Summary
Recovery of motor function after stroke may occur over weeks or months and is often attributed to neuronal reorganization. Functional imaging studies investigating patients who have made a good recovery after stroke have suggested that recruitment of other motor-related networks underlies this recovery. However, patients with less complete recovery have rarely been studied, or else the degree of recovery has not been taken into account. We set out to investigate the relationship between the degree of recovery after stroke and the pattern of recruitment of brain regions during a motor task as measured using functional MRI. We recruited 20 patients who were at least 3 months after their first ever stroke, and 26 right-handed age-matched control subjects. None of our patients had infarcts involving the hand region of the primary motor cortex. All subjects were scanned whilst performing an isometric, dynamic visually paced handgrip task. The degree of functional recovery of each patient was assessed using a battery of outcome measures. Single-patient versus control group analysis revealed that patients with poor recovery were more likely to recruit a number of motor-related brain regions over and above those seen in the control group during the motor task, whereas patients with more complete recovery were more likely to have ‘normal’ task-related brain activation. Across the whole patient group and across stroke subtypes, we were able to demonstrate a negative correlation between outcome and the degree of task-related activation in regions such as the supplementary motor area, cingulate motor areas, premotor cortex, posterior parietal cortex, and cerebellum. This negative correlation was also seen in parts of both contralateral and ipsilateral primary motor cortex. These results further our understanding of the recovery process by demonstrating for the first time a clear relationship between task-related activation of the motor system and outcome after stroke.
Keywords: stroke recovery, functional MRI, motor system, neuronal plasticity
Introduction
In humans, focal cerebral damage is followed by some degree of functional recovery (Twitchell, 1951). In animals, this phenomenon has been studied widely at both the systems and the neuronal level, and is attributed to neuronal reorganization (Schallert et al., 2000). This neuronal reorganization may be influenced in order to enhance functional outcome by a number of interventions, including motor practice, somatosensory input and pharmacological agents (Nudo et al., 1996; Feeney, 1997; Schallert et al., 2000). There is, understandably, much interest in translating this research into benefits for patients, but the tools available for studying the working human brain are very different from those available in animals. Functional imaging techniques provide such an opportunity. Most functional imaging studies of the motor system in previously hemiparetic patients have chosen to investigate patients described as ‘recovered’, inasmuch as they were able to perform the experimental motor task (often finger-to-thumb opposition). In these studies, task-related brain activation in patients over and above that found in control subjects has often been reported in the contralesional sensorimotor and premotor cortex, ipsilesional cerebellum, bilateral supplementary motor area (SMA) and parietal cortex (Chollet et al., 1991; Weiller et al., 1992, 1993; Cramer et al., 1997; Seitz et al., 1998). It was therefore assumed that increased activation in these areas had mediated the recovery process. Subsequently, two studies in which patients were scanned early after stroke and then again some months later demonstrated an overall reduction of much of this task-related activation (Marshall et al., 2000; Calautti et al., 2001). All of these patients made functional improvements between scans, suggesting the recovery process was accompanied by reductions in task-related activation, not an increase, as had initially been hypothesized. Feydy et al. (2002), described differential evolution of motor-related activation in patients with different rates of recovery, but one study found no correlates of functional improvement outside the ipsilateral cerebellum, in which increases in task-related activation were seen with recovery (Small et al., 2002). Thus, the relationship of motor-related brain activations to motor recovery after stroke remains unclear. Before this technique can be useful in understanding more about recovery and the effects that various interventions have on the underlying physiological process, we need to understand more about these task-related changes in brain activation in individual patients. In order to investigate this relationship specifically, we performed a cross-sectional functional MRI (fMRI) study in a number of previously hemiparetic patients with a variable degree of recovery at least 3 months after their stroke. In addition, each patient had undergone a battery of functional assessments in order that changes in motor-related brain activation could be related to recovery.
Methods
Subjects
Patients were recruited from the outpatient and inpatient services at the National Hospital for Neurology and Neurosurgery, London. All patients had suffered from first-ever stroke ≥3 months previously, resulting in weakness of at least the wrist and finger extensors, and hand interossei [to <4+ on the Medical Research Council (MRC) scale], for at least 48 h after onset of symptoms. Exclusion criteria consisted of (i) carotid artery occlusion or stenosis ≥70%; (ii) language or cognitive deficits sufficient to impair cooperation in the study; and (iii) inability to perform the motor task due to complete paralysis of handgrip. All patients received post-stroke rehabilitation therapy appropriate to their clinical needs.
The age-matched control group was recruited from the volunteer database at the Wellcome Department of Imaging Neuroscience. Their results have been fully reported previously (Ward and Frackowiak, 2003). They reported no history of neurological illness or psychiatric history and were not taking regular medication. Neurological and rheumatological examinations were normal in all control subjects.
All patients and control subjects were right-handed according to the Edinburgh handedness scale (Oldfield, 1971). Full written consent was obtained from all subjects in accordance with the Declaration of Helsinki. The study was approved by the Joint Ethics Committee of the Institute of Neurology and National Hospital for Neurology and Neurosurgery, UCL Hospitals NHS Trust, London.
Behavioural evaluation
Patients were evaluated using a battery of outcome measures designed to assess different aspects of functional recovery after stroke. All patients were scored on the following outcome measures on the same day as MRI: (i) Rankin disability scale; (ii) Barthel activities of daily living (ADL) index; (iii) Orpington Prognostic Stroke Scale (OPSS); (iv) motricity index (MI) for upper and (v) lower limbs; (vi) nine-hole peg test (NHPT); (vii) grip strength; (viii) Action Research Arm Test (ARAT); and (ix) timed 10 metre walk. In comparing these scores, the Rankin disability scale and OPSS were converted such that increasing scores reflected improvement, by subtracting the measured score from the maximum score possible for that scale. The NHPT was performed by measuring the time to place nine pegs with each hand. If patients failed to place all nine pegs within 60 s, the number of pegs successfully placed was recorded. Scores were recorded as pegs per second for each hand (averaged over three trials). The score for the impaired hand was corrected within subject by dividing by the score for the unimpaired hand. Maximum grip strength was measured using the same manipulandum as for the MRI scanning. The maximum of three trials was taken as the maximum grip strength for each hand. The score for the impaired hand was again corrected within subject by dividing by the score for the unimpaired hand (Sunderland et al., 1989).
Thus, for each outcome measure there were 20 scores, one for each patient. Each set of outcome measures was normalized (giving unit variance and zero mean), creating nine sets of outcome measures or vectors (one each for Barthel ADL, Rankin disability scale, OPSS, etc.). In order to obtain one representative vector of outcome (overall outcome score), we performed a principal components analysis on the whole data set. The first principal component (a vector of 20 values, one for each patient, explaining most of the variability within the data set) was taken as the vector representing differential outcomes across the patient group, with which activation patterns across the patient group was correlated.
Motor paradigm
Patients performed a dynamic isometric handgrip task using the impaired hand. Control subjects performed the task with their dominant and non-dominant hands in separate sessions and in a randomized counterbalanced order, to provide control data for patients with dominant or non-dominant affected hands. Handgrips were performed using an MRI-compatible manipulandum consisting of two force transducers (Honeywell FSG15N1A; Honeywell, NJ, USA) situated between two moulded plastic bars (width 6 cm). Compression of the two bars with an isometric handgrip resulted in the generation of a differential voltage signal, linearly proportional to the force exerted, which was fed into a signal conditioner (CED 1902 Cambridge Electronic Design, Cambridge, UK). This signal was digitized (CED 1401 Cambridge Electronic Design, Cambridge, UK) and fed into a computer running Cogent 2000 (Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/Cogent2000.html). The dynamic change in recorded signal was projected in real time onto a screen as a column whose height varied linearly with change in voltage and hence force. Prior to scanning, but whilst lying in the scanner, subjects were asked to grip the manipulandum with maximum force to generate a maximum voluntary contraction (MVC). During a continuous scanning session, subjects performed paced isometric dynamic handgrips in blocks of 20 s, alternating with 20 s rest. A total of 24 blocks of handgrip and 24 rest blocks were performed per session. Target forces and rates of handgrip were constant within each 20 s block, but were varied between blocks in a randomized, counterbalanced order. Target forces during scanning were set at 10, 20, 40 and 60% of MVC for each subject, and were indicated by a horizontal bar on the screen. The required rate of handgrip was indicated visually by a cross displayed at the bottom of the screen for 0.3 s at a rate of 0.67 Hz (control subjects). Patients performed at 40% of their maximum rate (up to a maximum of 0.67 Hz). The appearance of the cross indicated that the subject was to perform a single brief handgrip, to be continued until the column representing force applied came into contact with the horizontal bar on the screen, at which point the grip could be released. Subjects were specifically asked to attend to this continuous feedback. Prior to scanning, subjects were trained until comfortable with the task.
All patients performed the motor task outside the scanner in order that they might be observed for the presence of associated movements or mirror movements. To aid this assessment, patients held two identical handgrip manipulanda, one in each hand, during the performance of repetitive handgrip with the affected hand. These simultaneous recordings from both hands enabled us to detect true mirror movements (Nelles et al., 1998). In addition, surface EMG electrodes were positioned on the biceps, triceps and latissimus dorsi bilaterally, to detect more proximal muscle activation.
Data acquisition
A Siemens Vision system (Siemens, Erlangen, Germany), operating at 2 T, was used to acquire both T1-weighted anatomical images (1 × 1 × 1.5 mm voxels) and T2*-weighted MRI transverse echoplanar images (64 × 64 3 × 3 mm2 pixels, TE = 40 ms) with blood oxygenation leveldependent (BOLD) contrast. Each echoplanar image comprised 48 contiguous axial slices 1.8 mm thick, taken every 3 mm, positioned to cover the whole cerebrum. A total of 270 volumes were acquired continuously during each session, with an effective repetition time (TR) of 3.649 s per volume. The first six volumes were discarded to allow for T1 equilibration effects.
Image analysis
Imaging data were analysed using Statistical Parametric Mapping (SPM99; Wellcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm/) (Friston et al., 1995a; Worsley and Friston, 1995) implemented in Matlab5 (Mathworks, Natick, MA, USA). All volumes were realigned spatially to the first volume in order to correct for interscan movement. No subject moved >2 mm in any direction, but some of this movement was task-related. In order to remove some of this unwanted movement-related variance without removing variance attributable to the motor task, realigned images were processed using the ‘unwarp’ toolbox in SPM99 (Andersson et al., 2001), which is predicated on the assumption that susceptibility × movement interaction is responsible for a sizeable part of the residual movement-related variance. Given the observed variance (after realignment) and the realignment parameters, estimates of how deformations changed with subject movement were made, which were subsequently used to minimize movement-related variance.
