Abstract
Current neurocognitive models of attention-deficit/hyperactivity disorder (ADHD) suggest that neural circuits involving both attentional and affective processing make independent contributions to the phenomenology of the disorder. However, a clear dissociation of attentional and affective circuits and their behavioral correlates has yet to be shown in medication-naïve children with ADHD. Using resting-state functional connectivity MRI (rs-fcMRI) in a cohort of medication naïve children with (N=22) and without (N=20) ADHD, we demonstrate that children with ADHD have reduced connectivity in two neural circuits: one underlying executive attention (EA) and the other emotional regulation (ER). We also demonstrate a double dissociation between these two neural circuits and their behavioral correlates such that reduced connectivity in the EA circuit correlates with executive attention deficits but not with emotional lability, while on the other hand, reduced connectivity in the ER circuit correlates with emotional lability but not with executive attention deficits. These findings suggest potential avenues for future research such as examining treatment effects on these two neural circuits as well as the potential prognostic and developmental significance of disturbances in one circuit vs the other.
Keywords: ADHD, Functional Connectivity, Emotional Lability, Attention, Prefrontal Cortex
1. INTRODUCTION
A common neuropsychiatric disorder affecting between 3 and 8% of school-age children, Attention-Deficit/Hyperactivity Disorder (ADHD) is associated with significant negative long-term outcomes, including poor academic performance, substance abuse, incarceration, and underemployment (Barkley 2002; Biederman et al 2008). Several neurocognitive models for the pathogenesis of ADHD have been proposed, suggesting variable deficits in behavioral inhibition (Barkley 1997), emotional regulation (Posner et al 2011a), and temporal processing (Toplak et al 2006) as well as delays in cortical maturation (Shaw et al 2007). One particular neurocognitive model has gained substantial neuropsychological and neurobiological support: the dual pathway model (Sonuga-Barke 2002; Sonuga-Barke et al 2008). This model posits that the principal affected neural circuit in some children with ADHD underlies executive attention (EA)— a neurocognitive domain that mediates top-down control processes, including the inhibition of behavioral urges, set-shifting, and reorienting of attentional resources (Barkley 1997; Nigg et al 2005). Anatomically, the EA system is subserved by distributed cortical and subcortical networks, including the dorsolateral prefrontal cortex (DLPFC), the dorsal anterior cingulate cortex (dACC), the supramarginal gyrus, the dorsal caudate nucleus, frontal eye fields, and the supplementary motor cortex (Castellanos & Proal 2011; Goldman-Rakic 1995). At the same time, the dual pathway model posits that primary deficits in children with ADHD are not always in executive attention; for others, the principal neurocognitive deficit is in motivation and emotional regulation (ER). The ER system is subserved by frontolimbic circuits, consisting of the subgenual and orbitofrontal cortices, amygdala, hippocampus, and ventral striatum (Cardinal et al 2002). The dual pathway model, moving away from prior hypotheses suggesting that a single core neurocognitive deficit is likely in all children with ADHD, posits that the clinical phenotype of ADHD may be the shared outcome of anomalies in either EA or ER systems or the collective effects of anomalies in both systems.
The dual pathway model has significant support in neuropsychological as well as neuroimaging studies of ADHD. For example, neuropsychological studies of children with ADHD have demonstrated dissociable deficits in executive attention and motivation using neuropsychological tasks such as the Stroop and Stop-Signal Tasks (indexing executive attention) and the Choice Delay and Delay Frustration Tasks (indexing motivational deficits) (Bitsakou et al 2006; Posner et al 2009; Solanto et al 2001; Sonuga-Barke & Taylor 2006; Thorell 2007). A recent study demonstrated that these two domains may be further distinguished in children with ADHD from a third neuropsychological domain – temporal processing (Sonuga-Barke et al 2010). Structural MRI studies of children with ADHD demonstrate volumetric abnormalities in regions of the ER and EA networks, including increased volumes in the hippocampus (Plessen et al 2006) and reduced volumes in the basal ganglia (Aylward et al 1996; Qiu et al 2009). Functional neuroimaging studies of adults and children with ADHD, incorporating within-subject designs, likewise demonstrate abnormal task-related activations in both EA and ER systems (Carmona et al 2011; Posner et al 2011a).
