Abstract
Gastric inhibitory polypeptide, or GIP, has been postulated as the major enteric hormonal mediator of insulin release. The release of immuno-reactive GIP (IR-GIP) after oral glucose and its role in insulin release was studied in normal men by the glucose clamp technique. In 24 subjects studied with the hyperglycemic clamp, blood glucose was maintained at 125 mg/dl above basal for 2 h via a primed-continuous IV glucose infusion coupled to a servo-controlled negative feedback system. 40 g glucose per m2 surface area was ingested at 60 min, and the blood glucose was maintained at the steady-state hyperglycemic level. Plasma IR-GIP and insulin (IRI) levels were measured throughout the 2-h period. IR-GIP levels changed little when IV glucose alone was given; the mean basal value was 305±34 (SEM) pg/ml. After oral glucose, IR-GIP levels began to rise within 10 min and reached a peak within 40 min of 752±105 pg/ml. Plasma IRI responded initially to the square wave of hyperglycemia in the typical biphasic pattern. After oral glucose, plasma IRI levels rose strikingly above the elevated levels produced by hyperglycemia alone, reaching a peak of 170±15 μU/ml within 45 min. The time course of the rise in IR-GIP and IRI was nearly identical.
To assess whether the maintenance of euglycemia would affect this process, the euglycemic clamp was employed in 11 subjects to maintain basal blood glucose levels during a similar 2-h study. A primed-continuous insulin infusion, with a constant rate of 120 mU/m2 per min was given together with a servo-controlled glucose infusion. This resulted in hyper-insulinemia of ∼300 μU/ml. Glucose was ingested by six subjects at 60 min. Plasma IR-GIP responded to oral glucose similarly to the effect seen in the hyperglycemic studies. No increase in endogenous insulin release was seen despite the increase in IR-GIP when euglycemia