumour deposits (discontinuous extramural extension) ▸ Pathological staging (AJCC pTNM) ▸ Number of retrieved lymph nodes ▸ Treatment effect ▸ Macroscopic integrity of the mesorectum (for the rectum only) |
Number of patients with colorectal cancer undergoing surgery† | Surgical pathology report | Comprehensiveness and standardisation of surgical pathology report, comprehensiveness of staging evaluation, planning of further treatments | 18 19 63 64 | ||
| Treatment (n=16) | C&R | Proportion of patients with colorectal cancer who have been operated in emergency‡ | Number of patients with colorectal cancer who have been operated in emergency‡ | Number of patients with colorectal cancer undergoing surgery† |
Radiology and surgery report/discharge letter, surgical pathology report | Assessment of the patient's take charge | 65–67 | |
| C&R | Proportion of patients with colorectal cancer and dead within 30 days and 6 months from the surgery (postoperative mortality) | Number of patients with colorectal cancer and dead within 30 days from the surgery† Number of patients with colorectal cancer and dead within 6 months from the surgery |
Number of patients with colorectal cancer undergoing surgery† | Surgery report/discharge letter, surgical pathology report, access to regional Office of Population Registry Rosters for the assessment of patients’ vital status | Assessment of the quality of surgical procedure | 68–71 | ||
| C&R | Proportion of patients with colorectal cancer and postoperative multidisciplinary discussion | Number of patients with colorectal cancer for which there has been a multidisciplinary discussion after surgery† | Number of patients with colorectal cancer undergoing surgery† | Surgery, oncology, radiation oncology reports/discharge letters, multidisciplinary discussion documentation |
Planning of further diagnostic procedures and treatments | 72 73 | ||
| R | Proportion of patients with malignant rectal polyp (pT1) and complete endoscopic polypectomy | Number of patients with malignant rectal polyp (pT1) who have undergone a complete endoscopic polypectomy |
Number of patients with malignant rectal polyp (pT1) | Endoscopy report, endoscopic pathology reports | Assessment of the quality of surgical procedure | Proposed by the QC3 CRC-WG | ||
| R | Proportion of patients with low rectal* cancer who have undergone a surgical intervention with sphincter preservation |
Number of patients with low rectal* cancer who have undergone a surgical intervention with sphincter preservation | Number of patients with low rectal* cancer undergoing surgery† |
Surgical pathology report, surgery report/discharge letter | Assessment of the quality of surgical procedure | 7 74–76 | ||
| R | Proportion of patients with rectal cancer undergoing TEM with R0 resection | Number of patients with rectal cancer who have undergone TEM with R0 resection |
Number of patients with rectal cancer undergoing TEM | Surgical pathology report, surgery report/discharge letter | Assessment of the quality of surgical procedure | 77–79 | ||
| C&R | Proportion of patients with colorectal cancer and a number of resected lymph nodes ≥12 | Number of patients with colorectal cancer with a number of resected lymph nodes ≥12 | Number of patients with colorectal cancer undergoing surgery†, but no neo-adjuvant therapy |
Surgical pathology report, surgery report/discharge letter | Assessment of the quality of surgical procedure and pathology examination | 7 14 16 40 41 80–85 | ||
| C&R | Proportion of patients with colorectal cancer operated on with free margins | Number of patients with colon cancer who have undergone surgery and have free margins |
Number of patients with colorectal cancer undergoing surgery† | Surgical pathology report, surgery report/discharge letter | Assessment of the quality of surgical procedure | 7 86 87 | ||
| C&R | Proportion of patients with colorectal cancer and AJCC TNM clinical stage I (from T2N0M0) to III (any T, N1M0) undergoing a surgical resection with anastomosis | Number of patients with colorectal cancer and AJCC TNM clinical stage I (from T2N0M0) to III (any T, N1M0) who have undergone a surgical resection with anastomosis |
Number of patients with colorectal cancer and AJCC TNM stage I (from T2N0M0) to III | Radiology report, surgical pathology report,surgery report/discharge letter | Assessment of the quality of surgical procedure | 40 41 86 87 | ||
| C | Proportion of patients with colon cancer and AJCC TNM stage II (T3N0M0, T4N0M0) high-risk (presence of at least one of the following factors: LN<12, G3, lymph-vascular or perineural invasion, tumour obstruction, tumour perforation, pT4) or III undergoing adjuvant ChT | Number of patients with colon cancer and AJCC TNM stage II (T3N0M0, T4N0M0) high-risk (presence of at least one of the following factors: LN<12, G3, lymph-vascular or perineural invasion, tumour obstruction, tumour perforation, pT4) or III, who have undergone adjuvant ChT |
Number of patients with colon cancer and AJCC TNM stage II high-risk or III, undergoing surgery† | Radiology report, surgical pathology report, surgery, oncology reports/discharge letters | Assessment of the quality of oncological treatment | 16 18 40–41 88–91 | ||
| C | Proportion of patients with colon cancer AJCC TNM stage II high-risk or stage III undergoing adjuvant ChT within 8 weeks from surgical resection | Number of patients with colon cancer and AJCC TNM stage II high-risk or III, who have undergone adjuvant ChT within 8 weeks from surgical resection | Number of patients with colon cancer and AJCC TNM stage II high-risk or III undergoing surgery† and adjuvant ChT |