Despite these advances, significant questions about the dual pathway model remain. First, if the model is correct, anomalies in both the EA and ER systems should be independent of one another. It is impossible to determine, without demonstrating such independence, whether an anomaly in one pathway is merely the product of a disturbance in the other pathway (ie, deficits in executive attention could produce deficits in emotional regulation). To date, few MRI studies have conducted the within-group analyses necessary to demonstrate the independence of anomalies in EF and ER circuits. Second, the few MRI studies of that have used within-group analyses to examine the EA and ER circuits have relied on ADHD cohorts with either current or prior psychotropic medication exposure (Carmona et al 2011; Posner et al 2011b; Scheres et al 2007). We aim to test whether dissociable anomalies in the EA and ER circuits are detectable in medication-naïve children with ADHD.
We attempted to address these gaps in the literature by testing the dual pathway model in a cohort of medication-naïve children with ADHD, using resting-state functional connectivity MRI analysis (rs-fcMRI). Rs-fcMRI assesses the temporal coherence of neural activity across disparate brain regions. Brain regions in which neural activity is highly correlated are termed “functionally connected.” Using this technique, we tested two hypotheses. First, as posited by the dual pathway model, we predicted that in children with ADHD, functional connectivity would be abnormal in both EA and ER circuits. Second, we hypothesized that the anomalous connectivity in EA and ER circuits would be independent and that this independence would be demonstrable such that: a) connectivity in the EA and ER systems would not correlated with each other, b) connectivity in the EA system would correlate with behavioral measures of executive attention, but not with measures of emotional lability, and c) connectivity in the ER system would correlate with behavioral measures of emotional lability, but not with measures of executive attention.
2. METHODS
The Institutional Review Board of the New York State Psychiatric Institute (NYSPI) approved the study procedures. Child participants provided informed assent, and a legal guardian provided written informed consent.
2.1 Participants
Our cohort comprised 22 children with ADHD and 20 healthy control participants (HC) between the ages of 7 and 12 years. The ADHD patients fulfilled DSM-IV criteria for ADHD-Combined Type, ADHD-Predominantly Hyperactive, or ADHD-Predominantly Inattentive. Healthy control participants were recruited from a larger epidemiological study being conducted by Columbia University’s Mailman School of Public Health. Healthy control participants had no active DSM-IV Axis I psychiatric disorder and were group matched to the ADHD patients by age and gender (Table 1). In both the ADHD and HC groups, no study participant had prior exposure to psychotropic medication. Diagnoses were made using the parent and child versions of the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS) (Kaufman et al 1996) and confirmed by a board-certified child psychiatrist. Additional exclusion criteria for both groups included: neurological illness or significant head trauma (ie, loss of consciousness > 2 minutes); serious medical problems; and MRI contraindications (e.g., braces).
TABLE 1.
Demographic and Clinical Characteristics of the Sample
| ADHD | Healthy Controls |
Test Statistic (d.f.) |
P value | |
|---|---|---|---|---|
| Age in years | 10.0±1.6 | 10.5±1.4 | t(40)=1.2 | 0.2 |
| Gender | 17 males; 5 females |
15 males; 5 females |
Χ2(1,40)=0.9 | 0.6 |
| Hollingshead Index of Social Position |
26.4±8.8 | 31.2±14.6 | Mann Whitney U(1,40)=98.0 |
0.2 |
| FSIQ | 95.8±17.7 | 99.9±19.0 | t(40)=0.8 | 0.5 |
| Race/Ethnicity | 2 Cau/14 His/6 AA | 12 His/8 AA | Χ2(1,40)=2.4 | 0.3 |
| ADHD Diagnoses | 19 ADHD-C; 3 ADHD-I |
|||
| Comorbid Diagnoses | 6 ODD; 1 SAD |
AA, African American; Cau, Caucasian; His, Hispanic; ADHD-C, Attention Deficit Hyperactivity Disorder- Combined Type; ADHD-I, Attention Deficit Hyperactivity Disorder-Inattentive Type; ODD, Oppositional Defiant Disorder; SAD, Social Anxiety Disorder; FSIQ, Full-scale IQ estimated by the Wechsler Abbreviated Scale of Intelligence (WASI).
