Abstract
We studied the time interval between starting tuberculosis treatment and commencing antiretroviral treatment (ART) in HIV-infected patients (n=1433; median CD4 count 71 cells/μL, IQR,32-132) attending three South African township ART services between 2002-2008. The overall median delay was 2.66 months (IQR,1.58-4.17). In adjusted analyses, delays varied between treatment sites but were shorter for patients with lower CD4 counts and those treated in more recent calendar years. During the most recent period (2007-2008), 4.7%, 19.7% and 51.1% of patients started ART within 2, 4 and 8 weeks of TB treatment, respectively. Operational barriers must be tackled to permit further acceleration of ART initiation as recommended by 2010 WHO ART guidelines.
Keywords: tuberculosis, antiretroviral, timing, delay, Africa
INTRODUCTION
The high mortality risk of patients with HIV-associated tuberculosis (TB) is reduced by 64%-95% by antiretroviral therapy (ART).1 However, the optimal time to start ART during TB treatment to has for a long time remained unclear. Findings from observational studies and recent randomized controlled trials have demonstrated that delayed ART initiation is associated with increased mortality risk across a wide spectrum of baseline CD4 cell counts.2-6 The World Health Organization (WHO) has updated ART guidelines on several occasions between 2002 and 2010, recommending progressively higher CD4 cell count thresholds for ART eligibility and more rapid initiation of ART during TB treatment.7 Guidelines published in 2010 recommend ART be given to all patients with HIV-associated TB regardless of CD4 cell count and that this be started as soon as possible after TB treatment is tolerated and not later than 8 weeks.7
The operational feasibility of early initiation of ART in TB patients under routine programme conditions in resource-limited settings is not known, however. The timing of ART may be influenced by many factors, including delays associated with HIV testing and CD4 cell count measurement, constraints within the health system that contribute to delays in referral and access to ART clinics, and changes in programmatic efficiency and clinical expertise over time. In this study we quantified and explored factors associated with delays between starting TB treatment and starting ART among TB patients enrolling in three large community-based ART services in townships in South Africa.
METHODS
Antiretroviral treatment cohorts
Provision of ART within three primary care clinics in the townships of Khayelitsha, Gugulethu and Masiphumelele in Cape Town, South Africa, started between 2001 and 2003.8-13 ART was provided free of charge under the South African national guidelines for patients with WHO stage 4 disease and all those with blood CD4 cell counts <200 cells/μL.14 The huge burden and complications of TB in these services has been previously reported.9,15-17 TB treatment and ART in South Africa are typically delivered by separate primary care clinics.16 TB was treated using standardised rifampicin-based regimens of 6 months duration for new TB cases and 8 months for retreatment cases. The total treatment period, however, was longer for those with treatment interruptions or drug resistant disease.
Data sources
Each of the clinics participates in the International epidemiological Databases to Evaluate AIDS (IeDEA) in Southern Africa network (see www.iedea-sa.org). Prospective clinical databases have been maintained for all patients enrolling in these services.9,11,12 All sites obtained ethical approval from relevant local institutions before contributing anonymized patient data collected between 2002 and 2008 to this collaborative analysis. Data were included from patients aged over 18 years who had a notified diagnosis of TB, initiated ART for the first time during the course of TB treatment and were followed up for a minimum period of 10 months from TB diagnosis during which ART could be started. For each patient, demographic details, TB classification, WHO clinical stage, blood CD4 cell counts and dates of TB diagnosis and ART initiation were recorded. Where details of TB diagnoses were missing, these were sought from clinic TB registers and the Cape Town electronic TB register.
Statistical analyses
Patient characteristics were summarised by calendar period of starting TB treatment. Data were aggregated into four sequential calendar periods, 2002-2004, 2005, 2006 and 2007-2008, such that there were at least 200 patients represented in each period. The time from start of TB treatment to start of ART was the outcome of primary interest
Accelerated failure time (AFT) models were used to determine crude and adjusted associations between hypothesized risk factors and time to initiation of ART. The AFT metric was chosen because of its specific reference to time and impact on time and because the proportional hazards assumption was not satisfied by the data. The Weibull, Generalized Gamma, Log-logistic, Lognormal and Exponential AFT models were each considered. The Gamma model had the lowest values for the Akaike’s and Bayesian Information Criteria (AIC & BIC). The Wald test on the two shape parameters confirmed that none of the simpler distributions were appropriate. The Gamma model had the best model fit based on a graph plotting the Cox-Snell residuals against the cumulative hazard of time. The coefficients of the generalized Gamma AFT model were transformed to acceleration coefficients by taking the exponent of the negative co-efficient. These acceleration co-efficients measure the relative acceleration (and associated shortening of time) to ART initiation.