To correct for the different acquisition times of signals, the signal measured in each slice was shifted relative to the acquisition of the middle slice using sinc interpolation in time. For control subjects, resulting volumes were then normalized to a standard echoplanar image template based on the Montreal Neurological Institute (MNI) reference brain in Talairach space (Talairach and Tournoux, 1988), and resampled to 3 × 3 × 3 mm3 voxels. This normalization process may result in incorrect normalization (and therefore incorrect localization of activations) in brains with abnormal structure. In order to take account of this in our stroke patients, a mask of the lesion was created using MRIcro software (MRIcro, Nottingham University; http://www.psychology.nottingham.ac.uk/staff/cr1/mricro.html). This mask was then incorporated into the normalization step for all patients (Brett et al., 2001). All normalized images were then smoothed with an isotropic 8 mm full-width half-maximum Gaussian kernel to account for intersubject differences and allow valid statistical inference according to Gaussian random field theory (Friston et al., 1995b). The time series in each voxel were high-pass filtered at 1/100 Hz to remove low-frequency confounds, and scaled to a grand mean of 100 over voxels and scans within each session.
Statistical analysis was performed in two stages. In the first stage, using a single-subject fixed effects model, all handgrips were defined as a single-event type, and modelled as delta functions. The orthogonalized first- and second-order expansions of the peak force exerted (expressed as a percentage of MVC) during each handgrip were included as covariates (Buchel et al., 1998). The resulting covariates were convolved with a canonical synthetic haemodynamic response function, and were used in a general linear model (Friston et al., 1995a) together with a single covariate representing the mean (constant) term over scans. The parameter estimates for each covariate resulting from the least mean-squares fit of the model to the data were calculated, and statistical parametric maps of the t statistic, SPM{t}, resulting from linear contrasts of each covariate (Friston et al., 1995a) were generated and stored as separate images for each subject.
In order to address the null hypothesis that there was no difference in the task-related activation pattern in an individual patient compared with the normal population (as represented by the data acquired using the control group during the use of the dominant or non-dominant hand as appropriate for each patient), random effects analyses were performed (Friston et al., 1999). The data for this second stage of analysis comprised two samples: (i) the pooled parameter estimates for the covariate representing the main effects of handgrip across control subjects; and (ii) the parameter estimate for the same covariate in a single patient. Thus, contrast images for each subject were entered into two-sample t tests (control group compared with a single patient). The SPM{t} values were thresholded at P < 0.05, corrected for multiple comparisons across the whole brain.
The second experimental question related to whether a correlation existed between the voxel-wise task-related change in BOLD signal and outcome across subjects. For all group correlation analyses, the images of patients with left-sided infarcts (right-hand weakness) were flipped about the mid-sagittal line, such that all subjects were considered to have right-sided infarcts. This question was addressed in two ways. The first method used a fixed effects model to make inferences about whether such a correlation exists within subgroups of patients with the same cerebral infarct location. The inference drawn from these results applied only to the patients in the analysis. Secondly, we used a random effects model incorporating all patients in order to extend the inference into the population from which our sample was drawn.
In the subgroup analyses, multi-subject fixed effects models were employed, using the same covariates as described for single-subject analysis. We examined for voxels in which there was a linear correlation between recovery scores and the parameter estimates for the covariates representing the main effects of handgrip across subjects. The specific contrast across the appropriate covariates was weighted according to the mean corrected recovery scores. The resulting SPM{t} values were thresholded at P < 0.05, corrected for multiple comparisons across the whole brain.
In the random effects model, we performed a linear regression analysis within SPM99, in which the variables consisted of the contrast images representing the main effect of handgrip for each patient, and a measure of recovery for each patient (mean corrected across the group). For significant voxels, the correlation coefficient for the plot of parameter estimate against recovery for each subject, together with the corresponding P value, was calculated.
All SPM{t} values were transformed to the unit normal Z distribution to create a statistical parametric map (SPM{Z}). All t tests carried out within SPM were one-tailed.
Anatomical identification was carefully performed by superimposing the maxima of activation foci both on the MNI brain and on the normalized structural images of each subject, and labelling with the aid of the atlas of Duvernoy (1991).
Results
Clinical data
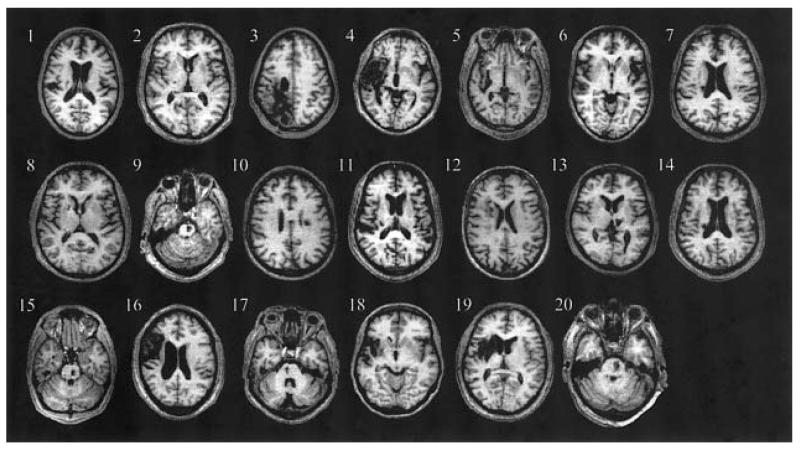
The control group were aged between 21 and 80 years [mean (SD) 47.6 (15.8) years], comprising seventeen male subjects (range 27–80 years, mean (SD) 50.2 (16.5) years] and nine female subjects [range 26–66 years, mean (SD) 44.7 (14.0)]. Twenty stroke patients were recruited [range 28–72 years, mean (SD) 53.2 (14.5)]. Patient characteristics are listed in Table 1. Fourteen patients had experienced left hemiparesis and six right hemiparesis. The site of cerebral infarction was determined from the T1-weighted structural MRI. Eight patients were found to have had infarcts involving the internal capsule, four patients had pontine infarcts (pons), and six patients had infarcts in the middle cerebral artery territory (MCA), specifically in a striatocapsular distribution with extension to the insular cortex (Fig. 1). In addition, one patient had suffered from a thalamic haemorrhage and one from haemorrhagic infarction of the posterior middle cerebral artery territory. No patients had lesions involving the hand representation of the primary motor cortex (M1).
Table 1.
Patient characteristics
| Patient | Age (years) |
Sex | Affected hand |
Site of lesion | Time to scan |
Initial severity* |
Previous medical history |
Medication |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | M | L | R IC | 13 weeks | 3 | NIDDM | Aspirin, simvastatin, gliclazide, metformin |
| 2 | 56 | M | L | R IC | 29 weeks | 4+ | Nil | Aspirin |
| 3 | 39 | M | L | R posterior MCA haemorrhagic infarct |
6 years | 0 | Epilepsy | Sodium valproate |
| 4 | 69 | M | L | R MCA | 3 years | 0 | Hyperthyroidism (treated) |
Aspirin, thyroxine |
| 5 | 49 | M | L | R MCA | 18 weeks | 0 | Hypertension | Aspirin, losartan, bendrofluazide |
| 6 | 29 | F | R | L MCA | 13 weeks | 2(1) | Nil | Aspirin |
| 7 | 58 | M | L | R IC | 25 weeks | 2 | Hypertension | Aspirin, bendrofluazide |
| 8 | 63 | M | L | R IC | 21 weeks | 4+ (4) | Hypertension | Aspirin, atenolol, amlodipine, simvastatin |
| 9 | 71 | M | R | L pons | 28 weeks | 2 | IHD, COPD | Aspirin, glyceryl trinitrate spray |
| 10 | 52 | M | R | L IC | 27 weeks | 4+ | Hypertension | Aspirin, atenolol, ramipril |
| 11 | 72 | M | L | L IC | 3 years | 1 | Mild asthma | Aspirin |
| 12 | 72 | F | L | R IC | 15 weeks | 1 | Hypertension, | Aspirin, bendrofluazide, atorvastatin |
| 13 | 50 | M | R | L thalamic haemorrhage |
27 weeks | 0 | Hypertension, gout |
Atenolol, lisinopril, allopurinol, omeprazole |
| 14 | 60 | M | L | R IC | 14 weeks | 4+ | Hypertension | Aspirin, bendrofluazide |
| 15 | 29 | M | R | L pons | 36 weeks | 0 | Nil | Aspirin, fluoxetine |
| 16 | 46 | M | L | R MCA | 27 weeks | 0 | Hypertension | Aspirin, amlodipine, atenolol, atorvastatin |
| 17 | 61 | M | L | R pons | 14 weeks | 2 (0) | Hypertension | Aspirin, atenolol |
| 18 | 28 | F | L | R MCA | 4 years | 0 | Nil | Aspirin |
| 19 | 38 | M | L | R MCA | 35 weeks | 0 | Nil | Aspirin |
| 20 | 71 | M | R | L pons | 20 weeks | 0 | Hypertension | Aspirin, atenolol |
Initial severity refers to the MRC grade for wrist extension (and finger extension in brackets if different) as recorded in the medical records at the time of stroke. M = male; F = female; R = right; L = left; IC = internal capsule; NIDDM = non-insulin-dependent diabetes mellitus; IHD = ischaemic heart disease; COPD = chronic obstructive airways disease.
Fig. 1.
Axial structural T1-weighted MRI scans at the level of maximum infarct volume for each patient performed at the time of the fMRI.
The degree of functional recovery at the time of scanning was variable, as measured by the selected outcome scores (Table 2). The first principal component of the data set comprising scores for each outcome measure across patients accounted for 81.2% of the variance within this data set, and was taken as the vector of the overall degree of recovery across the group.
Table 2.