Parents completed the DuPaul Barkley ADHD Rating Scale (DuPaul 1991), Conners’ Parent Rating Scales Revised (Conners et al 1998), the Child Behavior Checklist (Achenbach & Rescorla 2001), and the Hollingshead Index of Social Position (Hollingshead 1975). All participants were administered the Edinburgh Handedness Inventory (Oldfield 1971), and the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1999). The participants with ADHD had IQ assessments done within one week of the MRI scan. The HC participants had IQ assessments done as part of the larger epidemiological study from which they were recruited; their IQ assessments were within 2 years of the MRI scan. ADHD and HC participants did not differ significantly in estimated IQ or socioeconomic status (Table 1).
2.2 MRI Pulse Sequences
Images were acquired at the New York State Psychiatric Institute on a GE Signa 3.0T whole body scanner. T1-weighed sagittal localizing images were acquired, followed by a 3D spoiled gradient recall (SPGR) image for coregistration with axial echoplanar images. Axial echoplanar images (TR = 2200 msec, TE = 30 msec, 90° flip angle, receiver bandwidth = 62.5 kHz, single excitation per image, slice thickness = 3.5 mm, no gaps, 24 × 24 cm field of view, 64 × 64 matrix) were obtained to provide an effective resolution of 3.75 × 3.75 × 3.5 mm and whole-brain coverage. For resting-state image acquisition, participants were instructed to remain still with their eyes closed and to let their minds wander freely. Two 5-minute resting-state scans were obtained for each participant.
2.3 Image Processing
Standard image preprocessing methods were used, employing SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/) with the conn_toolbox (http://www.nitrc.org/projects/conn) for functional connectivity analysis. The functional images were slice time and motion corrected, coregistered with a high-resolution anatomical scan, normalized into the Montreal Neurological Institute (MNI) space, resampled at 2 mm3, and smoothed with a Gaussian kernel of 6 mm3 FWHM (Friston et al 1995). Connectivity preprocessing procedures followed the component-based noise correction method described elsewhere (Behzadi et al 2007) to minimize non-neural influences on fMRI signal (a detail description of this approach is provided in Supplemental Text S1). We took several additional steps to minimize the likelihood that the connectivity findings were confounded by subjects’ head motion during scanning (Supplemental Text S2). One ADHD participant was removed from the MRI analyses because of excessive head motion.
Following preprocessing, the resting-state BOLD time series were correlated voxel by voxel for each participant across the complete resting-time series. Fisher z transformation was applied. We generated connectivity maps for each subject with the seed region in the dorsolateral prefrontal cortex (DLPFC; Brodmann area 46) and the ventral striatum (VS) bilaterally. We used masks for each of these 4 regions (right and left DLPFC, and right and left VS) and averaged the fMRI signal intensity across the voxels in each mask. A mask for the DLPFC is provided by the conn_toolbox; for the VS, we created a spherical mask (radius = 4 mm) centered on the stereotactic coordinates (MNI coordinates: Left VS: x, y, z=−9, 9, −8; Right VS: x, y, z=9, 9, −8) delineated in prior rs-fcMRI studies (Di Martino et al 2008; Fitzgerald et al 2011; Harrison et al 2009).
2.4 Hypothesis Testing
To test our hypothesis that children with ADHD vs HC participants would demonstrate abnormal connectivity in EA and ER circuits, we entered each participant’s seed-based connectivity map into a second-level, random effects factorial model using SPM8. We used separate factorial models for each of the 4 seed-based connectivity maps (ie, left DLPFC, right DLPFC, left VS, and right VS). We used the DLPFC seeds to probe the EA circuit and the VS seeds to probe the ER circuit. We treated Group as the single factor with two levels: ADHD and HCs. We anatomically restricted our analyses to the EA and ER networks by masking each of the second-level analyses with the mean connectivity maps generated from the respective seed region (DLPFC for the EA network and VS for the ER network). These network of interest analyses are presented in the results section below. Voxelwise, whole-brain exploratory analyses are presented in the supplemental material (Supplemental Text – S3).