RESULTS
Patient characteristics
During the analysis period, 1433 patients who started ART during TB treatment were eligible for inclusion. Patients were adults with a median age of 33 years (IQR,29-39) and 60% were women. Sixty one per cent were recorded as having pulmonary disease alone and 39% had extrapulmonary disease with or without concomitant pulmonary involvement. Treatment for recurrent TB was received by 29% of patients. CD4 counts were available for 1152 (80.4%) patients with a median of 71 cells/μL (IQR, 32-132). When stratified by calendar period, patient characteristics did not vary substantially (Supplemental Digital Content 1) although the proportion of patients who were male tended to increase over time and the proportion of patients receiving a retreatment TB regimen decreased over time.
Time to initiation of ART
The time between starting TB treatment and starting ART was highly variable with a median of 2.66 months (IQR,1.58-4.17). Using accelerated failure time (AFT) models (Table 1), crude analyses showed that delays were shorter among younger patients, those with a first episode of TB, sputum smear-positive disease, lower CD4 cell counts and treatment in later calendar periods. However, in fully adjusted models, only three variables remained significantly associated with time to ART: the blood CD4 cell count, calendar year and the treatment site (Table 1).
Table 1.
| Crude Accelerators |
95%CI | p-value† | Adjusted Accelerators |
95%CI | p-value† | |
|---|---|---|---|---|---|---|
| Age category at start of TB treatment | 0.008 | |||||
| ≥ 40 (baseline) | 1 | |||||
| 25-39 | 1.12 | 1.04 - 1.21 | 0.003 | |||
| 18-24 | 1.15 | 1.02 - 1.30 | 0.022 | |||
| Gender | ||||||
| Male | 1 | |||||
| Female (baseline) | 1.01 | 0.94 - 1.07 | 0.879 | |||
| Clinic | <0.001 | <0.001 | ||||
| 2 (baseline) | 1 | 1 | ||||
| 1 | 1.08 | 1.00 - 1.15 | 0.046 | 1.39 | 1.29 - 1.48 | <0.001 |
| 3 | 1.34 | 1.21 - 1.48 | <0.001 | 1.56 | 1.42 - 1.71 | <0.001 |
| Year started TB treatment | <0.001 | <0.001 | ||||
| 2002-2004 (baseline) | 1 | 1 | ||||
| 2005 | 1.08 | 0.98 - 1.18 | 0.111 | 1.08 | 0.99 - 1.19 | 0.079 |
| 2006 | 1.34 | 1.23 - 1.46 | <0.001 | 1.22 | 1.12 - 1.33 | <0.001 |
| 2007-2008 | 1.83 | 1.65 - 2.03 | <0.001 | 1.39 | 1.25 - 1.54 | <0.001 |
| CD4 count categories* (cells/μL) | <0.001 | <0.001 | ||||
| >=200 (baseline) | 1 | 1 | ||||
| 100-199 | 1.16 | 1.03 - 1.32 | 0.017 | 1.20 | 1.07 - 1.34 | 0.002 |
| 50-99 | 1.29 | 1.14 - 1.47 | <0.001 | 1.34 | 1.19 - 1.50 | <0.001 |
| 0-49 | 1.70 | 1.49 - 1.94 | <0.001 | 1.75 | 1.55 - 1.98 | <0.001 |
| Classification of TB | ||||||
| Rtetreatment (baseline) | 1 | |||||