was maintained. However, in five of seven subjects given insulin whose blood glucose concentration rose by 20 mg/dl or more after oral glucose, there was an increase in plasma insulin concentration associated with the elevation in IR-GIP. Thus, the effect of glucose-released IR-GIP on insulin secretion is dependent upon the presence of some degree of hyper-glycemia and is not inhibited in the presence of marked hyperinsulinemia.
Full text
PDF


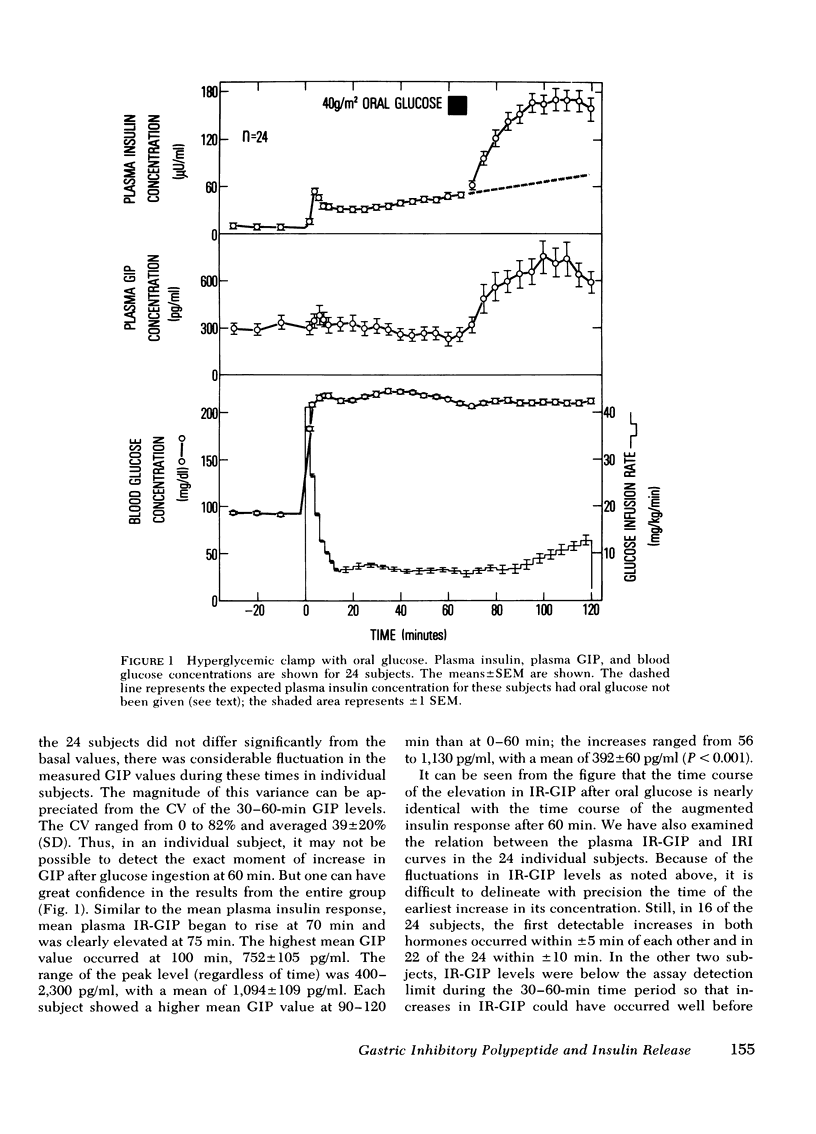
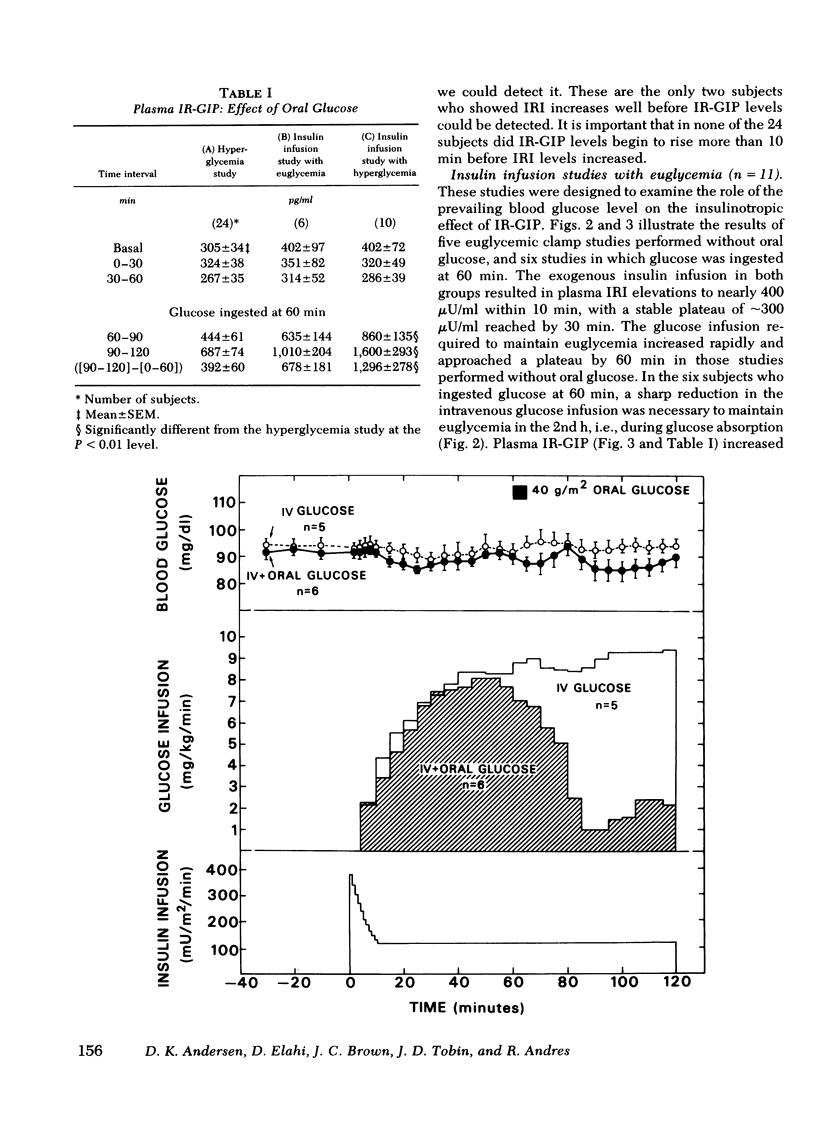
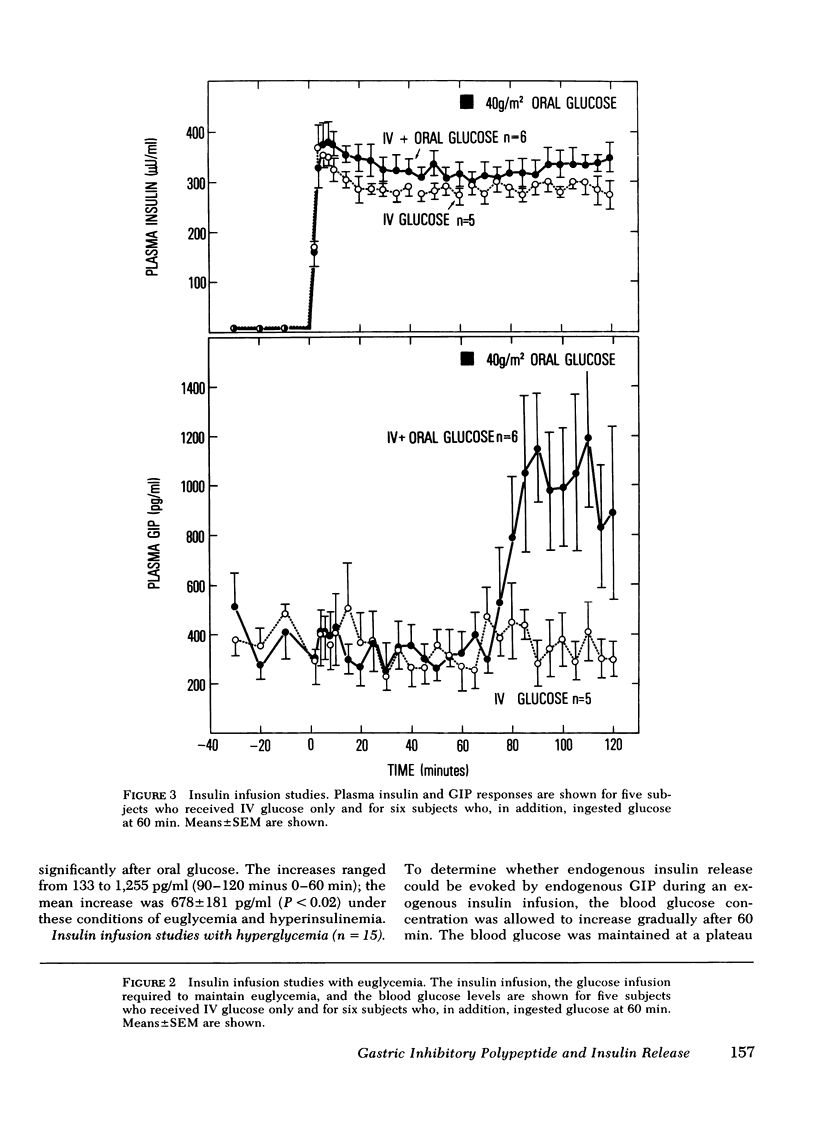
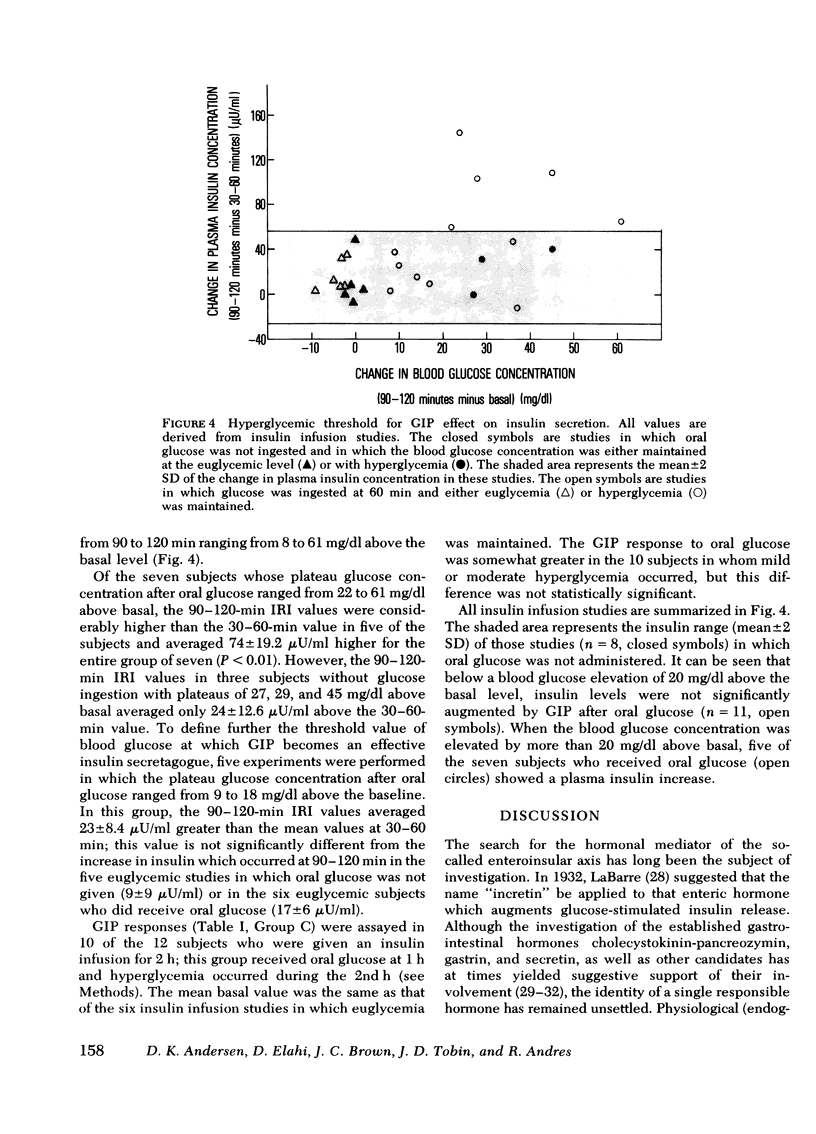


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres R. Aging and diabetes. Med Clin North Am. 1971 Jul;55(4):835–846. doi: 10.1016/s0025-7125(16)32479-8. [DOI] [PubMed] [Google Scholar]
- Botha J. L., Vinik A. I., Brown J. C. Gastric inhibitory polypeptide (GIP) in chronic pancreatitis. J Clin Endocrinol Metab. 1976 May;42(5):791–797. doi: 10.1210/jcem-42-5-791. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Dryburgh J. R., Ross S. A., Dupré J. Identification and actions of gastric inhibitory polypeptide. Recent Prog Horm Res. 1975;31:487–532. doi: 10.1016/b978-0-12-571131-9.50017-7. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Mutt V., Pederson R. A. Further purification of a polypeptide demonstrating enterogastrone activity. J Physiol. 1970 Jul;209(1):57–64. doi: 10.1113/jphysiol.1970.sp009155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. C., Pederson R. A., Jorpes E., Mutt V. Preparation of highly active enterogastrone. Can J Physiol Pharmacol. 1969 Jan;47(1):113–114. doi: 10.1139/y69-020. [DOI] [PubMed] [Google Scholar]
- Buffa R., Polak J. M., Pearse A. G., Solcia E., Grimelius L., Capella C. Identification of the intestinal cell storing gastric inhibitory peptide. Histochemistry. 1975 Jun 5;43(3):249–255. doi: 10.1007/BF00499706. [DOI] [PubMed] [Google Scholar]