Radiology report, surgical pathology report, surgery, oncology reports/discharge letters | Assessment of the quality of oncological treatment | 18 92 | ||
| C&R | Proportion of patients with colorectal cancer and histology of the primary tumour or metastases obtained before the beginning of ChT |
Number of patients with colorectal cancer and histology of the primary tumour or metastases obtained before the beginning of ChT | Number of patients with colorectal cancer undergoing primary ChT | Radiology and pathology reports, oncology report/discharge letter | Assessment of the quality of oncological treatment | 40 41 | ||
| C&R | Proportion of patients with colorectal cancer and unresectable metastases undergoing first-line ChT or bio-ChT | Number of patients with colorectal cancer and unresectable metastases who have undergone a first-line ChT or bio-ChT | Number of patients with colorectal cancer and unresectable metastases |
Radiology and pathology reports, oncology report/discharge letter | Assessment of the quality of oncological treatment | 93–96 | ||
| C&R | Proportion of patients with colorectal cancer and hepatic metastases primarily unresectable turned into resectable metastases after neo-adjuvant ChT | Number of patients with colorectal cancer and hepatic metastases primarily unresectable turned into resectable metastases after neo-adjuvant ChT | Number of patients with colorectal cancer and unresectable hepatic metastases undergoing neo-adjuvant ChT |
Radiology report, oncology report/discharge letter | Assessment of the quality of oncological treatment | 96 | ||
| R | Proportion of patients with locally advanced rectal cancer (T3-4 and/or any T, N+ and M0) undergoing neo-adjuvant RT±ChT | Proportion of patients with locally advanced rectal cancer (T3-4 and/or any T, N+ and M0) who have undergone neo-adjuvant RT±ChT |
Number of patients with locally advanced rectal cancer undergoing surgery† | Endoscopic pathology report, radiology report, radiation oncology and oncology reports/discharge letters | Assessment of the quality of oncological and radio-oncological treatment | 97 98 | ||
| R | Proportion of patients with rectal cancer and undergoing neo-adjuvant RT±ChT operated within 6–8 weeks after the end of neo-adjuvant RT±ChT | Number of patients with rectal cancer who have undergone neo-adjuvant RT±ChT and were operated within 6–8 weeks after the end of neo-adjuvant RT±ChT |
Number of patients with rectal cancer undergoing neo-adjuvant RT±ChT followed by surgery† | Endoscopic pathology report, radiology report, radiation oncology and oncology reports/discharge letters, surgical pathology report | Assessment of the quality of oncological and radio-oncological treatment | 18 98 | ||
| Outcome (n=2) | C&R | Analysis of overall survival at 1, 3, 5 and 10 years from diagnosis | Number of patients with colorectal cancer who survive at 1, 3, 5 and 10 years from diagnosis | Number of patients with colorectal cancer | Access to regional Office of Population Registry Rosters for the assessment of patients vital status | Assessment of overall survival | 7 99 | |
| C&R | Analysis of disease-free survival at 1, 3, 5 and 10 years from the curative treatment | Number of patients with colorectal cancer who are disease-free at 1, 3, 5 and 10 years from the curative treatment | Number of patients with colorectal cancer curatively treated | Reports/discharge letters coming from all hospital units/department (ie, surgery, medicine, oncology, radio-oncology) | Assessment of disease-free survival | 7 99 |
*Low rectum: 4–7.5 cm from the dentate line.100
†Surgery excludes endoscopic resection and colostomy.
‡Emergency: within 24 h from the onset of symptoms.
ACR, American College of Radiology; AJCC, American Joint Committee on Cancer; C, colon; C&R, colon-rectum; ChT, chemotherapy; CT, computed tomography; MRI, magnetic resonance imaging; R, rectum; RT, radiotherapy; TEM, transanal endoscopic microsurgery; WHO, World Health Organization.
Discussion
In the preliminary phase of the QC3 project shown in this paper, we developed a panel of evidence-based CRC QIs which are suitable for implementation in a population-based setting.
To develop the QC3 QIs, we used a formal iterative process, the RAND/UCLA Appropriateness Methodology widely diffused and validated within other QoCC research.23 24 Owing to the evidence that research studies demonstrated that single-discipline panels select different indicators than do multidisciplinary panels and to maximise the applicability of QC3 CRC QIs, we constituted a working group which could offer a multidisciplinary perspective on practice, including specialists, professionals, clinicians and researchers coming from both public and private hospitals.27–33 Moreover, we have used a further validation step enrolling an independent national/international academic AB. This choice was due to the aim of measuring QoCC within a Swiss region, with a point of view on the population-based data collection and evaluation, and of obtaining results which will be comparable with national and international data. We believe that the expertise and multidisciplinary representativeness of the QC3 CRC-WG and of the AB will surely increase the quality, acceptance and translation of QIs into daily clinical practice.