Patient outcome scores
| Patient | Barthel | Rankin | OPSS | ARAT | MI (UL) | MI (LL) |
10 m walk |
NHPT | Grip strength |
Ashworth scale |
Sensory loss | Mirror movements |
Associated movements |
Overall outcome score (mean corrected) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (0–20) | (5–0) | (1.6–6.8) | (0–57) | (0–100) | 0–100 | (m/s) | (% contralateral side) | (5–0) | ||||||
| 1 | 20 | 2 | 2 | 51 | 93 | 92 | 0.6 | 66.7 | 75.2 | 0 | No | No | No | 0.06 |
| 2 | 20 | 1 | 1.6 | 57 | 100 | 100 | 1.4 | 75.4 | 102.2 | 0 | Face, hand | No | No | 0.2 |
| 3 | 20 | 3 | 2.4 | 14 | 61 | 59 | 0.8 | 0 | 40.7 | 2 | UL,LL | No | WF | −0.22 |
| 4 | 18 | 3 | 2.4 | 24 | 70 | 100 | 0.9 | 0 | 58.2 | 1 | UL,LL | No | No | −0.12 |
| 5 | 10 | 4 | 3.2 | 30 | 58 | 59 | 0 | 3.7 | 35.5 | 0 | UL,LL | No | No | −0.36 |
| 6 | 20 | 1 | 2 | 57 | 100 | 100 | 1.6 | 78.4 | 90.2 | 1 | No | No | No | 0.18 |
| 7 | 19 | 2 | 2.8 | 38 | 78 | 62 | 0.4 | 64.1 | 77.1 | 3 | No | No | WF,EF | −0.08 |
| 8 | 20 | 1 | 1.6 | 57 | 100 | 100 | 1.5 | 107.3 | 91.2 | 0 | No | No | No | 0.21 |
| 9 | 20 | 1 | 2 | 57 | 100 | 100 | 1.1 | 88.4 | 98.2 | 1 | No | No | No | 0.18 |
| 10 | 20 | 1 | 1.6 | 57 | 100 | 100 | 2.3 | 102.8 | 103 | 0 | No | No | No | 0.26 |
| 11 | 10 | 3 | 2 | 10 | 58 | 59 | 0.5 | 0 | 54.9 | 2 | No | No | WF | −0.28 |
| 12 | 20 | 1 | 1.6 | 57 | 92 | 100 | 1.1 | 55.9 | 57.8 | 0 | Hand | No | No | 0.11 |
| 13 | 20 | 2 | 1.6 | 57 | 92 | 100 | 1.3 | 86.7 | 96.7 | 0 | No | No | No | 0.16 |
| 14 | 20 | 2 | 1.6 | 57 | 92 | 100 | 1 | 75 | 91,5 | 0 | Hand | No | No | 0.13 |
| 15 | 20 | 1 | 1.6 | 57 | 100 | 100 | 1.4 | 80.6 | 98.3 | 0 | No | No | No | 0.2 |
| 16 | 15 | 4 | 3.2 | 14 | 58 | 59 | 0 | 11.7 | 17.8 | 1 | No | No | WF | −0.37 |
| 17 | 16 | 4 | 2.8 | 49 | 77 | 92 | 0 | 86.2 | 54.3 | 3 | No | No | No | −0.11 |
| 18 | 20 | 1 | 2 | 56 | 92 | 100 | 1.2 | 29.1 | 85.6 | 2 | No | No | No | 0.11 |
| 19 | 20 | 1 | 1.6 | 57 | 100 | 100 | 1.5 | 68.1 | 106.9 | 0 | No | No | No | 0.2 |
| 20 | 9 | 4 | 4 | 15 | 48 | 62 | 0 | 0 | 26.8 | 1 | UL, LL | No | WF, EF | −0.45 |
| Contribution of score to overall outcome score (%) |
10.46 | 11.40 | 10.89 | 11.36 | 12.19 | 10.95 | 10.79 | 10.38 | 11.58 | |||||
Minimum to maximum scores (or units) are expressed under each outcome score. ARAT = Action Research Arm Test; MI = motricity index; NHPT = nine-hole peg test; WF = wrist flexion; EF = elbow flexion; UL = upper limb; LL = lower limb.
Behavioural results
All controls and patients were able to perform the task adequately. No subjects displayed mirror movements at bedside observation. When performing the motor paradigm outside the scanner, there was no evidence of mirror movements or surface EMG activity in the biceps, triceps or latissimus dorsi muscles on the side opposite the previously paretic hand. However, a number of patients did exhibit synergistic flexion of the wrist and elbow, as well as shoulder adduction during, rehearsal of the motor paradigm (Table 2). A 100 mm visual analogue scale (VAS) (0 = no effort, 100 = maximum effort) used after scanning suggested no significant differences existed in the perceived effortfulness of the task across subjects (range 10–33, mean 22.2). There was no correlation between the rating for effort and the overall recovery score for each patient (r2 = 0.08, not significant).
Main effects of handgrip: control group
Activations were seen in a network of regions for the main effect of isometric dynamic handgrip compared with rest, which was similar for the dominant and non-dominant hands. The most lateralized activations were in the contralateral sensorimotor cortex and ipsilateral superior cerebellum. Other activations were bilaterally distributed, including the dorsolateral premotor cortex (PMd) and the ventrolateral premotor cortex (PMv), supplementary motor area (SMA and pre-SMA), cingulate motor areas (CMA), inferior parietal cortex and intraparietal sulcus, insula cortex, cerebellar vermis, and both inferior and superior cerebellar hemispheres. Within this network a number of regions were identified in which increases in activation correlated with increasing age, including the left inferior sensorimotor cortex (for either hand), right inferior frontal gyrus and intraparietal sulcus (for either hand), ipsilateral M1, ipsilateral dorsal premotor cortex and contralateral cingulate sulcus.
Comparison of single patients with control group
In the comparison of the main effects of handgrip for single stroke patients compared with the control group, the task-related activation patterns of five patients were indistinguishable from those of the control group (at a threshold of P < 0.05, corrected for multiple comparisons across the whole brain). These patients were more likely to have made a good recovery and, in general, patients with poorer outcome were more likely to activate a number of brain regions over and above those activated in the control group performing the same task (Table 3, Fig. 2). These regions were often bilaterally distributed, involving sensorimotor, premotor, posterior parietal, prefrontal and insular cortices, SMA, CMA and cerebellum. Increased putaminal activation was seen in only four patients and thalamic overactivation in one.
Table 3.
Task-related activity for stroke patients compared with control group
| Patients ranked by outcome score (poorer outcome towards the left, better outcome towards the right) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | 20 | 16 | 5 | 11 | 3 | 17 | 4 | 7 | 1 | 18 | 12 | 14 | 13 | 9 | 6 | 15 | 2 | 19 | 8 | 10 |
| SMC, contralateral | + | + | + | + | + | |||||||||||||||
| SMC, ipsilateral | + | + | + | + | + | + | ||||||||||||||
| PMd, contralateral | + | + | + | + | + | |||||||||||||||
| PMd, ipsilateral | + | + | + | + | + | + | + | + | ||||||||||||
| PMv, contralateral | + | + | ||||||||||||||||||
| PMv, ipsilateral | + | + | + | + | + | + | ||||||||||||||
| CMA, contralateral | + | + | + | + | + | + | ||||||||||||||
| CMA, ipsilateral | + | + | + | |||||||||||||||||
| SMA, contralateral | + | + | + | + | + | |||||||||||||||
| SMA, ipsilateral | + | + | + | + | + | |||||||||||||||
| PPC, contralateral | + | + | + | + | + | + | ||||||||||||||
| PPC, ipsilateral | + | + | + | + | + | + | + | + | + | |||||||||||
| PFC, contralateral | + | + | + | + | + | + | ||||||||||||||
| PFC, ipsilateral | + | + | + | + | + | + | + | + | ||||||||||||
| Insula cortex, contralateral | + | + | + | + | ||||||||||||||||
| Insula cortex, ipsilateral | + | + | + | |||||||||||||||||
| Cerebellum, contralateral | + | + | + | + | + | + | + | + | + | |||||||||||
| Cerebellum, ipsilateral | + | + | + | + | + | + | + | + | + | |||||||||||
| Cerebellar vermis | + | + | + | + | + | + | + | |||||||||||||
| Putamen, contralateral | + | + | + | + | ||||||||||||||||
| Putamen, ipsilateral | + | + | ||||||||||||||||||
| Thalamus, contralateral | + | |||||||||||||||||||
| Thalamus, ipsilateral | + | + | ||||||||||||||||||
The table illustrates the finding that patients with poorer outcome activated more brain regions than patients with better outcome. Each column shows the areas of increased task-related activation for an individual patient compared with controls. Patients (represented by patient number; see Table 1) are ranked by outcome. + = increased activation present in patient compared with control group (voxels significant at P < 0.05, corrected for multiple comparisons across the whole brain volume). PPC = posterior parietal cortex; PFC = prefrontal cortex. SMC = sensorimotor cortex
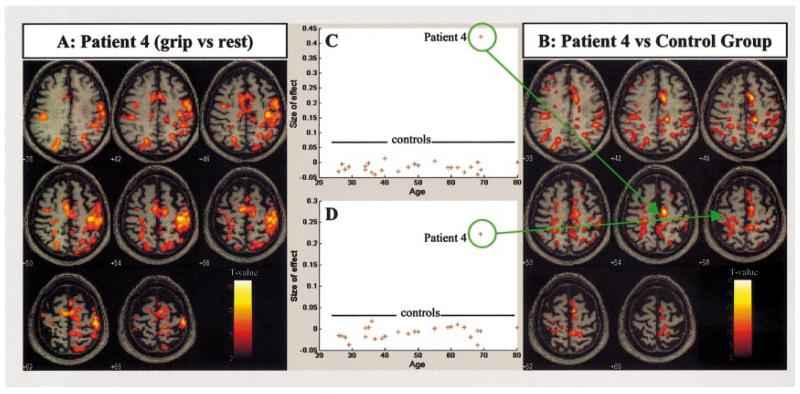
Fig. 2.
Results for a single stroke patient (Patient 4) with only moderate recovery. (A) The main effects of left (affected) handgrip compared with rest and displayed on the patient’s own structural T1-weighted anatomical image in axial section. The front of the brain is upwards and the right side of the brain displayed on the right. (B) The comparison of the main effects of left handgrip for a single subject (Patient 4) versus the control group. The display is as in (A). Plots of the task-related parameter estimates for main effects of left handgrip are shown for each subject in the control group and Patient 4 for (C) the right supplementary motor area (x = 18, y = −12, z = 54), and (D) the ipsilateral (contralesional) deep central sulcus (x = −16, y = −26, z = 54).
Correlation with recovery in stroke subgroups
Patients were grouped according to the site of the infarct: internal capsule (n = 8), MCA (n = 6) and pons (n = 4). The overall vector of outcomes within each subgroup was determined. The first principle components (overall recovery scores) accounted for 78.3% (internal capsule), 80.3% (MCA) and 82.3% (pons) of the variance in each data set.
There were no positive correlations with overall recovery in any of the subgroups. However, there were significant negative correlations with overall recovery across all subgroups (Table 4, Fig. 3). Regions correlating negatively with outcome were distributed bilaterally, in M1, primary somatosensory cortex (S1), PMd, PMv, SMA, pre-SMA, the rostral (RCZ) and caudal (CCZ) cingulate motor areas, posterior parietal cortex, prefrontal regions, superior temporal gyrus and sulcus, insula cortex, middle and superior occipital gyri, cerebellar hemispheres (lobules V, VI, VIIB, VIIIB, CrusI) and vermis. Negative correlations in thalamic activation with recovery were seen only in the MCA group, and in caudate activation only in the pontine group.
Table 4.