| New TB case | 1.11 | 1.03 - 1.19 | 0.004 | |||
| Type of TB | 0.018 | |||||
| Smear-Negative Pulmonary TB (baseline) | 1 | |||||
| Extra-Pulmonary TB | 1.05 | 0.97 - 1.13 | 0.262 | |||
| Smear-Pbstive Pulmonary TB | 1.12 | 1.04 - 1.22 | 0.005 |
The p-value in line with the heading of each variable with more than 2 categories wascalculated using the Waid Test
CD4 count taken closest start of TB treatment that satisfied criteria of being taken not earlier than 183 daysbefore or 91 daysafter start of TB treatment and not more than 7 days after start of ART
Shape parametersof multivariate gamma model: kappa = 0.7 (95%CI: 0.57-0.84); sigma = 0.42 (95%CI: 0.39-0.45) for all strata other than CD4count lessthan 50 cells/μL(sgma = 0.59, 95%CI:0.50-0.69) and clinic 1 (sigma = 0.51, 95%CI: 0.44-0.60)
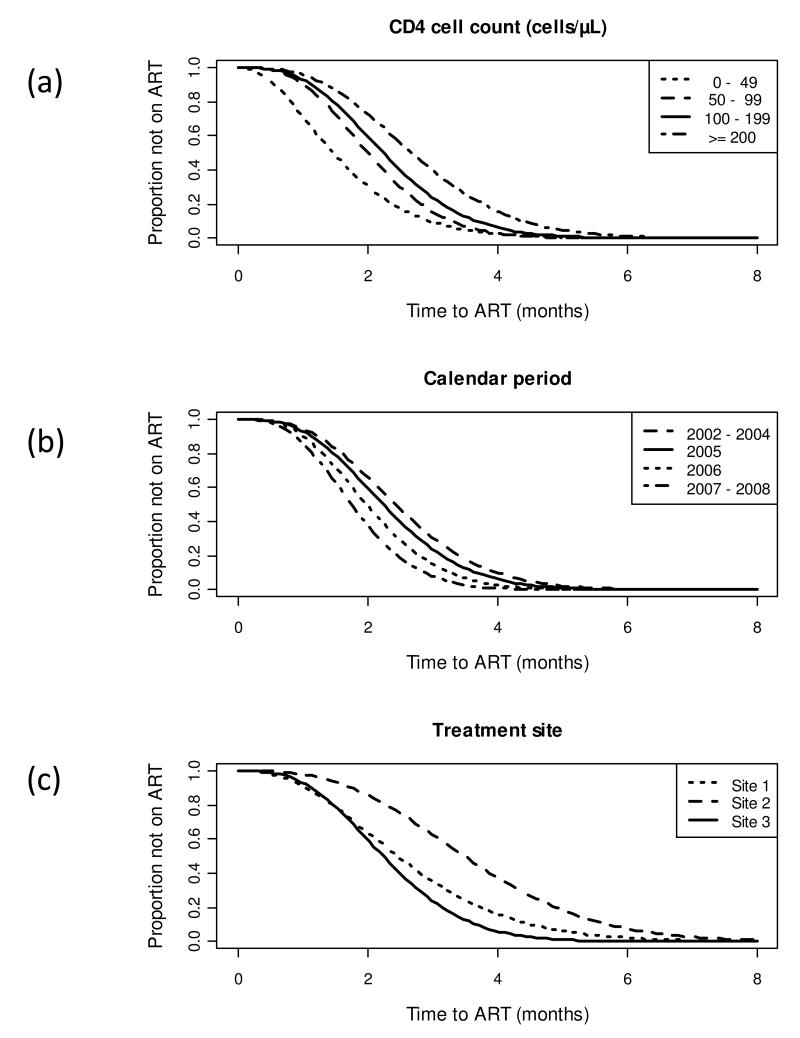
The longest predicted delays between start of TB treatment and ART initiation were among those with blood CD4 cell counts of ≥200 cells/μL who started TB treatment during the calendar period 2002-2004 at site 2; the average time was 4.73 months (95%CI, 4.12-5.33). Time to ART initiation was significantly accelerated among those with lower CD4 counts (Figure 1a). In patients with CD4 counts of 100-199, 50-99 and <50 cells/μL, time to starting ART was accelerated by 20%, 34% and 75%, respectively (Table 1), giving mean times of 3.95, 3.53 and 2.77 months. Similarly, mean times were accelerated among patients treated in calendar periods 2005, 2006 and 2007-2008 by 8%, 22% and 39%, respectively, giving mean times of 4.36, 3.87 and 3.41 months (Figure 1b). Considerable heterogeneity in time to ART start was also observed between the three different treatment sites (Figure 1c), although the associations with CD4 cell count and calendar period were observed at all three sites. The shortest predicted delays between start of TB treatment and ART initiation were among those with a blood CD4 cell count of <50 cells/μL who started TB treatment during the calendar period 2007-2008 at site 3; the average time was 1.28 months (95% CI, 1.15 - 1.42)
Figure 1.
Gamma Survival Curves showing the proportion of patients who have yet to start antiretroviral treatment (survival) with increasing duration of tuberculosis treatment (analysis time in months). Data are adjusted for other co-variates shown in Table 1 with the reference group in each plot representing patients with CD4 cell counts in the range 100-199 cells/μL cared for at treatment site 3 in 2005. Data are shown stratified by (a) blood CD4 cell count; (b) calendar year of starting treatment and (c) treatment site.