We next examined the relationship between altered EA and ER connectivity and clinical symptoms in the participants with ADHD. We calculated Pearson’s correlations between: (1) DLPFC connectivity and (2) executive attention as measured by the Perseveration T-Score on the Conners’ Continuous Performance Task (CPT) (Conners 2000). A description of the CPT is provided in the Supplemental Text - S4. We did not use the more traditional CPT measure of impulsivity, Commission Errors, because our cohort lacked sufficient variability on this measure to meaningfully test for bivariate correlations. We also calculated Pearson’s correlations between: (1) VS connectivity and (2) symptoms of emotional lability as measured by the Emotional Lability subscale on the Conners’ Parent ADHD Rating Scale (Conners et al 1998). We then tested our hypothesis that there would be a double dissociation in behavioral correlates of EA and ER connectivity. We did this by testing first whether DLPFC connectivity would correlate with symptoms of emotional lability, and, second, by testing whether VS connectivity would correlate with executive attention.
2.5 Potential Confounds
We conducted sensitivity analyses by covarying for a) the presence of a comorbid disorder; b) IQ; and c) ADHD subtype (Supplemental Material S5).
2.6 Statistical Threshold
For the connectivity analyses, we corrected for multiple statistical comparisons using the conjoint requirement of 100 continuous voxels (from the normalized images) with each voxel meeting an alpha level of <0.01. Using Monte Carlo simulations, we calculated that this conjoint requirement yields an effective alpha of <0.01. This correction accounts the additional four statistical tests necessary to examine the four seed regions we used in our connectivity analyses.
3. RESULTS
3.1 Resting-State Connectivity
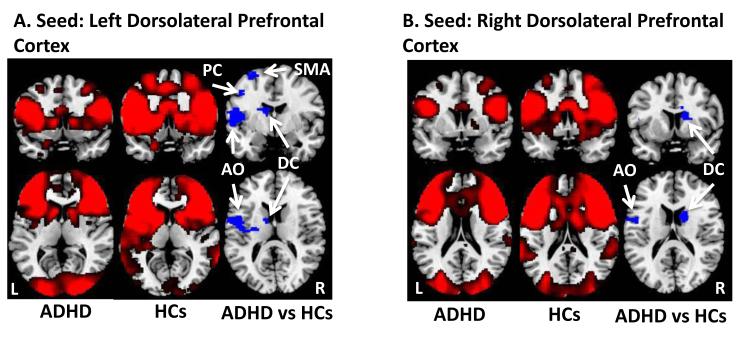
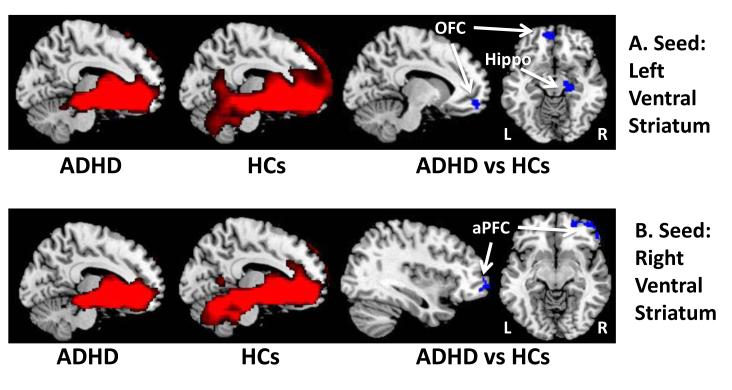
We generated voxel-wise whole-brain maps of resting-state functional connectivity generated from seed regions in the DLPFC and VS bilaterally. With the seed in the DLPFC, resting-state connectivity maps in both groups demonstrated significant connectivity with the dorsal caudate, supramarginal gyrus, dACC, supplemental motor area, and anterior operculum (Figure 1A & 1B). Connectivity maps with the seed in the VS in both groups demonstrated significant connectivity with the anterior prefrontal and orbitofrontal cortices, the amygdala, and the hippocampus (Figure 2).