Inverse correlation with recovery within stroke subgroups
| Internal capsule (n = 8) |
Middle cerebral artery territory (n = 6) |
Pons (n = 4) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Talairach coordinates in MNI space |
Correlation analysis |
Talairach coordinates in MNI space |
Correlation analysis |
Talairach coordinates in MNI space |
Correlation analysis |
||||||||||||||||
| Region | Side | x | y | z | Z | r 2 | P | Side | x | y | z | Z | r 2 | P | Side | x | y | z | Z | r 2 | P |
| Central sulcus (Ml) |
CL | 42 | −18 | 44 | >8.0 | 0.79 | 0.003 | CL | 30 | −28 | 72 | >8.0 | 0.78 | 0.0l9 | CL | 28 | −28 | 66 | 6.58 | 0.77 | 0.1 |
| IL | −56 | −4 | 40 | >8.0 | 0.79 | 0.003 | CL | 40 | −24 | 50 | >8.0 | 0.71 | 0.034 | IL | −28 | −26 | 64 | 6.85 | 0.75 | 0.1 | |
| IL | −48 | −10 | 50 | 6.77 | 0.53 | 0.041 | IL | −26 | −30 | 60 | 7.33 | 0.91 | 0.003 | IL | −32 | −26 | 56 | >8.0 | 0.77 | 0.1 | |
| IL | −34 | −28 | 52 | 5.73 | 0.6 | 0.025 | IL | −52 | −8 | 48 | 5.95 | 0.72 | 0.033 | ||||||||
| Postcentral gyrus/sulcus (Sl) |
CL | 20 | −44 | 72 | 6.47 | 0.7 | 0.009 | CL | 22 | −38 | 66 | 5.26 | 0.8 | 0.017 | CL | 24 | −46 | 56 | >8.0 | 0.84 | 0.08 |
| IL | −38 | −26 | 48 | 5.75 | 0.79 | 0.018 | CL | 16 | −34 | 72 | 5.66 | 0.88 | 0.06 | ||||||||
| IL | −40 | −16 | 44 | 6.53 | 0.88 | 0.005 | |||||||||||||||
| PMd | CL | 34 | −10 | 64 | >8.0 | 0.84 | 0.001 | CL | 24 | −16 | 74 | >8.0 | 0.54 | 0.049 | CL | 22 | −14 | 56 | 6.85 | 0.8 | 0.1 |
| IL | −38 | −2 | 58 | >8.0 | 0.71 | 0.009 | CL | 24 | 4 | 70 | >8.0 | 0.6 | 0.048 | CL | 20 | −22 | 72 | 5.87 | 0.86 | 0.08 | |
| IL | −26 | −12 | 50 | 5.11 | 0.77 | 0.022 | IL | −12 | −18 | 76 | 6.13 | 0.78 | 0.1 | ||||||||
| PMv | CL IL |
42 −52 |
l0 6 |
18 40 |
7.44 >8.0 |
0.82 0.48 |
0.002 0.049 |
CL | 56 | 0 | 22 | 6.28 | 0.84 | 0.011 | |||||||
| SMA | CL | 12 | −10 | 76 | >8.0 | 0.59 | 0.026 | CL | 2 | −18 | 62 | >8.0 | 0.8 | 0.0l6 | CL | 8 | −18 | 56 | >8.0 | 0.77 | 0.1 |
| IL | −8 | −4 | 74 | 7.47 | 0.71 | 0.008 | IL | −2 | 0 | 64 | >8.0 | 0.64 | 0.046 | IL | −4 | −20 | 62 | >8.0 | 0.81 | 0.1 | |
| M | 0 | −8 | 68 | >8.0 | 0.71 | 0.008 | |||||||||||||||
| Pre-SMA | M | 0 | 2 | 68 | >8.0 | 0.58 | 0.028 | CL | 4 | 22 | 56 | 6.08 | 0.98 | <0.001 | CL IL |
l0 −6 |
26 30 |
58 58 |
6.19 6.72 |
0.77 0.77 |
0.1 0.1 |
| CCZ | CL IL |
10 −2 |
−6 −l2 |
48 48 |
>8.0 6.14 |
0.85 0.56 |
0.001 0.032 |
CL | 4 | −2 | 46 | >8.0 | 0.65 | 0.038 | |||||||
| RCZ | CL IL |
6 −10 |
l4 26 |
34 36 |
5.27 6.5 |
0.58 0.79 |
0.027 0.003 |
||||||||||||||
| M | 0 | 6 | 44 | 6.82 | 0.67 | 0.012 | M | 0 | 18 | 36 | >8.0 | 0.67 | 0.05 | ||||||||
| Superior parietal cortex |
CL | 24 | −76 | 52 | 6.56 | 0.62 | 0.019 | ||||||||||||||
| IL | −14 | −70 | 56 | 6.82 | 0.62 | 0.02 | |||||||||||||||
| Inferior parietal cortex |
CL | 66 | −20 | 20 | 7.73 | 0.81 | 0.002 | CL | 64 | −30 | 28 | >8.0 | 0.65 | 0.047 | |||||||
| CL | 58 | −32 | 48 | 7.39 | 0.62 | 0.019 | IL | −62 | −24 | 40 | 5.97 | 0.68 | 0.043 | ||||||||
| CL | 44 | −64 | 50 | 6.02 | 0.51 | 0.048 | |||||||||||||||
| IL | −50 | −62 | 24 | 5.53 | 0.51 | 0.047 | |||||||||||||||
| Intraparietal sulcus |
CL | 32 | −72 | 52 | 6.34 | 0.77 | 0.004 | CL | 34 | −50 | 50 | 6.75 | 0.66 | 0.05 | |||||||
| IL | −24 | −56 | 50 | 5.99 | 0.61 | 0.021 | |||||||||||||||
| Middle frontal gyrus (BA9/46) |
CL | 38 | 40 | 32 | 6.93 | 0.68 | 0.042 | CL | 46 | 30 | 26 | 7.71 | 0.81 | 0.1 | |||||||
| IL | −24 | 36 | 28 | >8.0 | 0.85 | 0.07 | |||||||||||||||
| Inferior frontal gyrus (BA45) |
IL | −46 | 46 | −4 | 7.25 | 0.61 | 0.021 | CL | 50 | 26 | l0 | 6.56 | 0.85 | 0.08 | |||||||
| Inferior frontal gyrus (BA44) |
CL | 46 | 20 | 10 | >8.0 | 0.86 | 0.07 | ||||||||||||||
| Inferior frontal sulcus |
CL | 52 | 16 | −6 | 7.16 | 0.62 | 0.02 | ||||||||||||||
| Superior temporal gyrus |
CL | 64 | −8 | 6 | 7.15 | 0.59 | 0.025 | CL | 50 | 12 | −10 | 7.71 | 0.93 | 0.03 | |||||||
| IL | −62 | −6 | 2 | >8.0 | 0.6 | 0.023 | |||||||||||||||
| Superior temporal sulcus |
CL | 62 | −32 | −6 | 6.38 | 0.77 | 0.004 | CL | 54 | −24 | −6 | 5.96 | 0.95 | 0.001 | |||||||
| IL | −54 | −24 | 0 | 7.08 | 0.73 | 0.007 | IL | −52 | −6 | −2 | 7.76 | 0.66 | 0.05 | ||||||||
| Middle temporal gyrus |
CL | 52 | −54 | 2 | >8.0 | 0.89 | 0.001 | ||||||||||||||
| IL | −60 | −52 | −2 | >8.0 | 0.65 | 0.016 | IL | −42 | 16 | −36 | >8.0 | 0.79 | 0.1 | ||||||||
| Insula cortex | CL | 40 | 20 | −2 | 6.25 | 0.56 | 0.033 | ||||||||||||||
| IL | −48 | 2 | 0 | >8.0 | 0.71 | 0.009 | IL | −26 | 30 | 0 | 7.29 | 0.89 | 0.05 | ||||||||
| Middle occipital gyrus |
CL | 42 | −88 | 2 | 6.9 | 0.88 | 0.001 | CL | 44 | −80 | −4 | 7.8 | 0.76 | 0.025 | CL | 38 | −62 | 4 | 7.18 | 0.95 | 0.02 |
| Cerebellum (CrI) | CL | 14 | −84 | −22 | >8.0 | 0.72 | 0.008 | IL | −38 | −82 | −20 | 5.81 | 0.72 | 0.031 | |||||||
| CL | 44 | −64 | −28 | 7.33 | 0.75 | 0.005 | |||||||||||||||
| IL | −48 | −54 | −40 | 7.38 | 0.78 | 0.004 | |||||||||||||||
| IL | −8 | −86 | −20 | 6.55 | 0.55 | 0.036 | |||||||||||||||
| IL | −28 | −62 | −32 | 6.01 | 0.56 | 0.027 | |||||||||||||||
| Cerebellum (VI) | IL | −28 | −36 | −32 | 6.1 | 0.78 | 0.004 | CL | 16 | −60 | −12 | >8.0 | 0.66 | 0.048 | |||||||
| IL | −26 | −72 | −18 | >8.0 | 0.91 | 0.003 | |||||||||||||||
| Cerebellum (V) | CL | 26 | −30 | −24 | 6.23 | 0.84 | 0.011 | ||||||||||||||
| Cerebellum (VIIB) |
IL | −46 | −58 | −48 | 7.58 | 0.66 | 0.048 | ||||||||||||||
| Cerebellum (VIIIB) |
IL | −24 | −42 | −50 | 7.58 | 0.7 | 0.009 | ||||||||||||||
| Cerebellar vermis |
M | 2 | −62 | −32 | 5.72 | 0.59 | 0.026 | M | −2 | −66 | −20 | >8.0 | 0.97 | <0.001 | |||||||
| M | 4 | −80 | −32 | 7.5 | 0.78 | 0.019 | |||||||||||||||
| Thalamus (ventral posterolateral) |
IL | −16 | −22 | 8 | 6.14 | 0.98 | <0.001 | ||||||||||||||
| Thalamus (mediodorsal) |
CL | 2 | −6 | 8 | 5.13 | 0.76 | 0.024 | ||||||||||||||
| Caudate | CL | 20 | 16 | 14 | 7.15 | 0.88 | 0.06 | ||||||||||||||
Table shows regions in which there was a negative correlation between recovery and task-related activation across stroke subtypes. Coordinates represent voxels significant at P < 0.05, corrected for multiple comparisons across the whole brain volume. The correlation coefficient (r2) and the corresponding P value for the correlation analysis are also given. IL = ipsilateral; CL = contralateral; M = midline; BA = Brodmann area; CCZ = caudal cingulate sulcus; RCZ = rostral cingulate sulcus.
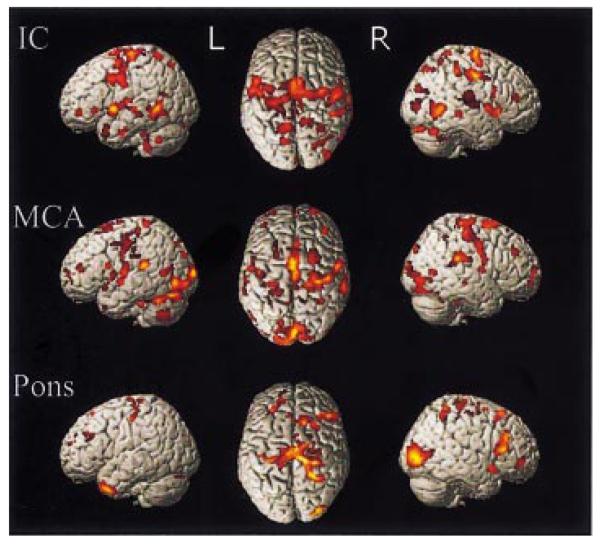
Fig. 3.