The selected QIs are representative of the main steps of the diagnostic-therapeutic process. The diagnosis QIs reflect the importance of a preoperative evaluation and staging, reliable evaluation of the tumour localisation and local invasion and particularly for the rectal cancers, of a feasible and effective surgery. The first indicator of the ‘diagnosis’ group is important to understand what happens in a territory where there is no organised screening programme for CRCs, but only an opportunistic screening strategy. Actually, the tumour is detected because the physician submits the patients older than 50 years old to a fecal occult blood test (FOBT) or colonoscopy control or if a patient, being aware of the possible risk, asks his family doctor to undergo screening examinations. These are interesting data to be evaluated, also in the hypothesis of a CRC screening programme implementation. We therefore believe that a higher proportion of patients diagnosed through screening (FOBT or colonoscopy in asymptomatic patients) would represent a higher diagnostic quality, since the therapeutic approach and, consequently, the patients’ outcome (in terms of recurrence and survival) would be more favourable, as reported in the literature.20 34–39 The pathology QIs reflect the importance of good communication between clinicians and pathologists in terms of the patient's clinical history and consequent evaluation of the effectiveness of a neo-adjuvant therapy; moreover, there is a need for standardisation of the pathological report following international guidelines (eg, take at least three samples of the tumour during macroscopy), not leaving any items unexplained or implicit. In particular, the third QI reported in table 1 (pathology section) refers to the surgical pathology report, which derives from the surgical curative intervention and should be as complete as possible to be useful for the future decision about the patient's treatment. Our intent is to calculate it not only for all the listed items considered together, but also for each item analysed individually: for example, the proportion of patients with CRC and a definitive pathological report including the surgical intervention description; the proportion of patients with CRC and a definitive pathological report including the tumour size; the proportion of patients with CRC and a definitive pathological report including the resection margins; the proportion of patients with CRC and a definitive pathological report including the pathological staging (AJCC pTNM); etc. The treatment QIs cover the general issues of surgery, such as emergency, postoperative mortality and a multidisciplinary discussion of the clinical case; furthermore, they focus on the debate of the retrieved lymph nodes, on the timing between radiotherapy and surgery, on the adjuvant chemotherapy and on the attitude towards the metastatic patients. The two main items of the outcome chapter refer to the overall and disease-free survival. Although it is necessary to wait for a certain follow-up period (ie, 1, 3, 5–10 years from the date of diagnosis for the calculation of overall survival, and from the date of curative treatment for the calculation of disease-free survival), they will represent the overall resume of the diagnostic and treatment quality of CRC patients. Our intent will be to analyse the overall and disease-free survival according to some of the proposed QIs (such as QI concerning the pathological characteristics of the tumours, QI of the adjuvant chemotherapy in patients with colon cancer and AJCC TNM stage II high-risk or III, QI of colorectal patients operated on with free margins, QI of locally advanced rectal cancer patients undergoing neo-adjuvant radio±chemotherapy, etc). We will finally compare our results with other regional and national reality, favouring the interpretation of each single QI. Concerning the QIs about follow-up, AB did not finally include any of them. Indeed, although the follow-up procedures are suggested by several international guidelines, they are based on levels II–III evidence and controversies remain regarding the selection of optimal strategies for following up patients after potentially curative CRC surgery.40–43
The first limitation of the current work is the level of evidence found in the literature. For some QIs, strong evidence of their validity was not available from randomised controlled trials. However, this situation is common to many aspects of healthcare, and it was the very reason that the expert panel methodology was developed—specifically, to identify the processes that are most likely to be valid measures of quality when the highest level of evidence is not available.19 23 44 Second, we may have missed some studies during the literature search, and consequently, some QIs may not have been proposed to the QC3 CRC-WG since the beginning of the QIs revision process. However, this limitation should have been overcome by the fact that the members of the QC3 CRC-WG were likely to be very familiar with the literature, and had the opportunity to suggest other QIs based on their experience and literature search.7 27 28 45 Thus, we integrated the best research evidence with clinical expertise, as reported by Sackett et al46 A further limit could be the feasibility of measuring QIs in terms of data collection and calculation, which is immediately the next step. Actually, the QIs selected by both the QC3 CRC-WG and the AB represent an ideal set of criteria to measure the quality of CRC care; at the same time, they both were concerned about the feasibility, validity and reliability of clinical data collection, necessary for the calculation of each single QI at the population-based level. This is the reason why most of the identified QC3 QIs are common to many QoCC studies.1 2 4 7 14–20 Besides the traditional Delphi process, the panellists had the chance to comment on the single QI from a population-based cancer registration and data collection point of view. Therefore, in order to warrant an accurate measurement, those QIs reaching more than 70% of the agreement, confirming their scientific and clinical value, but evaluated at least by one of the experts as not feasible and difficult to be collected at the population-based level, were definitely excluded. In addition, we performed a retrospective preliminary pilot collection on the detailed and necessary incidence data of CRC which occurred in 2011, realising that the measurement of most QIs is feasible, whereas for some selected QIs the retrieval of variables would need additional efforts; some preliminary results were presented in national and international conferences and congresses, receiving positive feedback in both the clinical and epidemiological settings.47–50 Only the definitive results will give us the proportion of missing information, whose magnitude will be assessed.