Figure 1.
Resting-state functional connectivity maps with seeds in the left and right dorsolateral prefrontal cortex. A. Compared to healthy controls, children with ADHD had weaker functional connections between the left dorsolateral prefrontal cortex and the left anterior operculum (AO), left supplemental motor area (SMA), left dorsal caudate (DC), and left precentral gyrus (PC). B. Compared to healthy controls, children with ADHD also had weaker functional connections between right dorsolateral prefrontal cortex and the left anterior operculum (AO), and right dorsal caudate (DC). L, left; R, right
Figure 2.
Resting-state functional connectivity maps with seeds in the left and right ventral striatum. A. Compared to healthy controls, children with ADHD had weaker functional connections between the left ventral striatum and the left orbitofrontal cortex (OFC) and right hippocampus (Hippo). B. Compared to healthy controls, children with ADHD also had weaker functional connections between right ventral striatum and the right anterior prefrontal cortex (aPFC). L, left; R, right
3.2 Hypothesis Testing
Dorsolateral Prefrontal Cortex
Direct comparison of the 2 groups with a seed in the left DLPFC demonstrated that compared to HC participants, participants with ADHD have weaker connections between the left DLPFC and the left anterior operculum, left supplemental motor area, left dorsal caudate, left precentral gyrus, right dorsal anterior cingulate cortex and left supramarginal gyrus (Figure 1A, Table 2). We detected similar findings with the seed in the right DLPFC; compared to HC participants, youths with ADHD have weaker connections between right DLPFC and the left anterior operculum, left inferior frontal gyrus, and the right dorsal caudate (Figure 1B, Table 2). Conversely, compared to HC participants, youths with ADHD have stronger connections between the left DLPFC and the middle occipital gyrus bilaterally and left middle frontal gyrus (Table 2). With the seed in the right DLPFC, we detected similar regions with increased connectivity in participants with ADHD vs HCs (Table 2). Ventral Striatum. With a seed in the left VS, participants with ADHD have weaker connections between the VS and left orbitofrontal cortex and right hippocampus (Figure 2A, Table 2). With a seed in the right VS, we found that compared to HC participants, participants with ADHD have weaker connections between the VS and the right anterior prefrontal cortex (Figure 2B, Table 2).
TABLE 2.
| MNI Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Connection Strength with Seed in the Left Dorsolateral Prefrontal Cortex |
x | y | z | Hemisphere | Brodmann Area |
Cluster Size |
Peak t Value |
| Healthy Controls > ADHD | |||||||
| Anterior Operculum | −54 | 0 | 10 | L | 44 | 1014 | 4.42 |
| Supplemental Motor Area | −24 | −2 | 68 | L | 6 | 360 | 3.83 |