SPM{Z}s representing voxels for in which there was a negative (linear) correlation between recovery and task-related BOLD signal within different stroke subtypes. Results are surface-rendered onto a canonical brain. The brain is shown (from left to right) from the left side, from above (left hemisphere on the left) and from the right. All voxels are significant at P < 0.05, corrected for multiple comparisons across the whole brain.
Correlation with recovery across all stroke patients
We performed a regression analysis between task-related activation and an overall outcome score (first principle component, 81.2% of variance), as well as each of the individual outcome scores, across the whole patient group. We found no significant positive correlation with task-related activity. As in the subgroup analyses, there was a significant negative linear correlation with the overall principle components analysis-derived outcome score across patients (Table 5, Figs 4 and 5). Thus, the regions which are increasingly likely to be activated by patients with poorer outcome include the bilateral PMd, cingulate sulcus, and dorsolateral prefrontal cortex, contralateral SMA and pre-SMA, contralateral insular cortex, bilateral (but more extensively contralesional) cerebellar hemispheres and vermis, together with a number of regions in close proximity to the sensorimotor cortex bilaterally. In the contralateral hemisphere the regions showing this negative correlation were in the central sulcus (z coordinates 52 and 40), but in the ipsilateral hemisphere they included not only the central sulcus (z coordinates 30 and a large cluster from z = 44 to z = 68), but also the postcentral gyrus and postcentral sulcus.
Table 5.
Inverse correlation with overall recovery across all patients
| Talairach coordinates in MNI space |
Correlation analysis |
||||||
|---|---|---|---|---|---|---|---|
| Region | Side | x | y | z | Z | r 2 | P |
| Central sulcus | IL | −54 | −8 | 36 | 4.41 | 0.67 | <0.0001 |
| CL | 38 | −26 | 52 | 4.02 | 0.6 | 0.0001 | |
| CL | 52 | −12 | 40 | 4.22 | 0.64 | <0.0001 | |
| Posterior central sulcus | IL | −38 | −24 | 48 | 4.53 | 0.69 | <0.0001 |
| Postcentral gyrus | IL | −22 | −42 | 66 | 4.59 | 0.70 | <0.0001 |
| IL | −62 | −20 | 30 | 4.25 | 0.64 | <0.0001 | |
| Precentral gyrus (BA 4/6) | IL | −18 | −18 | 64 | 4.16 | 0.63 | <0.0001 |
| PMd | IL | −24 | −14 | 52 | 4.21 | 0.64 | <0.0001 |
| CL | 28 | 4 | 64 | 4.43 | 0.67 | <0.0001 | |
| CCZ | CL | 6 | −8 | 52 | 4.98* | 0.76 | <0.0001 |
| IL | −8 | 2 | 62 | 4.73 | 0.72 | <0.0001 | |
| RCZ | IL | −10 | 52 | −4 | 3.89 | 0.58 | 0.0001 |
| SMA | CL | 4 | −12 | 64 | 4.23 | 0.64 | <0.0001 |
| IL | −2 | −2 | 70 | 4.18 | 0.63 | <0.0001 | |
| Pre-SMA | CL | 8 | 20 | 52 | 4.55 | 0.69 | <0.0001 |
| Insula | CL | 44 | 22 | 2 | 4.28 | 0.65 | <0.0001 |
| DLPFC | CL | 34 | 34 | 28 | 4.03 | 0.60 | 0.0001 |
| IL | −34 | 34 | 20 | 4.01 | 0.60 | 0.0001 | |
| Cerebellum (CrI) | IL | −32 | −76 | −24 | 4.98* | 0.76 | <0.0001 |
| Cerebellum (VIIB) | IL | −20 | −66 | −42 | 4.93 | 0.75 | <0.0001 |
| Cerebellum (VI) | IL | −26 | −30 | −32 | 5.11* | 0.77 | <0.0001 |
| CL | 24 | −62 | −20 | 4.65 | 0.71 | <0.0001 | |
| Cerebellar vermis | M | 4 | −62 | −38 | 4.66 | 0.71 | <0.0001 |
The table shows regions in which there was a negative correlation between recovery and task-related activation across all stroke patients. Coordinates represent peak voxels within significant cluster (P < 0.05, corrected for multiple comparisons across the whole brain volume).
Voxels significant at P < 0.05, corrected for multiple comparisons across the whole brain volume. The correlation coefficient (r2) and corresponding P value for the correlation analysis are also given. IL = ipsilateral; CL = contralateral; M = midline; BA = Brodmann area; CCZ = caudal cingulate sulcus; RCZ = rostral cingulate sulcus.
Fig. 4.
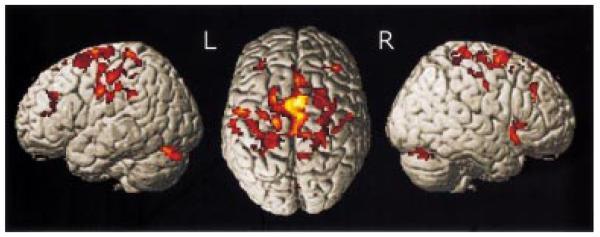
SPM{Z} representing regions in which there was a linear inverse correlation between recovery and task-related BOLD signal across all stroke subtypes (internal capsule infarcts, MCA infarcts, pons infarcts). Results are surface-rendered onto a canonical brain. The brain is shown (from left to right) from the left side, from above (left hemisphere on the left) and from the right. All clusters are significant at P < 0.05, corrected for multiple comparisons across the whole brain.
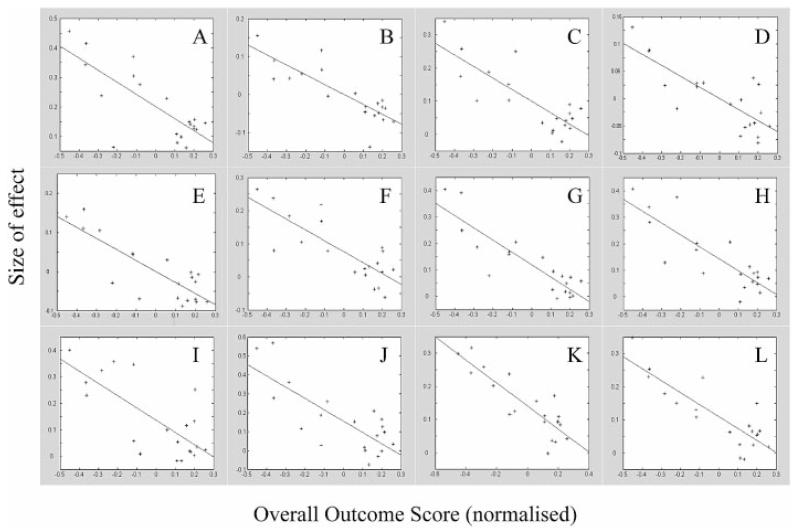
Fig. 5.
Plots of parameter estimates (size of effect) against relative recovery score (normalized) for all stroke patients in different areas of the brain. Correlation coefficient and associated P value for peak voxel in each region (see Table 5) shown. (A) Contralateral M1 (x = 38, y = −26, z = 52) (r2 = 0.60, P = 0.0001). (B) Ipsilateral inferior M1 (r2 = 0.67, P < 0.0001). (C) Contralateral lateral dorsal premotor cortex (r2 = 0.64, P < 0.0001). (D) Ipsilateral lateral dorsal premotor cortex (r2 = 0.67, P < 0.0001). (E) Ipsilateral posterior central sulcus (primary sensory cortex) (r2 = 0.69, P < 0.0001). (F) Contralateral insula cortex (r2 = 0.65, P < 0.0001). (G) ipsilateral cerebellum (VI) (r2 = 0.77, P < 0.0001). (H) Contralateral cerebellum (VI) (r2 = 0.71, P < 0.0001). (I) Contralateral supplementary motor area (r2 = 0.64, P < 0.0001). (J) Ipsilateral supplementary motor area (r2 = 0.63, P < 0.0001). (K) Contralateral caudal cingulate sulcus (r2 = 0.76, P < 0.0001). (L) Ipsilateral caudal cingulate sulcus (r2 = 0.72, P < 0.0001).
There were no significant correlations between task-related activity and the second and third principal components (6.7 and 3.7% of variance, respectively) of the outcome scores across patients.
When examining for the same correlation post hoc using single outcome scores as the independent variable, similar results were obtained (Table 6), particularly with the SMA (bilateral or contralateral), sensorimotor cortex (bilateral or ipsilateral) and cerebellum (bilateral or ipsilateral).
Table 6.
Inverse correlation with individual outcome scores
| Region | Barthel | Rankin | OPSS | ARAT | MI (UL) | MI (LL) | 10m walk | NHPT | Grip |
|---|---|---|---|---|---|---|---|---|---|
| SMC | B | B | IL | IL | B | B | B | B | |
| PMd | CL | IL | CL | IL | IL | ||||
| PMv | |||||||||
| SMA | B | CL | B | B | B | CL | CL | ||
| CMA | CL | B | B | ||||||
| PPC | IL | IL | IL | ||||||
| PFC | IL | B | B | B | |||||
| Insula | CL | CL | |||||||
| Temporal lobe | IL | CL | IL | B | IL | ||||
| V5 | CL | CL | |||||||
| V1 | B | B | B | B | B | ||||
| Cerebellum | B | B | B | B | IL | IL | IL | IL | |
| Vermis | M | M | M | M | |||||
| Putamen | |||||||||
| Thalamus |
A significant negative correlation between task-related activity and each outcome score across patients is indicated for each region (IL = ipsilateral; CL = contralateral; B = bilateral; M = midline) if the cluster is significant at P < 0.05, corrected for multiple comparisons across the whole brain volume. NHPT = nine-hole peg test; SMC = sensorimotor cortex; UL = upper limb; LL = lower limb.
Linear and non-linear correlations between BOLD signal and force of handgrip
The statistical parametric maps derived from the parameter estimates for the first-order (linear) and second-order (non-linear) polynomial expansions of the handgrip force demonstrate voxels in which the BOLD signal exhibited a higher-order relationship to force of handgrip. In the control group, linear increases with either hand were seen in the contralateral sensorimotor cortex, cerebellar vermis, ipsilateral cerebellar hemisphere (inferior cerebellum during dominant handgrip, superior cerebellum for non-dominant handgrip) and ipsilateral ventroposterior lateral thalamus. No second-order relationships were consistently demonstrated with use of the non-dominant hand, but were seen with dominant hand use. Positive second-order effects were seen in the contralateral rostral cingulate sulcus and intraparietal sulcus, ipsilateral superior frontal sulcus, caudal cingulate sulcus and dorsolateral prefrontal cortex, and bilaterally in the insula cortex. A negative second-order effect was seen in a cluster with peak voxel in the right medial orbitofrontal cortex.