The selected QC3 CRC QIs will be applied in a population-based setting, where age is an extremely important determinant of treatment. The elderly are rarely included in the randomised clinical trials with the consequence of a possible ‘underuse of treatment’.25 51 52 At a broad European level, national audit registries in surgical oncology have led to improvements with a great impact and they offered the possibility, as for our project, to perform research on patients that are usually excluded from clinical trials such as the elderly and comorbid patients.53 54 Evidence suggests that the relative benefits of treatment for the elderly are similar to those seen for cancer patients in general, though decision-making for treatment becomes more complex as life expectancy, coexisting illnesses and functional status all need to be considered.25 51 52 Applying these QIs, and if all these items will be satisfied, we can affirm having a real good quality process of CRC care for the whole population. The foreseeable future in quality evaluation and improvement of healthcare will quite likely involve a more frequent use of QIs by regulatory and accrediting agencies, stakeholders, clinicians, individual hospitals and healthcare providers, as well as patients. This underlines the fact that QIs should be defined, developed and tested with scientific evidence-based rigour in a careful and transparent manner, taking into account their degree of relevancy, validity, reliability and feasibility.30 32 Although QIs have been defined in several different ways, all authors agreed that the final aim was the improvement of patients’ outcome.31 33 55
The systematic trend analysis of QIs allows the assessment of immediate changes and improvements in the diagnostic-therapeutic process, which could be translated into a short-term benefit for patients, without waiting for survival analysis typically needing some years to produce, because of the patients’ follow-up. Furthermore, this system of evaluation and autoevaluation could favour the surveillance and monitoring of the comprehensive level of oncological care in the region, the clinical performance homogeneity, the possible weakness of the clinical network and finally the corrective interventions to be adopted to improve QoCC.
With this study, we hope to increase the awareness of the value of QIs in healthcare so as to encourage more uniform practices and improve provider documentation of medical care in our region; moreover, we hope that the standardisation of QIs among different regions will help to define the threshold of a minimal standard of care.
Supplementary Material
Footnotes
Correction notice: This paper has been corrected since it was first published. One of the authors, Andrea Bordoni, did not have an affiliation number after their name.
Acknowledgements: We are particularly grateful to the QC3 CRC AB Members, who contribute to critically review the QC3 CRC QIs, for their precious collaboration: Professor Franco Cavalli, Scientific Director, Istituto Oncologico della Svizzera Italiana (IOSI), Bellinzona, Switzerland; Professor Gian Dorta, Director, Digestive Endoscopy Department, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland; Professor Jean Faivre, Director, Registre Bourguignon des Cancers Digestifs, Dijon Cedex, France; Professor Stefano Fanti, Director, PET Center, Policlinico S. Orsola-Malpighi, Bologna, Italy; Professor Roberto Labianca, Director, Oncology and Haematology Department, Ospedali Riuniti, Bergamo, Italy; Professor Sebastiano Martinoli, Director, General Surgery Department, Clinica Luganese, Lugano, Switzerland; Professor Philip Quirke, Director, Leeds Institute of Molecular Medicine (LIMM), Section of Pathology, Wellcome Trust Brenner Building, St James's University Hospital, Leeds, UK; Professor Emmanuel Tiret, Chef, Pôle Digestif des Hôpitaux Univesitaires Paris Est, Service de Chirurgie Générale et Digestive, Hôpital Saint-Antoine, Paris, France; Professor Vincenzo Valentini, Director, Unità Operativa Complessa Radioterapia 1, Policlinico Universitario Agostino Gemelli, Rome, Italy; Professor Dominik Weishaupt, Director, Radiology Department, Stadtspital Triemli, Zürich, Switzerland.
Collaborators: Members of the QC3 CRC Working Group are listed as following: Jessica Barizzi, Clinical Pathology, Cantonal Institute of Pathology, Locarno, Switzerland; Florian Bihl, Gastroenterology Department, Ospedale Regionale Bellinzona e Valli, Bellinzona, Switzerland; Dimitri Christoforidis, General Surgery Department, Ospedale Regionale di Lugano, Lugano, Switzerland; Alessandra Franzetti-Pellanda, Radiation Oncology Department, Clinica Luganese, Lugano, Switzerland; Luca Giovanella, Nuclear Medicine Department, Istituto Oncologico della Svizzera Italiana (IOSI), Bellinzona, Switzerland; Jürgen Heinkel, Radiology Department, Ospedale La Carità, Locarno, Switzerland; Massimo Maffei, Gastroenterology Department, Ospedale Regionale di Lugano, Lugano, Switzerland; Luca Mazzucchelli, Clinical Pathology, Cantonal Institute of Pathology, Locarno, Switzerland; Bernard Miazza, Gastroenterology Department, Ospedale Regionale di Lugano, Lugano, Switzerland; Angelo Pelloni, General Surgery Department, Ospedale La Carità, Locarno, Switzerland; Cristiana Quattropani, Gastroenterology Department, Clinica Luganese, Lugano, Switzerland; Raffaele Rosso, General Surgery Department, Ospedale Regionale di Lugano, Lugano, Switzerland; Piercarlo Saletti, Medical Oncology Department, Istituto Oncologico della Svizzera Italiana (IOSI), Bellinzona, Switzerland; Maria Carla Valli, Radiation Oncology Department, Istituto Oncologico della Svizzera Italiana (IOSI), Bellinzona, Switzerland; Marco Varini, Medical Oncology Department, Clinica Sant'Anna, Lugano, Switzerland; Rolf Wyttenbach, Radiology Department, Ospedale Regionale Bellinzona e Valli, Bellinzona, Switzerland.