| Dorsal Caudate | −10 | −2 | 18 | L | NA | 155 | 3.78 |
| Precentral Gyrus | −44 | −8 | 44 | L | 4 | 388 | 3.66 |
| Dorsal Anterior Cingulate | 12 | 16 | 42 | R | NA | 158 | 3.50 |
| Supramarginal Gyrus | −56 | −48 | 30 | L | 40 | 216 | 3.11 |
| ADHD>Controls | |||||||
| Middle Occipital Gyrus | 30 | −94 | −4 | R | 19 | 1371 | 4.54 |
| Middle Occipital Gyrus | −24 | −102 | 4 | L | 19 | 1202 | 4.04 |
| Middle Frontal Gyrus | −38 | 52 | 4 | L | 10 | 165 | 3.37 |
|
Connection Strength with Seed in the
Right Dorsolateral Prefrontal Cortex |
|||||||
| Controls> ADHD | |||||||
| Anterior Operculum | −54 | −2 | 12 | L | 44 | 207 | 3.68 |
| Inferior Frontal Gyrus | −24 | 30 | −6 | L | 47 | 167 | 3.42 |
| Caudate, dorsal | 16 | 2 | 20 | R | NA | 265 | 3.10 |
| ADHD>Controls | |||||||
| Middle Occipital Gyrus | −22 | −96 | 0 | L | 19 | 851 | 4.04 |
| Middle Occipital Gyrus | 32 | −92 | −8 | R | 18 | 1188 | 3.65 |
| Middle Frontal Gyrus | −42 | 48 | 2 | L | 10 | 120 | 3.36 |
|
Connection Strength with Seed in the
Left Ventral Striatum |
|||||||
| Controls> ADHD | |||||||
| Hippocampus | 18 | −20 | −12 | R | 35 | 128 | 3.86 |
| Orbitofrontal Gyrus | −6 | 54 | −12 | L | 11 | 183 | 3.41 |
| ADHD>Controls | |||||||
| None | |||||||
|
Connection Strength with Seed in the
Right Ventral Striatum |
|||||||
| Controls> ADHD | |||||||
| Anterior Prefrontal Cortex | 36 | 62 | −4 | R | 11 | 116 | 3.28 |
| ADHD>Controls | |||||||
| None | |||||||
We conducted additional voxel-wise sensitivity analyses of the DLPFC and VS seed maps which included comorbid disorders as a covariate (Table 1). After adding this covariate, the findings remained statistically significant (Supplemental Table 1); however, a consistent pattern emerged indicating that including this covariate reduced the magnitude of the statistical differences between children with ADHD and HC participants (Supplemental Material S3).
3.4 Dissociation of Attentional and Affective Circuits
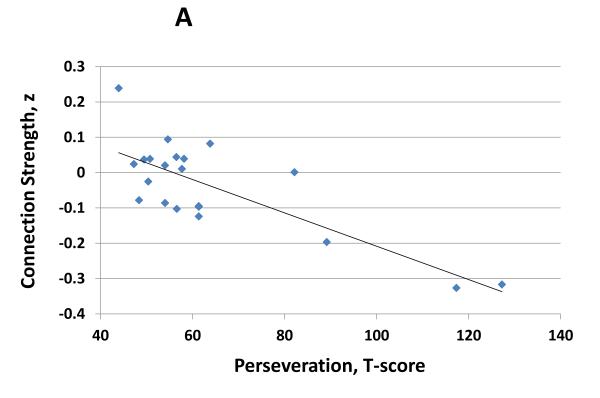
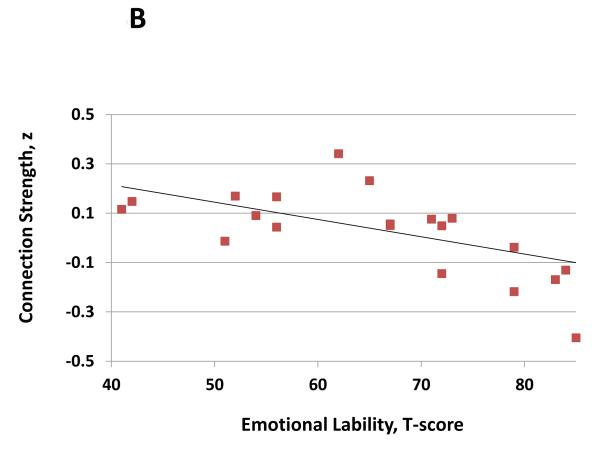
Reduced connectivity between the right DLPFC and right dorsal caudate (x, y, z = −6, 8, 14) was associated with deficits in executive attention as determined by the Perseveration T-Score on the Continuous Performance Task (Figure 3A, r = −0.79, df = 19; P < 0.001). However, the right DLPFC / dorsal caudate connection did not correlate with symptoms of emotional lability (r = 0.12, df = 19; P = 0.6). Conversely, connectivity between the left VS and the left orbitofrontal cortex (OFC; x, y, z = −14, 54, −20) correlated with symptoms of emotional lability (Figure 3B; r = −0.63; df = 19; P = 0.002), but not with deficits in executive attention (r = 0.03, df = 19; P = 0.9). Lastly, right DLPFC / dorsal caudate connectivity was not correlated with left VS / OFC connectivity (r = 0.04, df = 19; P = 0.9).