- Böttger I., Dobbs R., Faloona G. R., Unger R. H. The effects of triglyceride absorption upon glucagon, insulin, and gut glucagon-like immunoreactivity. J Clin Invest. 1973 Oct;52(10):2532–2541. doi: 10.1172/JCI107444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K. F., Nestel P. J. Effect of long-chain triglyceride on human insulin secretion. Diabetes. 1972 Sep;21(9):923–929. doi: 10.2337/diab.21.9.923. [DOI] [PubMed] [Google Scholar]
- Cataland S., Crockett S. E., Brown J. C., Mazzaferri E. L. Gastric inhibitory polypeptide (GIP) stimulation by oral glucose in man. J Clin Endocrinol Metab. 1974 Aug;39(2):223–228. doi: 10.1210/jcem-39-2-223. [DOI] [PubMed] [Google Scholar]
- Chisholm D. J., Young J. D., Lazarus L. The gastrointestinal stimulus to insulin release. I. Secretin. J Clin Invest. 1969 Aug;48(8):1453–1460. doi: 10.1172/JCI106111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleator I. G., Gourlay R. H. Release of immunoreactive gastric inhibitory polypeptide (IR-GIP) by oral ingestion of food substances. Am J Surg. 1975 Aug;130(2):128–135. doi: 10.1016/0002-9610(75)90360-8. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W., Ebert R., Arnold R., Freichs H., Brown J. C. Gastric inhibitory polypeptide (GIP), gastrin and insulin: response to test meal in coeliac disease and after duodeno-pancreatectomy. Diabetologia. 1976 Jul;12(3):279–286. doi: 10.1007/BF00422096. [DOI] [PubMed] [Google Scholar]
- Crockett S. E., Cataland S., Falko J. M., Mazzaferri E. L. The insulinotropic effect of endogenous gastric inhibitory polypeptide in normal subjects. J Clin Endocrinol Metab. 1976 Jun;42(6):1098–1103. doi: 10.1210/jcem-42-6-1098. [DOI] [PubMed] [Google Scholar]
- Crockett S. E., Mazzaferri E. L., Cataland S. Gastric inhibitory polypeptide (GIP) in maturity-onset diabetes mellitus. Diabetes. 1976 Oct;25(10):931–935. doi: 10.2337/diab.25.10.931. [DOI] [PubMed] [Google Scholar]