Contributors: VB, AB, AS and LM have directly participated in the planning of the manuscript; VB, AB, AS and the QC3 Colorectal Working Group have directly participated in the conduct of the project; VB, AB, AS and LO have directly participated in the reporting, acquisition of data or analysis and interpretation of data and VB and AB are responsible for the overall content as guarantors of the work. All the authors have drafted and revised the paper critically for important intellectual content, and that they have given final approval of the version published.
Funding: This work was supported by Krebsforschung Schweiz, grant number KFS—02668-08-2010, and by Advisory Board Research Ente Ospedaliero Cantonale Bellinzona, grant number ABREOC 10/2010. The funding sources have not any involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the report and in the decision to submit the paper for publication.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Contributor Information
Collaborators: Jessica Barizzi, Florian Bihl, Dimitri Christoforidis, Alessandra Franzetti-Pellanda, Luca Giovanella, Jürgen Heinkel, Massimo Maffei, Luca Mazzucchelli, Bernard Miazza, Angelo Pelloni, Cristiana Quattropani, Raffaele Rosso, Piercarlo Saletti, Maria Carla Valli, Marco Varini, and Rolf Wyttenbach
References
- 1.Malin JL, Schneider EC, Epstein AM, et al. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol 2006;24:626–34 [DOI] [PubMed] [Google Scholar]
- 2.Schneider EC, Malin JL, Kahn KL, et al. Developing a system to assess the quality of cancer care: ASCO's national initiative on cancer care quality. J Clin Oncol 2004;22:2985–91 [DOI] [PubMed] [Google Scholar]
- 3.Campbell SM, Roland MO, Buetow SA. Defining quality of care. Soc Sci Med 2000;51:1611–25 [DOI] [PubMed] [Google Scholar]
- 4.Neuss MN, Desch CE, McNiff KK, et al. A process for measuring the quality of cancer care: the Quality Oncology Practice Initiative. J Clin Oncol 2005;23:6233–9 [DOI] [PubMed] [Google Scholar]
- 5.Duvalko KM, Sherar M, Sawka C. Creating a system for performance improvement in cancer care: Cancer Care Ontario's clinical governance framework. Cancer Control 2009;16:293–302 [DOI] [PubMed] [Google Scholar]
- 6.Mainz J, Hansen AM, Palshof T, et al. National quality measurement using clinical indicators: the Danish National Indicator Project. J Surg Oncol 2009;99:500–4 [DOI] [PubMed] [Google Scholar]
- 7.Gagliardi AR, Simunovic M, Langer B, et al. Development of quality indicators for colorectal cancer surgery, using a 3-step modified Delphi approach. Can J Surg 2005;48:441–52 [PMC free article] [PubMed] [Google Scholar]
- 8.Peppercorn JM, Weeks JC, Cook EF, et al. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet 2004;363:263–70 [DOI] [PubMed] [Google Scholar]
- 9.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 2010;46:765–81 [DOI] [PubMed] [Google Scholar]
- 10.NICER. 2012. Secondary. http://www.nicer.org.
- 11.Bordoni A, Lorez M, Bouchardy C, et al. Trends in colorectal cancer survival in Switzerland. Bull Suisse Cancer 2012;1/2012:51–4 [Google Scholar]
- 12.Bouchardy C, Lutz JM, Kühni C, et al. I tumori in Svizzera. Situazione e sviluppi dal 1983 al 2007. Neuchâtel: Ufficio Federale di Statistica (UFS), 2011 [Google Scholar]
- 13.Sant M, Allemani C, Santaquilani M, et al. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer 2009;45:931–91 [DOI] [PubMed] [Google Scholar]
- 14.Desch CE, Benson AB, III, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol 2005;23:8512–19 [DOI] [PubMed] [Google Scholar]
- 15.Desch CE, McNiff KK, Schneider EC, et al. American Society of Clinical Oncology/National Comprehensive Cancer Network Quality Measures. J Clin Oncol 2008;26:3631–7 [DOI] [PubMed] [Google Scholar]
- 16.QOPI Summary of the measures. Spring 2011. Secondary summary of the measures. Spring: 2011. http://qopi.asco.org/Methodology [Google Scholar]
- 17.Jacobsen PB, Shibata D, Siegel EM, et al. Measuring quality of care in the treatment of colorectal cancer: the Moffitt quality practice initiative. J Oncol Pract 2007;3:60–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malafa MP, Corman MM, Shibata D, et al. The Florida Initiative for Quality Cancer Care: a regional project to measure and improve cancer care. Cancer Control 2009;16:318–27 [DOI] [PubMed] [Google Scholar]
- 19.McGory ML, Shekelle PG, Ko CY. Development of quality indicators for patients undergoing colorectal cancer surgery. J Natl Cancer Inst 2006;98:1623–33 [DOI] [PubMed] [Google Scholar]