Comparison with 2010 WHO guidelines
WHO ART guidelines (2010 revision) recommend that ART should be started as soon as possible within 2 to 8 weeks of starting TB treatment, regardless of CD4 cell count. We examined the timing of ART among patients treated in the most recent calendar period, 2007-2008. The proportions starting ART within 2, 4, 6 and 8 weeks of TB treatment were 4.7% (n=11), 19.7% (n=46), 35.6% (n=83) and 51.1% (n=119), respectively. For those with the most advanced immunodeficiency (CD4 count less than 50 cells/μL) treated in this period, the corresponding percentages were 6.2% (n=5), 33.3% (n=27), 61.7% (n=50) and 77.8% (n=63).
DISCUSSION
The use of ART must be carefully optimised to maximise the benefit for patients with HIV-associated TB. This study found that the time interval between starting TB treatment and starting ART among patients treated under routine programme conditions was prolonged, but highly variable and strongly associated with three key factors. Delays varied between different clinics but were shorter for those with lower CD4 counts and for those treated in more recent calendar periods. However, delays must be reduced much further as recommended by current WHO guidelines.
The numbers of patients referred to ART clinics with a TB diagnosis has increased over time such that these comprised over one third of all referrals to ART services in Cape Town by 2008.9,16 In addition, intensive TB screening just prior to ART initiation has found that up to 25% of other referrals also have sputum culture-positive TB.18,19 These patients typically have low CD4 counts and high mortality risk. Data from a randomised controlled trial conducted in Cambodia (the CAMELIA trial)5 found that TB patients with advanced immunodeficiency had 35% greater mortality if they received ART after 2 months of TB treatment rather than starting ART within the first 2 weeks, showing the need for rapid initiation in this group.5
Overall, we found a strong graded relationship between CD4 count and timing of ART with progressively more rapid ART initiation among those in lower CD4 count strata. These data may reflect growing clinical expertise and recognition of the need for greater rapidity of ART among those with advanced immunodeficiency.10,15 However, despite local and international guidelines, only one third of patients with CD4 counts of <50 cells/μL treated in 2007-2008 received ART within the first one month of TB treatment, which may suggest the existence of constraints within the care pathway (such as referral delays between TB and ART clinics)16 to achieving more rapid ART initiation.
Delays in initiation of ART diminished substantially between sequential calendar periods and this may reflect growing awareness over time of the high mortality risk associated with delays in ART10 as well as more efficient HIV testing procedures. After 2005, a traditional model of voluntary counselling and testing (VCT) for HIV with very poor uptake was replaced by a model of provider initiated testing and counselling (PITC), which has achieved testing rates of over 90% in local TB clinics.16 PITC is likely to have resulted in increases in the numbers of TB patients referred for ART and the rapidity with which this is done.
There were differences in the timing of ART among patients attending the three separate treatment sites that were independent of other variables. This may reflect differences in referral processes and patient accessibility to ART services or differences in the clinical decision making process. Further operational research studies are needed to better characterise the various component delays associated with different models of care. In particular, as individual sites move towards integration of TB treatment and provision of ART, the impact on timing of ART should be investigated and timing might be used as an indicator the quality of care.
The 2010 WHO ART guidelines and South African national ART guidelines recommend ART be started as soon as TB treatment is tolerated and not later than after 8 weeks.20 However, in the most recent calendar period (2007-2008), only one half of patients started ART within an 8-week time frame. Much progress now needs to be made in identifying the operational barriers to more rapid ART initiation. Integration of TB care and ART in the same clinic would be likely to decrease these delays. However, this may also be associated with increased risks of TB immune reconstitution disease,5,17,21 which will require appropriate management, and nosocomial TB transmission,22 which require improved infection control measures.
Strengths of this study include the large number of patients analysed; the patient populations attending primary care ART cohorts were typical of public sector ART clinics in the southern African region; careful prospective recording of patient data, and inclusion of patients enrolled over a 7 year period permitting analysis of temporal trends. Weaknesses of the study include the retrospective study design and lack of identification of the constituent delays contributing to the overall delay in starting ART. The clinical consequences of delays in ART initiation have not been demonstrated and patients who died or were lost to follow-up prior to initiating ART have not been quantified. Further operational research is needed to study the impact of interventions to accelerate ART access and the time to starting ART should be prospectively monitored within programmes.
In conclusion, the timing of ART among TB patients attending three large ART services in townships around Cape Town was very heterogeneous. However, delays decreased substantially over a 7 year period and those with lower CD4 cell counts received ART more rapidly. New WHO ART guidelines and national ART guidelines now recommend much greater acceleration of ART initiation among all TB patients, which is likely to require models of care with much better integration of TB and ART treatment.