- Dobbs R., Faloona G. R., Unger R. H. Effect of intravenously administered glucose on glucagon and insulin secretion during fat absorption. Metabolism. 1975 Jan;24(1):69–75. doi: 10.1016/0026-0495(75)90008-6. [DOI] [PubMed] [Google Scholar]
- Dupre J., Ross S. A., Watson D., Brown J. C. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973 Nov;37(5):826–828. doi: 10.1210/jcem-37-5-826. [DOI] [PubMed] [Google Scholar]
- Dupré J., Beck J. C. Stimulation of release of insulin by an extract of intestinal mucosa. Diabetes. 1966 Aug;15(8):555–559. doi: 10.2337/diab.15.8.555. [DOI] [PubMed] [Google Scholar]
- Ebert R., Arnold R., Creutzfeldt W. Lowering of fasting and food stimulated serum immunoreactive gastric inhibitory polypeptide (GIP) by glucagon. Gut. 1977 Feb;18(2):121–127. doi: 10.1136/gut.18.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert R., Creutzfeldt W., Brown J. C., Frerichs H., Arnold R. Response of gastric inhibitory polypeptide (GIP) to test meal in chronic pancreatitis--relationship to endocrine and exocrine insufficiency. Diabetologia. 1976 Dec;12(6):609–612. doi: 10.1007/BF01220638. [DOI] [PubMed] [Google Scholar]
- Falko J. M., Crockett S. E., Cataland S., Mazzaferri E. L. Gastric inhibitory polypeptide (GIP) stimulated by fat ingestion in man. J Clin Endocrinol Metab. 1975 Aug;41(2):260–265. doi: 10.1210/jcem-41-2-260. [DOI] [PubMed] [Google Scholar]
- Gastrointestinal hormones and insulin secretion. Scand J Gastroenterol. 1972;7(4):289–292. doi: 10.3109/00365527209180744. [DOI] [PubMed] [Google Scholar]
- Kuzio M., Dryburgh J. R., Malloy K. M., Brown J. C. Radioimmunoassay for gastric inhibitory polypeptide. Gastroenterology. 1974 Mar;66(3):357–364. [PubMed] [Google Scholar]