- 20.Siegel EM, Jacobsen PB, Malafa M, et al. Evaluating the quality of colorectal cancer care in the state of Florida: results from the Florida Initiative for Quality Cancer Care. J Oncol Pract 2012;8:239–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donabedian A. Evaluating the quality of medical care. Milbank Q 1966;83:691–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fink A, Kosecoff J, Chassin M, et al. Consensus methods: characteristics and guidelines for use. Am J Public Health 1984;74:979–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995;311:376–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brook RH. The Rand/UCLA appropriateness method. In: McCormic KA, Moore SR, Siegel RA. eds. Clinical practice guideline development: methodology perspectives. Rockville, MD: Agency for Health Care Policy and Research, 1994:59–70 [Google Scholar]
- 25.Krzyzanowska MK, Barbera L, Elit L, et al. Identifying population-level indicators to measure the quality of cancer care for women. Int J Qual Health Care 2011;23:554–64 [DOI] [PubMed] [Google Scholar]
- 26.Blind K, Cuhls K, Grupp H. Personal attitudes in the assessment of the future of science and technology: a fact on analysis approach. Technol Forecast Soc Change 2001:131–49 [Google Scholar]
- 27.Leape LL, Park RE, Kahan JP, et al. Group judgments of appropriateness: the effect of panel composition. Qual Assur Health Care 1992;4:151–9 [PubMed] [Google Scholar]
- 28.Campbell SM, Hann M, Roland MO, et al. The effect of panel membership and feedback on ratings in a two-round Delphi survey: results of a randomized controlled trial. Med Care 1999;37:964–8 [DOI] [PubMed] [Google Scholar]
- 29.Coulter I, Adams A, Shekelle P. Impact of varying panel membership on ratings of appropriateness in consensus panels: a comparison of a multi- and single disciplinary panel. Health Serv Res 1995;30:577–91 [PMC free article] [PubMed] [Google Scholar]
- 30.Wollersheim H, Hermens R, Hulscher M, et al. Clinical indicators: development and applications. Neth J Med 2007;65:15–22 [PubMed] [Google Scholar]
- 31.Mainz J. Defining and classifying clinical indicators for quality improvement. Int J Qual Health Care 2003;15:523–30 [DOI] [PubMed] [Google Scholar]
- 32.Mainz J. Developing evidence-based clinical indicators: a state of the art methods primer. Int J Qual Health Care 2003;15(Suppl 1):i5–11 [DOI] [PubMed] [Google Scholar]
- 33.Rubin HR, Pronovost P, Diette GB. From a process of care to a measure: the development and testing of a quality indicator. Int J Qual Health Care 2001;13:489–96 [DOI] [PubMed] [Google Scholar]
- 34.Wilkins T, Reynolds PL. Colorectal cancer: a summary of the evidence for screening and prevention. Am Fam Physician 2008;78:1385–92 [PubMed] [Google Scholar]
- 35.Sikka V, Ornato JP. Cancer diagnosis and outcomes in Michigan EDs vs other settings. Am J Emerg Med 2012;30:283–92 [DOI] [PubMed] [Google Scholar]
- 36.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008;134:1570–95 [DOI] [PubMed] [Google Scholar]
- 37.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-update based on new evidence. Gastroenterology 2003;124:544–60 [DOI] [PubMed] [Google Scholar]
- 38.Majumdar SR, Fletcher RH, Evans AT. How does colorectal cancer present? Symptoms, duration, and clues to location. Am J Gastroenterol 1999;94:3039–45 [DOI] [PubMed] [Google Scholar]
- 39.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012;366:2345–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labianca R, Nordlinger B, Beretta GD, et al. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol 2010;21(Suppl 5):v70–7 [DOI] [PubMed] [Google Scholar]
- 41.NCCN Colon Cancer. 2.2011 : NCCN, 2011 [Google Scholar]
- 42.Pfister DG, Benson AB, III, Somerfield MR. Clinical practice. Surveillance strategies after curative treatment of colorectal cancer. N Engl J Med 2004;350:2375–82 [DOI] [PubMed] [Google Scholar]
- 43.Li Destri G, Di Cataldo A, Puleo S. Colorectal cancer follow-up: useful or useless? Surg Oncol 2006;15:1–12 [DOI] [PubMed] [Google Scholar]
- 44.Campbell SM, Braspenning J, Hutchinson A, et al. Research methods used in developing and applying quality indicators in primary care. Qual Saf Health Care 2002;11:358–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ayanian JZ, Landrum MB, Normand SL, et al. Rating the appropriateness of coronary angiography—do practicing physicians agree with an expert panel and with each other? N Engl J Med 1998;338:1896–904 [DOI] [PubMed] [Google Scholar]
- 46.Sackett DL, Starus SE, Richardson WS, et al. Evidence-based medicine: how to practice and teach. 2nd edn. London: Churchill Livingstone, 2000 [Google Scholar]
- 47.Bordoni A, Spitale A, Mazzucchelli L, et al. Qualità delle cure contro il cancro (QC3) nel territorio della Svizzera Italiana. Risultatai preliminari dei tumori colorettali incidenti nel 2011. XXXVII GRELL Annual Meeting; 16–18 May 2012; Porto, Portugal. [Google Scholar]
- 48.Bordoni A, Bianchi Galdi V, Mazzucchelli L, et al. QoCC Study: indicators of quality of cancer care in Southern Switzerland. 33rd IACR Annual Meeting; 11–13 October 2011; Balaclava, Mauritius.