Supplementary Material
ACKNOWLEDGMENTS
IeDEA Southern Africa is funded by the National Institute of Allergy and Infectious Diseases (grant 5 U01 AI069924-02). This study was also supported by a grant from the United States President’s Emergency Plan for AIDS Relief (PEPFAR). SDL is funded by the Wellcome Trust, London, UK. RW is funded in part by the National Institutes of Health, USA, RO1 grant (A1058736-01A1) and a CIPRA grant 1U19AI53217-01. MC, LC, AB & FL are funded in part by the National Institutes of Health, USA, grant 5U01AI069924-05.
Footnotes
Conflicts of Interest The authors have no conflicts of interest to declare.
Additional Files Submitted Supplemental Digital Content 1
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- (1).Lawn SD, Kranzer K, Wood R. Antiretroviral Therapy for Control of the HIV-associated Tuberculosis Epidemic in Resource-Limited Settings. Clin Chest Med. 2009;30:685–99. doi: 10.1016/j.ccm.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Lawn SD, Torok ME, Wood R. Optimum time to start antiretroviral therapy during HIV-associated opportunistic infections. Curr Opin Infect Dis. 2010;24:34–42. doi: 10.1097/QCO.0b013e3283420f76. Ref Type: Generic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43:42–6. doi: 10.1097/01.qai.0000230521.86964.86. [DOI] [PubMed] [Google Scholar]
- (4).Velasco M, Castilla V, Sanz J, et al. Effect of Simultaneous Use of Highly Active Antiretroviral Therapy on Survival of HIV Patients With Tuberculosis. J Acquir Immune Defic Syndr. 2009;50:148–52. doi: 10.1097/QAI.0b013e31819367e7. [DOI] [PubMed] [Google Scholar]
- (5).Blanc F-X, Sok T, Laureillard D, et al. Significant enhancement in survival with early (2 weeks) vs late (8 weeks) initiation of highly active antiretroviral treatment (HAART) in severely immunosuppressed HIV-infected adults with newly diagnosed tuberculosis. Abstracts of the XVIII International AIDS Conference; Vienna, Austria. July 2010; International AIDS Society; Abstract THLBB1. [Google Scholar]
- (6).Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).World Health Organization . Antiretroviral therapy for HIV infection in adults and adolescents.Recommendations for a publc health approach (2010 revision) World Health Organization; Geneva: [Accessed on 19.12.10]. at the following URL: http://www.who.int/hiv/pub/arv/adult/en/index.html. [Google Scholar]
- (8).Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–95. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- (9).Boulle A, Van CG, Hilderbrand K, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–72. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- (10).Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–8. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- (11).Nglazi M, Lawn SD, Kaplan R, et al. Changes in programmatic outcomes during 7 years of scale-up at a community-based antiretroviral treatment service in South Africa. J Acquir Immune Defic Syndr. 2010;56:e1–8. doi: 10.1097/QAI.0b013e3181ff0bdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Sanne I, Orrell C, Fox MP, et al. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): a randomised non-inferiority trial. Lancet. 2010;376:33–40. doi: 10.1016/S0140-6736(10)60894-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Fox MP, Sanne IM, Conradie F, et al. Initiating patients on antiretroviral therapy at CD4 cell counts above 200 cells/microl is associated with improved treatment outcomes in South Africa. AIDS. 2010;24:2041–50. doi: 10.1097/QAD.0b013e32833c703e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).National Department of Health . National antiretrovial treatment guidelines. first edition South African Department of Health; 2004. [Google Scholar]
- (15).Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- (16).Lawn SD, Fraenzel A, Kranzer K, Caldwell J, Bekker LG, Wood R. Provider initiated HIV testing increases access of patients with HIV-associated tuberculosis to antiretroviral therapy. S Afr Med J. 2010 doi: 10.7196/samj.4392. in press. [DOI] [PubMed] [Google Scholar]
- (17).Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–41. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- (18).Lawn SD, Edwards DJ, Kranzer K, Vogt M, Bekker LG, Wood R. Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS. 2009;23:1875–80. doi: 10.1097/qad.0b013e32832e05c8. [DOI] [PubMed] [Google Scholar]
- (19).Bassett IV, Wang B, Chetty S, et al. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis. 2010;51:823–9. doi: 10.1086/656282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).National department of health SA . Clinical Guidelines for the Management of HIV & AIDS in Adults and Adolescents. South African Department of Health; 2010. [Google Scholar]
- (21).Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–73. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- (22).Bock NN, Jensen PA, Miller B, Nardell E. Tuberculosis infection control in resource-limited settings in the era of expanding HIV care and treatment. J Infect Dis. 2007;196(Suppl 1):S108–S113. doi: 10.1086/518661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.