- MCINTYRE N., HOLDSWORTH C. D., TURNER D. S. NEW INTERPRETATION OF ORAL GLUCOSE TOLERANCE. Lancet. 1964 Jul 4;2(7349):20–21. doi: 10.1016/s0140-6736(64)90011-x. [DOI] [PubMed] [Google Scholar]
- McGuire E. A., Helderman J. H., Tobin J. D., Andres R., Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976 Oct;41(4):565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- Moore B. On the treatment of Diabetus mellitus by acid extract of Duodenal Mucous Membrane. Biochem J. 1906;1(1):28–38. doi: 10.1042/bj0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dorisio T. M., Cataland S., Stevenson M., Mazzaferri E. L. Gastric inhibitory polypeptide (GIP). Intestinal distribution and stimulation by amino acids and medium-chain triglycerides. Am J Dig Dis. 1976 Sep;21(9):761–765. doi: 10.1007/BF01073027. [DOI] [PubMed] [Google Scholar]
- Ohneda A., Parada E., Eisentraut A. M., Unger R. H. Characterization of response of circulating glucagon to intraduodenal and intravenous administration of amino acids. J Clin Invest. 1968 Oct;47(10):2305–2322. doi: 10.1172/JCI105916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson R. A., Brown J. C. The insulinotropic action of gastric inhibitory polypeptide in the perfused isolated rat pancreas. Endocrinology. 1976 Sep;99(3):780–785. doi: 10.1210/endo-99-3-780. [DOI] [PubMed] [Google Scholar]
- Pederson R. A., Schubert H. E., Brown J. C. The insulinotropic action of gastric inhibitory polypeptide. Can J Physiol Pharmacol. 1975 Apr;53(2):217–223. doi: 10.1139/y75-032. [DOI] [PubMed] [Google Scholar]
- Perley M. J., Kipnis D. M. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest. 1967 Dec;46(12):1954–1962. doi: 10.1172/JCI105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi-Sunyer F. X., Hashim S. A., Van Itallie T. B. Insulin and ketone responses to ingestion of medium and long-chain triglycerides in man. Diabetes. 1969 Feb;18(2):96–100. doi: 10.2337/diab.18.2.96. [DOI] [PubMed] [Google Scholar]