- 49.Bordoni A, Spitale A, Mazzucchelli L, et al. QC3: quality of comprehensive cancer care in Southern Switzerland. 34th IACR Annual Meeting. Cork, Ireland: IACR, 2012 [Google Scholar]
- 50.Bordoni A, Spitale A, Mazzucchelli L, et al. Defining evidence-based clinical oncologic cares quality indicators. ENCR Scientific Meeting. Cork, Ireland: ENCR, 2012 [Google Scholar]
- 51.Bouchardy C, Rapiti E, Blagojevic S, et al. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol 2007;25:1858–69 [DOI] [PubMed] [Google Scholar]
- 52.Bouchardy C, Rapiti E, Fioretta G, et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol 2003;21:3580–7 [DOI] [PubMed] [Google Scholar]
- 53.Van Gijn W, Van de Velde CJ. 2010 SSO John Wayne clinical research lecture: rectal cancer outcome improvements in Europe: population-based outcome registrations will conquer the world. Ann Surg Oncol 2011;18:691–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Gijn W, Van de Velde CJ. Improving quality of cancer care through surgical audit. Eur J Surg Oncol 2010;36(Suppl 1):S23–6 [DOI] [PubMed] [Google Scholar]
- 55.Characteristics of clinical indicators. QRB Qual Rev Bull 1989;15:330–9 [DOI] [PubMed] [Google Scholar]
- 56.Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol 2008;47:20–31 [DOI] [PubMed] [Google Scholar]
- 57.Schneider PM, Vallbohmer D, Ploenes Y, et al. Evaluation of quality indicators following implementation of total mesorectal excision in primarily resected rectal cancer changed future management. Int J Colorectal Dis 2011;26:903–9 [DOI] [PubMed] [Google Scholar]
- 58.ACR. 2011. Practice Guidelines and Technical Standards. Secondary Practice Guidelines and Technical Standards. http://www.acr.org/guidelines.
- 59.Taylor FG, Quirke P, Heald RJ, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study that recruited consecutive patients with rectal cancer. Ann Surg 2011;253:711–9. [DOI] [PubMed] [Google Scholar]
- 60.Beets-Tan RG, Beets GL. Local staging of rectal cancer: a review of imaging. J Magn Reson Imaging 2011;33:1012–19 [DOI] [PubMed] [Google Scholar]
- 61.Bellows CF, Jaffe B, Bacigalupo L, et al. Clinical significance of magnetic resonance imaging findings in rectal cancer. World J Radiol 2011;3:92–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor FG, Quirke P, Heald RJ, et al. One millimetre is the safe cut-off for magnetic resonance imaging prediction of surgical margin status in rectal cancer. Br J Surg 2011;98:872–79 [DOI] [PubMed] [Google Scholar]
- 63.Lugli A, Tornillo L, Cathomas G, et al. Colon et rectum. In: Dirnhofer S, Bubendorf L, Lehr H-A, et al. eds. Recommandations pour la qualité—SSPath. Bâle: Sociéte Suisse de Pathologie, 2011:1–13. [Google Scholar]
- 64.Washington K, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Vers. 3.1.0.0. In: College of American Pathologist (CAP) ed. Cancer protocols and checklists, 2011:1–33. [Google Scholar]
- 65.MacDonald AJ, McEwan H, McCabe M, et al. Age at death of patients with colorectal cancer and the effect of lead-time bias on survival in elective vs emergency surgery. Colorectal Dis 2011;13:519–25 [DOI] [PubMed] [Google Scholar]
- 66.Ascanelli S, Navarra G, Tonini G, et al. Early and late outcome after surgery for colorectal cancer: elective versus emergency surgery. Tumori 2003;89:36–41 [DOI] [PubMed] [Google Scholar]
- 67.Biondo S, Marti-Rague J, Kreisler E, et al. A prospective study of outcomes of emergency and elective surgeries for complicated colonic cancer. Am J Surg 2005;189:377–83 [DOI] [PubMed] [Google Scholar]
- 68.Thompson GA, Cocks JR, Collopy BT, et al. Clinical indicators in colorectal surgery. J Qual Clin Pract 1996;16:31–5 [PubMed] [Google Scholar]
- 69.Morris EJ, Taylor EF, Thomas JD, et al. Thirty-day postoperative mortality after colorectal cancer surgery in England. Gut 2011;60:806–13 [DOI] [PubMed] [Google Scholar]
- 70.McArdle CS, McKee RF, Finlay IG, et al. Improvement in survival following surgery for colorectal cancer. Br J Surg 2005;92:1008–13 [DOI] [PubMed] [Google Scholar]
- 71.Rutten HJ, Den Dulk M, Lemmens VE, et al. Controversies of total mesorectal excision for rectal cancer in elderly patients. Lancet Oncol 2008;9:494–501 [DOI] [PubMed] [Google Scholar]
- 72.Rogers SO, Jr., Ayanian JZ, Ko CY, et al. Surgeons’ volume of colorectal cancer procedures and collaborative decision-making about adjuvant therapies. Ann Surg 2009;250:895–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kurtz JE, Heitz D, Serra S, et al. Adjuvant chemotherapy in elderly patients with colorectal cancer. A retrospective analysis of the implementation of tumor board recommendations in a single institution. Crit Rev Oncol Hematol 2010;74:211–17 [DOI] [PubMed] [Google Scholar]