No consistent first- or second-order effects in the patient group as a whole could be identified that were different from those in the control group, but there were significant differences within the patient group (Table 7). There was a negative correlation between the first-order increase in BOLD signal, as a function of increasing grip force, and outcome in the contralateral central sulcus (deep), postcentral gyrus, PMd, middle temporal gyrus, ipsilateral cerebellum and bilateral intraparietal sulcus. In other words, these regions were more likely to exhibit a linear increase in activity in response to increasing grip force in patients with poorer outcome. Furthermore, a number of regions were identified in which there was more likely to be a negative second-order relationship (an inverted U shape) between the BOLD signal and increasing handgrip force in patients with poorer recovery. These regions included a number of medial motor areas (SMA and cingulate sulcus), contralateral precentral gyrus [Brodmann area (BA) 4/6], postcentral gyrus, superior frontal sulcus (BA 6/8) and cerebellum (lobule VI), ipsilateral superior temporal sulcus and putamen, and bilateral intraparietal sulcus.
Table 7.
Relationship between task-related brain activation, peak handgrip force and outcome
| Talairach coordinates in MNI space |
|||||
|---|---|---|---|---|---|
| Region | Side | x | y | z | Z |
| (A) Force-related linear increases in BOLD signal in less well-recovered patients | |||||
| Central sulcus (deep) | CL | 32 | −32 | 40 | 3.91 |
| Postcentral gyrus | CL | 58 | −28 | 44 | 3.9 |
| PMd | CL | 22 | −2 | 58 | 4.1 |
| Intraparietal sulcus | CL | 40 | −48 | 42 | 3.57 |
| IL | −34 | −48 | 60 | 4.03 | |
| Middle temporal gyrus | CL | 54 | −68 | 4 | 5.4* |
| Cerebellum (VI) | IL | −12 | −80 | −18 | 4.91 |
| Cerebellar vermis | M | 4 | −84 | −28 | 4.42 |
| (B) Force-related negative second order effects on BOLD signal in less well recovered patients | |||||
| Precentral gyrus (BA 4/6) | CL | 36 | −12 | 46 | 4.91 |
| Postcentral gyrus | CL | 32 | −40 | 64 | 4.11 |
| Superior frontal sulcus (BA 6/8) | CL | 32 | 10 | 58 | 4.99 |
| SMA | CL | 8 | 0 | 66 | 3.76 |
| CCZ | CL | 6 | −6 | 44 | 4.32 |
| Intraparietal sulcus | CL | 36 | −54 | 56 | 3.9 |
| IL | −32 | −38 | 40 | 5.21* | |
| Superior temporal sulcus | IL | −50 | 2 | −22 | 4.01 |
| Putamen | IL | −20 | −8 | 8 | 3.78 |
| Cerebellum (VI) | CL | 26 | −46 | −32 | 4.19 |
The table shows regions in which there was a differential relationship between BOLD signal and peak force exerted during a handgrip in patients with different degrees of recovery. (A) Regions which were more likely to exhibit linear increases in BOLD signal as a function of increasing handgrip force in less well-recovered patients. (B) Regions in which a negative second order (inverted U-shaped) relationship was more likely to be seen in less well-recovered patients. Coordinates represent peak voxels within significant cluster (P < 0.05, corrected for multiple comparisons across the whole brain volume).
Voxels significant at P < 0.05, corrected for multiple comparisons across the whole brain volume. IL = ipsilateral; CL = contralateral; M = midline; BA = Brodmann area; CCZ = caudal cingulate sulcus.
Discussion
We have demonstrated that, in patients who have suffered from hemiparetic stroke, there are significant increases in task-related activation in a number of brain regions over and above that seen in the normal population performing the same task. Furthermore, by carefully measuring a number of outcome variables in each patient, we have demonstrated for the first time the relationship between task-related activations and post-stroke outcome in patients with infarcts that spare M1. Specifically, we found that patients with poorer outcome recruit more regions of the brain during the performance of a standard motor task, often bilaterally, and that there was a significant negative correlation between outcome and task-related brain activations.
Practical considerations
In order to address the key issue of whether differences in motor-related activation in previously hemiparetic patients is related to outcome, we studied patients with a broad range of recovery. In order to be able to do this, we chose handgrip as our motor paradigm, and modulated one aspect of the performance level of this task (i.e. peak force exerted during handgrip) by visual feedback. Because this task involves not only the generation of a motor output but also significant visuomotor control, we hypothesized that a large number of regions would be activated, so that we would be able to examine for differential effects of recovery in a wide network of brain regions. Handgrip is well suited to the study not only of previously paretic patients but also of patients in whom recovery is less than complete. In the natural history of recovery from hemiparesis, the ability to perform handgrip returns relatively early compared with fractionated finger movements (Heller et al., 1987), and compares well with other measures of upper limb function (Heller et al., 1987; Sunderland et al., 1989). Thus, patients with poor outcome were able to perform the task, and we were not reliant on selecting only patients who could perform fractionated finger movements.
However, the network subserving motor performance will be differentially engaged, depending not only on the task used but also on the level of performance. Any differences in functional imaging results detected in patients compared with normals, or indeed across patients with different degrees of recovery, can be attributed to either differences in neuronal or cognitive reorganization (Price and Friston, 1999). In studies in which patients (with impaired motor performance) are asked to perform a motor task with the same absolute parameters (e.g. rate of finger-tapping) as normal controls, patients will find the task more effortful or cognitively complex. Increases in activation in the patient group may then be attributable to neuronal reorganization (often termed plasticity) or increasing effort, and complex motor tasks are known to recruit a wider network of motor regions than simple motor tasks (Catalan et al., 1998). In order to control as much as possible for the degree of effort involved in performing our handgrip task, performance levels, as measured by peak forces exerted, were maintained across all subjects (controls and patients) by asking subjects to perform at a fixed percentage of their own MVC. That this strategy succeeded in controlling for effort across subjects is supported by the results of the VAS ratings for effort completed by each subject.
Nevertheless, there are some aspects of cognitive performance, such as attention, that are very difficult to measure, and the increased activation of some regions (particularly ‘non-motor’ areas) in patients with poor outcome may indeed be due to increased attention to the task. However, we argue that, by performing a motor task (handgrip) that is more reflective of intrinsic motor recovery rather than adaptation (Sunderland et al., 1989) and controlling for motor effort as much as possible, our results within known motor-related regions are more likely to reflect neuronal reorganization.
The second important aspect of our experimental design relates to the way in which the degree of recovery, or outcome, is defined. There are many scores that one could choose which reflect some aspect of outcome, but clearly not all are correlated with one another. If one wants to build an overall picture of the degree of recovery, the consensus view is that a range of measures should be used (Turton and Fraser, 1986; Duncan et al., 2000). Creating a single measure of outcome (at the time of scanning), so that patients can be viewed relative to one another, is difficult, not least because some scales are linear, some non-linear. However, by performing a principle components analysis we can say how much of the variance described by all the outcome scores is attributable to the first, second, third, etc. principal components of the data set. Our overall outcome rating across all patients accounts for 81.3% of the variance across all outcome scores, and for a similar amount across subgroups. We feel, therefore, that our approach provides a useful and robust measure of relative overall recovery or outcome. This is supported by our post hoc analysis, in which regression analyses were performed between task-related activity across the whole group and each individual (normalized) outcome score. There is clearly some variation, but an inverse relationship between activation and outcome is consistently seen in very similar brain regions, particularly the SMA, sensorimotor cortex and cerebellum (Table 6), reflecting correlations between individual scores.
Task-related activations in patients
A wide range of motor-related regions were activated during the task in all patients, as has been seen in other studies (Chollet et al., 1991; Weiller et al., 1992, 1993; Cramer et al., 1997; Cao et al., 1998; Seitz et al., 1998). In our study, patients with poorer recovery were more likely to activate a number of brain regions, including the premotor and parietal cortex, SMA and cerebellum, Furthermore, the relationship between activation in these regions and outcome was linear. In explaining these results, it is useful to consider recent advances in the understanding of the functional neuroanatomy of the premotor (both lateral and medial wall) regions (Strick, 1988; Dum and Strick, 1991, 1996). The notion of a hierarchical motor system, with premotor regions at the top and M1 acting as the common final pathway for the central control of movement, has been challenged by observations made supporting the existence of separate parallel motor networks involving cerebellar and basal ganglia afferents and their projections to cortical motor regions via the ventrolateral thalamus (Strick, 1988). Outputs from deep caudal cerebellar nuclei influence the arcuate (lateral) premotor area, those from deep rostral cerebellar nuclei influence M1, and those from the globus pallidus interna influence the SMA (Strick, 1988). Furthermore, each of these cortical motor regions receives input from different parietal regions. Thus, Strick (1988) proposed that these circuits are parallel and independent, interactions between them occurring at the level of the cortex. Subsequent experiments have indicated that there is a high degree of similarity between the corticospinal projections from the hand regions of M1, SMA and CMA (Dum and Strick, 1996; Rouiller et al., 1996), thus providing the substrate whereby a number of motor networks acting in parallel could generate an output to the spinal cord that is necessary for movement. The implication is that damage in one of these networks could be compensated for by activity in another, thus explaining the recruitment of these regions seen in recovered stroke patients.
However, our new findings indicate that bilateral recruitment of such regions occurs in stroke patients with poor outcome, not in those with good outcome. Recent work has demonstrated that the projections from M1 to the motoneurons in the spinal cord are more numerous and exert a greater excitatory effect than those from SMA (Maier et al., 2002), suggesting that, in the situation where SMA projection to spinal cord motoneurons augment or substitute for those from M1, the functional consequences may fall short of fractionated finger movements. Thus, in our patient group, in those with return of fractionated finger movements the cortico-motoneuronal input from M1 is presumably preserved, hence a ‘normal’ activation pattern, as input from other motor circuits is less important or suppressed. But in those in whom the M1 cortico-motoneuronal input is lost or impaired, there is greater reliance on other parallel motor circuits to generate an alternative input to the spinal motoneurons, resulting in increased task-related activation. However, because of the nature of these projections (Maier et al., 2002), functional recovery is incomplete. This hypothesis can be directly addressed using transcranial magnetic stimulation (TMS) in the same patients to examine the integrity of the corticospinal pathway, work that we are currently conducting. Support for the importance of M1 cortico-motoneuronal projections for full recovery comes from the relationship between preserved TMS-induced motor-evoked potentials in the hand and recovery of hand function after stroke (Heald et al., 1993; Cruz et al., 1999; Pennisi et al., 1999).
Task-related brain activation was not different from that in the normal population in six patients with good outcome (Table 3). It might be expected that the initial severity of their motor impairment was mild, or that they had partial internal capsule lesions. But this is not the case. Of the six, four had marked weakness of the wrist and finger extensors (grade 2 or less on the MRC grade of muscle power) at presentation, and three had striatocapsular infarcts. Post hoc analysis found no correlation between task-related activity in the chronic phase and the amount of motor improvement from the time of presentation.