- Ross S. A., Brown J. C., Dupré J. Hypersecretion of gastric inhibitory polypeptide following oral glucose in diabetes mellitus. Diabetes. 1977 Jun;26(6):525–529. doi: 10.2337/diab.26.6.525. [DOI] [PubMed] [Google Scholar]
- Schauder P., Brown J. C., Frerichs H., Creutzfeldt W. Gastric inhibitory polypeptide: effect on glucose-induced insulin release from isolated rat pancreatic islets in vitro. Diabetologia. 1975 Oct;11(5):483–484. doi: 10.1007/BF00429919. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Kramer K. J., Tobin J. D., Insel P. A., Liljenquist J. E., Berman M., Andres R. A model of the kinetics of insulin in man. J Clin Invest. 1974 May;53(5):1481–1492. doi: 10.1172/JCI107697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F. B., Shook D. F., O'Dorisio T. M., Cataland S., Mekhjian H. S., Caldwell J. H., Mazzaferri E. L. Localization of gastric inhibitory polypeptide release by intestinal glucose perfusion in man. Gastroenterology. 1977 Jan;72(1):49–54. [PubMed] [Google Scholar]
- Turner D. S., Shabaan A., Etheridge L., Marks V. The effect of an intestinal polypeptide fraction on insulin release in the rat in vitro and in vivo. Endocrinology. 1973 Dec;93(6):1323–1328. doi: 10.1210/endo-93-6-1323. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Ketterer H., Dupré J., Eisentraut A. M. The effects of secretin, pancreozymin, and gastrin on insulin and glucagon secretion in anesthetized dogs. J Clin Invest. 1967 Apr;46(4):630–645. doi: 10.1172/JCI105565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Ohneda A., Valverde I., Eisentraut A. M., Exton J. Characterization of the responses of circulating glucagon-like immunoreactivity to intraduodenal and intravenous administration of glucose. J Clin Invest. 1968 Jan;47(1):48–65. doi: 10.1172/JCI105714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]