- 74.Jarry J, Faucheron JL, Moreno W, et al. Delayed colo-anal anastomosis is an alternative to prophylactic diverting stoma after total mesorectal excision for middle and low rectal carcinomas. Eur J Surg Oncol 2010;37:127–33 [DOI] [PubMed] [Google Scholar]
- 75.Junginger T, Gonner U, Trinh TT, et al. Permanent stoma after low anterior resection for rectal cancer. Dis Colon Rectum 2010;53:1632–9 [DOI] [PubMed] [Google Scholar]
- 76.Neuman HB, Patil S, Fuzesi S, et al. Impact of a temporary stoma on the quality of life of rectal cancer patients undergoing treatment. Ann Surg Oncol 2010;18:1397–403. [DOI] [PubMed] [Google Scholar]
- 77.Ramirez JM, Aguilella V, Valencia J, et al. Transanal endoscopic microsurgery for rectal cancer. Long-term oncologic results. Int J Colorectal Dis 2011;26:437–43. [DOI] [PubMed] [Google Scholar]
- 78.Lezoche G, Guerrieri M, Baldarelli M, et al. Transanal endoscopic microsurgery for 135 patients with small nonadvanced low rectal cancer (iT1-iT2, iN0): short- and long-term results. Surg Endosc 2011;25:1222–9. [DOI] [PubMed] [Google Scholar]
- 79.Doornebosch PG, Tollenaar RA, De Graaf EJ. Is the increasing role of transanal endoscopic microsurgery in curation for T1 rectal cancer justified? A systematic review. Acta Oncol 2009;48:343–53 [DOI] [PubMed] [Google Scholar]
- 80.Elferink MA, Siesling S, Lemmens VE, et al. Variation in lymph node evaluation in rectal cancer: a Dutch Nationwide Population-Based Study. Ann Surg Oncol 2011;18:386–95. [DOI] [PubMed] [Google Scholar]
- 81.Elferink MA, Siesling S, Visser O, et al. Large variation between hospitals and pathology laboratories in lymph node evaluation in colon cancer and its impact on survival, a nationwide population-based study in the Netherlands. Ann Oncol 2011;22:110–17 [DOI] [PubMed] [Google Scholar]
- 82.Kelder W, Inberg B, Schaapveld M, et al. Impact of the number of histologically examined lymph nodes on prognosis in colon cancer: a population-based study in the Netherlands. Dis Colon Rectum 2009;52:260–7 [DOI] [PubMed] [Google Scholar]
- 83.Vather R, Sammour T, Kahokehr A, et al. Lymph node evaluation and long-term survival in Stage II and Stage III colon cancer: a national study. Ann Surg Oncol 2009;16:585–93 [DOI] [PubMed] [Google Scholar]
- 84.Lindebjerg J, Spindler KL, Ploen J, et al. The prognostic value of lymph node metastases and tumour regression grade in rectal cancer patients treated with long-course preoperative chemoradiotherapy. Colorectal Dis 2009;11:264–9 [DOI] [PubMed] [Google Scholar]
- 85.Choi HK, Law WL, Poon JT. The optimal number of lymph nodes examined in stage II colorectal cancer and its impact of on outcomes. BMC Cancer 2010;10:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 2001;93:583–96 [DOI] [PubMed] [Google Scholar]
- 87.Smith AJ, Driman DK, Spithoff K, et al. Guideline for optimization of colorectal cancer surgery and pathology. J Surg Oncol 2010;101:5–12 [DOI] [PubMed] [Google Scholar]
- 88.Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16 [DOI] [PubMed] [Google Scholar]
- 89.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704 [DOI] [PubMed] [Google Scholar]
- 90.Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol 2009;27:3117–25 [DOI] [PubMed] [Google Scholar]
- 91.Sobrero A. Lower GI. In: ESMO HIghlights 2010. http://www.esmo.org/Guidelines-Practice/ESMO-Spotlights [Google Scholar]
- 92.Des Guetz G, Nicolas P, Perret GY, et al. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer 2010;46:1049–55 [DOI] [PubMed] [Google Scholar]
- 93.Dienstmann R, Vilar E, Tabernero J. Molecular predictors of response to chemotherapy in colorectal cancer. Cancer J 2011;17:114–26 [DOI] [PubMed] [Google Scholar]
- 94.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408–17 [DOI] [PubMed] [Google Scholar]
- 95.Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 2011;29:1261–70 [DOI] [PubMed] [Google Scholar]
- 96.Van Cutsem E, Nordlinger B, Cervantes A. Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann Oncol 2010;21(Suppl 5):v93–7 [DOI] [PubMed] [Google Scholar]
- 97.Glimelius B, Holm T, Blomqvist L. Chemotherapy in addition to preoperative radiotherapy in locally advanced rectal cancer—a systematic overview. Rev Recent Clin Trials 2008;3:204–11 [DOI] [PubMed] [Google Scholar]
- 98.Glimelius B, Pahlman L, Cervantes A. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21(Suppl 5):v82–6 [DOI] [PubMed] [Google Scholar]
- 99.Landheer ML, Therasse P, Van de Velde CJ. The importance of quality assurance in surgical oncology in the treatment of colorectal cancer. Surg Oncol Clin N Am 2001;10:885–914, x [PubMed] [Google Scholar]
- 100.Wagner G. Tumor-Lokalisationsschlüssel. International Classification of Diseases for Oncology ICD-O, 2. Topographischer Teil. 5th edn Berlin: Springer-Verlag, 1993 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