In attempting to reconcile our results with those of previous studies, we suggest that patients in some previously reported studies may not have been fully recovered, as judged by a larger number of outcome measures, such as we used in this study. Indeed, three out of six patients in one study (Chollet et al., 1991) described residual slowness of finger movements. The results in these patients were similar to those of our patients with residual motor deficit. More recent studies have taken account of differential outcomes in the patients studied. Johansen-Berg et al. (2002a) described more bilaterally distributed motor cortex activations in patients with greater impairment, and Feydy et al. (2002) suggested that non-M1 lesioned patients with better recovery seemed more likely to focus task-related brain activations towards a relatively normal pattern over three separate fMRI sessions. Both these sets of results would be consistent with our own findings.
Task-related activity in ipsilateral M1
A great deal of the motor system is represented bilaterally (Tanji et al., 1988). Indeed, in our control group, task-related activations, other than in the contralateral sensor-imotor cortex and ipsilateral superior cerebellum, were largely bilateral. Thus, the recruitment of parallel motor networks involving premotor regions, and their connections with the cerebellum and parietal cortex, are likely to be bilateral, which is confirmed by our results. Recruitment of the contralesional (ipsilateral) M1, however, cannot be explained in this way. Anatomical studies suggest that both direct (corticospinal) and indirect (corticoreticulospinal) pathways from the ipsilateral M1 end in projections to axial and proximal stabilizing muscles rather than hand muscles (Brinkman and Kuypers, 1973; Carr et al., 1994). However, repetitive TMS to M1 results in errors in both complex and simple motor tasks using the ipsilateral hand (Chen et al., 1997), suggesting that the ipsilateral M1 may play a role in the planning and organization of hand movement. Some functional imaging studies have reported activation in the ipsilateral M1 (Cramer et al., 1999), whilst others have observed task-related deactivations in the ipsilateral M1 (Allison et al., 2000). In our control group, we observed only task-related relative deactivation of the ipsilateral M1, particularly in younger subjects, although this relative deactivation diminished with increasing age. In our patient group, activation in the ipsilateral (contralesional) M1, including the hand region, was observed in five less well-recovered patients (Table 3). We did not observe movement or surface EMG activity of the unaffected arm or hand to explain these activations. These results are similar to those in which TMS to the ipsilateral M1 elicited motor-evoked potentials (MEPs) in the affected arm only in stroke patients with poor outcome (Turton et al., 1996; Netz et al., 1997), findings which were thought to call into question the functional role of the ipsilateral M1. Furthermore, in a group of stroke patients shown to activate the ipsilateral M1 during movement of the affected hand, single-pulse TMS to M1 did not affect the performance of simple reaction-time finger movements (Johansen-Berg et al., 2002a). TMS to the ipsilateral PMd, however, did prolong reaction times, suggesting that the ipsilateral PMd, but not M1, is contributing significantly to motor recovery in these patients. Interestingly, however, we observed a negative correlation between task-related activity and recovery not in the hand region of the ipsilateral M1 but caudally in the postcentral gyrus and in the ventral central sulcus (z = 36). Furthermore, this latter region was situated deep in the central sulcus, possibly in the putative area 4p (Geyer et al., 1996). TMS to the motor hotspot for hand muscles would not affect these parts of the sensorimotor cortex. We conclude that parts of the ipsilateral M1 do generate a motor output to the affected hand. However, as with premotor regions, this recruitment occurs only in patients with a significant deficit, possibly as a result of loss of contralateral M1 cortico-motoneuronal projections, in whom a dependency on alternative motor projections has developed.
Task-related activity in contralateral M1
Shifts in the peak contralateral (ipsilesional) sensorimotor task-related activations in post-stroke patients have been observed in previous studies (Weiller et al., 1993; Pineiro et al., 2001). All our patients activated the contralateral M1, but only four patients (with poorer outcome) activated the contralateral M1 hand region over and above the control group. This might occur if they were gripping with greater force, but the absolute force exerted was less than in patients with good outcome (since the proportion of MVC used was the same across the group). Across the group, we observed a negative correlation between outcome and task-related activity in two parts of the contralateral M1. The first was situated medial to the hand region, deep in the central sulcus (x = 38, y = −26, z = 52), possibly representing the putative area 4p (Geyer et al., 1996), and the second ventral to the hand region (x = 52, y = −12, z = 40). The more dorsal of these is located within the region activated by the control group, so that handgrip in patients with poorer outcome was associated with greater, rather than new, activation in this region. The more ventral region was beyond that activated by the control group, suggesting a ventral shift in task-related M1 activation in some patients with poorer outcome. A similar enlargement in the motor output zone was observed as a consequence of degeneration of the corticospinal tract (Kew et al., 1994), possibly as a result of loss of recurrent inhibition onto surrounding pyramidal cells (Ghosh and Porter, 1988). Changes in cortical maps as a consequence of lesions to the corticospinal pathway may enable the lesioned brain to take advantage of the considerable redundancy within the somatotopy of M1 [in that a number of combinations of pyramidal cells may produce the same movement (Sanes and Donoghue, 2000)], to generate an output to the spinal cord via an intact portion of the pyramidal tract. One might also speculate that plastic changes in somatotopic representations in the SMA, CMA and premotor regions may occur (as a consequence of the lesion, or driven by a therapeutic intervention), resulting in stronger connections with different regions of M1 (i.e. with alternative representations of the hand region) in order to generate an output to the spinal cord. Some studies using TMS have demonstrated increases in motor cortex representation of hand muscles in stroke patients correlating with the degree of clinical improvement after a period of rehabilitation (Traversa et al., 1997). This finding is similar to the therapy-driven increases in premotor cortex activation (as demonstrated with fMRI), also associated with clinical improvement in stroke patients (Johansen-Berg et al., 2002b). One might speculate that these changes occurring within subjects do indeed facilitate functional improvement. However, a single-subject study using both fMRI and TMS cortical mapping demonstrated an asymmetrical enlargement and posterior shift of the motor representation in the affected hemisphere (Rossini et al., 1998). Although described as having a full motor recovery, this patient still had some weakness in the forearm and may not have been considered fully recovered in our study. Clearly, studies using both techniques in the same subjects together with thorough measures of recovery will be required to understand fully the contribution of each technique to our understanding of the recovery process.
Increases in task-related brain activation as a result of increased attention to a simple motor task have been observed in a number of motor regions, including the SMA, cingulate cortex, insula, postcentral sulcus and deep central sulcus (putative area 4p) (Johansen-Berg and Matthews, 2002). It could be argued, therefore, that our results are largely due to patients with poorer outcome needing to pay more attention to the task. We attempted to control for attention by incorporating visual feedback, to which all subjects were asked to attend. However, it is possible that increased attention to the task is a key strategy in the less well-recovered patient, increasing activation in a number of motor regions and thereby providing a substrate for activity-dependent plastic changes in somatotopic representations within the motor system, and consequently alternative motor pathways. This is very similar to the notion of patients accessing latent motor engrams, described by Luria (1963) and Meyer (1972). Evidence that attention can modulate motor performance can be seen in the effect that attending to a neglected limb has in patients with unilateral neglect (Robertson et al., 1997), and may also explain the beneficial effect that d-amphetamine is said to have on motor function (in the context of motor practice) in both animals and humans (Feeney, 1997).
Effect of lesion site
Patients with MCA infarcts had poorer outcomes overall than the other two groups, but within groups there was significant variability, as reflected by the differential activation patterns. However, there were no significant differences in the correlation analyses between groups. It may be that the numbers of patients in each group were too small to detect any significant differences. It is likely that our stroke patients were relatively homogeneous in terms of the relevant neuroanatomical structures affected by their infarct. None of our subjects had damage to the hand region of M1. A prime determinant of outcome may be the degree to which direct M1 cortico-motoneuronal projections are disrupted, with less recovery and greater recruitment of alternative motor circuits in those with some loss of these projections. One might hypothesize that in a cohort of patients with complete disruption of M1 cortico-motoneuronal projections, recruitment of premotor circuits involving SMA, CMA and the lateral premotor cortex would facilitate recovery, such that a positive correlation between outcome and task-related activation of these regions would be observed. This idea is partially supported by the observation of bilateral motor-related activation in both PMd and SMA, but not M1, in patients with good outcome after middle cerebral artery territory infarcts (Seitz et al., 1998). Furthermore, Feydy et al. (2002) demonstrated persistent compensatory recruitment in the ipsilateral (contralesional) hemisphere over three sessions in patients with infarcts affecting M1, in whom there was moderate to good outcome. In contrast, the findings in patients with non-M1 infarcts (a similar group to our patients) tended to show a focusing back to a relatively normal pattern of task-related brain activation in those with good outcome, but less focusing in those with poorer recovery. These findings would be consistent with our current data.
Alterations in linear and non-linear BOLD responses to increasing force
Using single-cell recordings in macaque monkeys, both linear and non-linear force-related firing rates in cortical neurons have been reported (Evarts et al., 1983). Studies in humans have demonstrated linear force-related responses in the contralateral sensorimotor cortex, caudal cingulate sulcus, SMA and ipsilateral cerebellum with functional imaging (Dettmers et al., 1995), and non-linear responses were observed in our control group. These non-linearities have been interpreted as either recruitment or saturation effects in different populations of neurons within functionally similar cortical regions. Dettmers and colleagues described an altered relationship between the force exerted in a finger press task and regional cerebral blood flow (rCBF) in several stroke patients with incomplete recovery (Dettmers et al., 1997). Ipsilesional sensorimotor cortex activity showed a binomial relationship with force compared with a logarithmic relationship in controls. Furthermore, linear increases in rCBF with increasing force, not seen in controls, were observed in SMA and the parietal cortex. These observations provide further evidence to support a role for alternative motor networks in patients regaining some motor function after stroke. We have extended this observation, and describe differential linear and non-linear force-related responses across the spectrum of recovery in a number of parallel motor networks. Although it is difficult to interpret these results in terms of their relationship to the recovery process, it is clear that after focal damage the motor system adapts not only in terms of what structures are engaged but also in how these structures respond.
Conclusions
We have correlated task-related activation and recovery of function for the first time in a functional imaging study involving stroke patients. We have been able to do this because of detailed measurement of a variety of aspects of functional recovery in each patient. This approach has revealed an inverse relationship between task-related brain activation and outcome in patients with infarcts that spare M1, which may at first seem counter-intuitive. We do not suggest that this relationship is causative. Instead, we suggest that the potential for recovery is limited, at least in part, by the degree of damage to the contralateral M1 cortico-motoneuronal projection to the spinal cord, which is critical for fractionated finger movements. Subsequent recovery is likely to be a function of neuronal reorganization within the remaining motor-related areas to generate alternative input to the spinal motoneurons. In short, greater reorganization occurs in those with greatest need, and in this sense the changes that we have described are likely to be driving the functional gains that have been made in each individual. These results further our understanding of the mechanisms that are likely to underlie functional recovery after stroke, but if we are to understand the dynamic process of neuronal reorganization, patients will have to be studied over time during the period of recovery.
Acknowledgements
We wish to thank Peter Aston and Eric Featherstone (Wellcome Department of Imaging Neuroscience) for the design and programming involved in creating the handgrip manipulandum, and the staff of the Acute Brain Injury Unit and Neurorehabilitation Unit at the National Hospital for Neurology and Neurosurgery, Queen Square, London, for their assistance. N.S.W. and R.S.J.F. are supported by the Wellcome Trust. N.S.W. was supported by the Stroke Association during part of this work. M.M.B.’s chair in stroke medicine is supported by the Reta Lila Weston Trust for Medical Research. A.J.T. holds the Garfield Weston Chair of Clinical Neurology and Neurological Rehabilitation.
Abbreviations
- ADL
activities of daily living
- ARAT
Action Research Arm Test
- BA
Brodmann area
- CMA
cingulate motor areas
- BOLD
blood oxygen level-dependent
- DLPFC
dorsolateral prefrontal cortex
- fMRI
functional magnetic resonance imaging
- M1
primary motor cortex
- MI
motricity index
- MCA
middle cerebral artery
- MNI
Montreal Neurological Institute
- MRI
magnetic resonance imaging
- MVC
maximum voluntary contraction
- OPSS
Orpington Prognostic Stroke Scale
- PMd
dorsal lateral premotor cortex
- PMv
ventral lateral premotor cortex
- S1
primary somatosensory cortex
- SMA
supplementary motor area
- SPM
statistical parametric mapping
- TMS
transcranial magnetic stimulation
- VAS
visual analogue scale
References
- Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC. Functional MRI cerebral activation and deactivation during finger movement. Neurology. 2000;54:135–42. doi: 10.1212/wnl.54.1.135. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–19. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Brinkman J, Kuypers HG. Cerebral control of contralateral and ipsilateral arm, hand and finger movements in the split-brain rhesus monkey. Brain. 1973;96:653–74. doi: 10.1093/brain/96.4.653. [DOI] [PubMed] [Google Scholar]
- Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage. 1998;8:140–8. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Calautti C, Leroy F, Guincestre JY, Baron JC. Dynamics of motor network overactivation after striatocapsular stroke: a longitudinal PET study using a fixed-performance paradigm. Stroke. 2001;32:2534–42. doi: 10.1161/hs1101.097401. [DOI] [PubMed] [Google Scholar]
- Cao Y, D’Olhaberriague L, Vikingstad EM, Levine SR, Welch KM. Pilot study of functional MRI to assess cerebral activation of motor function after poststroke hemiparesis. Stroke. 1998;29:112–22. doi: 10.1161/01.str.29.1.112. [DOI] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Stephens JA. Evidence for bilateral innervation of certain homologous motoneurone pools in man. J Physiol. 1994;475:217–27. doi: 10.1113/jphysiol.1994.sp020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M. The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain. 1998;121:253–64. doi: 10.1093/brain/121.2.253. [DOI] [PubMed] [Google Scholar]
- Chen R, Gerloff C, Hallett M, Cohen LG. Involvement of the ipsilateral motor cortex in finger movements of different complexities. Ann Neurol. 1997;41:247–54. doi: 10.1002/ana.410410216. [DOI] [PubMed] [Google Scholar]
- Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–27. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Finklestein SP, Schaechter JD, Bush G, Rosen BR. Activation of distinct motor cortex regions during ipsilateral and contralateral finger movements. J Neurophysiol. 1999;81:383–7. doi: 10.1152/jn.1999.81.1.383. [DOI] [PubMed] [Google Scholar]
- Cruz Martinez A, Tejada J, Diez Tejedor E. Motor hand recovery after stroke. Prognostic yield of early transcranial magnetic stimulation. Electromyogr Clin Neurophysiol. 1999;39:405–10. [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, et al. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol. 1995;74:802–15. doi: 10.1152/jn.1995.74.2.802. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Stephan KM, Lemon RN, Frackowiak RSJ. Reorganization of the executive motor system after stroke. Cerebrovasc Dis. 1997;7:187–200. [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–89. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16:6513–25. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan PW, Jorgensen HS, Wade DT. Outcome measures in acute stroke trials: a systematic review and some recommendations to improve practice. Stroke. 2000;31:1429–38. doi: 10.1161/01.str.31.6.1429. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy and MRI. Springer-Verlag; New York: 1991. [Google Scholar]
- Evarts EV, Fromm C, Kroller J, Jennings VA. Motor cortex control of finely graded forces. J Neurophysiol. 1983;49:1199–215. doi: 10.1152/jn.1983.49.5.1199. [DOI] [PubMed] [Google Scholar]
- Feeney DM. From laboratory to clinic: noradrenergic enhancement of physical therapy for stroke or trauma patients. Adv Neurol. 1997;73:383–94. [PubMed] [Google Scholar]
- Feydy A, Carlier R, Roby-Brami A, Bussel B, Cazalis F, Pierot L, et al. Longitudinal study of motor recovery after stroke. Recruitment and focusing of brain activation. Stroke. 2002;33:1610–7. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995a;2:189–210. [Google Scholar]
- Friston KJ, Ashburner L, Frith CD, Poline JB, Hether JD, Frackowiak RS. Spatial registration and normalization of images. Hum Brain Mapp. 1995b;3:165–89. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, et al. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–7. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Porter R. Corticocortical synaptic influences on morphologically identified pyramidal neurones in the motor cortex of the monkey. J Physiol. 1988;400:617–29. doi: 10.1113/jphysiol.1988.sp017139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald A, Bates D, Cartlidge NE, French JM, Miller S. Longitudinal study of central motor conduction time following stroke. 2. Central motor conduction measured within 72 h after stroke as a predictor of functional outcome at 12 months. Brain. 1993;116:1371–85. doi: 10.1093/brain/116.6.1371. [DOI] [PubMed] [Google Scholar]
- Heller A, Wade DT, Wood VA, Sunderland A, Hewer RL, Ward E. Arm function after stroke: measurement and recovery over the first three months. J Neurol Neurosurg Psychiatry. 1987;50:714–9. doi: 10.1136/jnnp.50.6.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Matthews P. Attention to movement modulates activity in sensorimotor areas, including primary motor cortex. Exp Brain Res. 2002;142:13–24. doi: 10.1007/s00221-001-0905-8. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews P. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA. 2002a;99:14518–23. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002b;125:2731–42. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- Kew JJ, Brooks DJ, Passingham RE, Rothwell JC, Frackowiak RS, Leigh PN. Cortical function in progressive lower motor neuron disorders and amyotrophic lateral sclerosis: a comparative PET study. Neurology. 1994;44:1101–10. doi: 10.1212/wnl.44.6.1101. [DOI] [PubMed] [Google Scholar]
- Luria AR. Restoration of function after brain injury. Persimmon Press; Oxford: 1963. [Google Scholar]
- Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex. 2002;12:281–96. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–61. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- Meyer DR. Access to engrams. Am Psychol. 1972;27:124–33. doi: 10.1037/h0032688. [DOI] [PubMed] [Google Scholar]
- Nelles G, Cramer SC, Schaechter JD, Kaplan JD, Finklestein SP. Quantitative assessment of mirror movements after stroke. Stroke. 1998;29:1182–7. doi: 10.1161/01.str.29.6.1182. [DOI] [PubMed] [Google Scholar]
- Netz J, Lammers T, Homberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120:1579–86. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–4. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pennisi G, Rapisarda G, Bella R, Calabrese V, Maertens De Noordhout A, Delwaide PJ. Absence of response to early transcranial magnetic stimulation in ischemic stroke patients: prognostic value for hand motor recovery. Stroke. 1999;30:2666–70. doi: 10.1161/01.str.30.12.2666. [DOI] [PubMed] [Google Scholar]
- Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM. Functional MRI detects posterior shifts in primary sensorimotor cortex activation after stroke: evidence of local adaptive reorganization? Stroke. 2001;32:1134–9. doi: 10.1161/01.str.32.5.1134. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Scanning patients with tasks they can perform. Hum Brain Mapp. 1999;8:102–8. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IH, Ridgeway V, Greenfield E, Parr A. Motor recovery after stroke depends on intact sustained attention: a 2-year follow-up study. Neuropsychology. 1997;11:290–5. doi: 10.1037//0894-4105.11.2.290. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Caltagirone C, Castriota-Scanderbeg A, Cicinelli P, Del Gratta C, Demartin M, et al. Hand motor cortical area reorganization in stroke: a study with fMRI, MEG and TCS maps. Neuroreport. 1998;9:2141–6. doi: 10.1097/00001756-199806220-00043. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Moret V, Tanne J, Boussaoud D. Evidence for direct connections between the hand region of the supplementary motor area and cervical motoneurons in the macaque monkey. Eur J Neurosci. 1996;8:1055–9. doi: 10.1111/j.1460-9568.1996.tb01592.x. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Schallert T, Leasure JL, Kolb B. Experience-associated structural events, subependymal cellular proliferative activity, and functional recovery after injury to the central nervous system. J Cereb Blood Flow Metab. 2000;20:1513–28. doi: 10.1097/00004647-200011000-00001. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Hoflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol. 1998;55:1081–8. doi: 10.1001/archneur.55.8.1081. [DOI] [PubMed] [Google Scholar]
- Small SL, Hlustik P, Noll DC, Genovese C, Solodkin A. Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain. 2002;125:1544–57. doi: 10.1093/brain/awf148. [DOI] [PubMed] [Google Scholar]
- Strick PL. Anatomical organization of multiple motor areas in the frontal lobe: implications for recovery of function. Adv Neurol. 1988;47:293–312. [PubMed] [Google Scholar]
- Sunderland A, Tinson D, Bradley L, Hewer RL. Arm function after stroke. An evaluation of grip strength as a measure of recovery and a prognostic indicator. J Neurol Neurosurg Psychiatry. 1989;52:1267–72. doi: 10.1136/jnnp.52.11.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; Stuttgart: 1988. [Google Scholar]
- Tanji J, Okano K, Sato KC. Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. J Neurophysiol. 1988;60:325–43. doi: 10.1152/jn.1988.60.1.325. [DOI] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Bassi A, Rossini PM, Bernardi G. Mapping of motor cortical reorganization after stroke. A brain stimulation study with focal magnetic pulses. Stroke. 1997;2:110–7. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- Turton AJ, Fraser CM. A test battery to measure the recovery of voluntary movement control following stroke. Int Rehabil Med. 1986;8:74–8. doi: 10.3109/03790798609166179. [DOI] [PubMed] [Google Scholar]
- Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–28. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–80. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RSJ. Age related changes in the neural correlates of motor performance. Brain. 2003;126:873–88. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992;31:463–72. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol. 1993;33:181–9. doi: 10.1002/ana.410330208. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited— again. Neuroimage. 1995;2:173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]