Figure 3.
A. Connection strength between the right dorsolateral prefrontal cortex and right dorsal caudate (x, y, z = −6, 8, 14) predicted deficits in executive attention in children with ADHD as determined Perseveration T-Score on the Conners’ Continuous Performance Task (r = −0.79, df = 19; p<0.001). B. Connection strength between the left VS and the left orbitofrontal cortex (x, y, z = −14, 54, −20) predicted symptoms of emotional lability in children with ADHD as determined emotional lability subscale on the Conners’ Parent ADHD Rating Form (r = −0.63; df = 19; p = 0.002).
4. DISCUSSION
In this study, we used rs-fcMRI to examine the functional connectivity in EA and ER circuits in medication-naïve children with ADHD. First, we found that children with ADHD have abnormal functional connectivity in both EA and ER circuits. Second, we found a double dissociation in the behavioral correlates of EA and ER circuit connectivity. On the one hand, reduced connectivity in the EA circuit correlated with executive attention deficits but not with emotional lability, while on the other hand, reduced connectivity in the ER circuit correlated with emotional lability but not with executive attention deficits.
The EA circuit is a disturbed system of interacting networks including the ventral and dorsal attentional networks as well as the frontostriatal system. The right lateralized ventral attentional network (VAN) is involved in monitoring the environment for behaviorally salient stimuli (Castellanos & Proal 2011); the dorsal attentional network (DAN) provides top-down control necessary for reorienting attentional resources (Castellanos & Proal 2011), and the frontostriatal system inhibits or facilitates behavioral response based on changing environmental demands (Marsh et al 2009). Anatomically, the VAN is composed of the frontal operculum, supramarginal gyrus, and the temporal-parietal junction (Castellanos & Proal 2011; Fox et al 2006) The DAN consists of intraparietal sulcus and frontal eye fields (Castellanos & Proal 2011; Fox et al 2006). Receiving input from the both the VAN and DAN, the DLPFC integrates attentional cues (Fox et al 2006) and via connections with the frontostriatal system, which consists of the DLPFC, dorsal caudate, putamen, and thalamus, controls behavioral responses (Marsh et al 2009; Posner & Petersen 1990). We found that compared with HC participants, children with ADHD have reduced connection strength between the DLPFC and multiple components of the EA network. Moreover, we found that the connection strength between the DLPFC and dorsal caudate strongly predicted executive attention deficits: weaker connections were associated with more perseverative errors. This is consistent with a central function of the EA circuit – to respond to changing environmental demands by inhibiting unwanted responses and facilitating preferred responses.
The ER circuit is composed of the ventral striatum, the subgenual and orbitofrontal cortices, the amygdala, and the hippocampus (Posner et al 2011a; Posner et al 2011b; Scheres et al 2007; Sheline et al 2010). A prior functional MRI study demonstrated attenuated responses to appetitive stimuli in the ER circuit in adult and adolescent patients with ADHD (Scheres et al 2007). Our findings are consistent with this finding, demonstrating that compared to HC participants, children with ADHD have reduced connectivity between the VS and the orbitofrontal cortex. Moreover, we found an inverse correlation between connectivity in the ER circuit and emotional lability such that emotional lability increased as the connectivity between the VS and left orbitofrontal cortex decreased. Electrophysiological stimulation studies in animals have demonstrated the regulatory role of the orbitofrontal cortex on VS activation (Del Arco & Mora 2008; Montaron et al 1996). We suspect that reduced connectivity between the orbitofrontal cortex and VS may underlie the limitations on the ability of children with ADHD to regulate emotions. Reduced connection strength between the orbitofrontal cortex and VS diminishes the regulatory control that the orbitofrontal cortex has over the VS and this results in heightened emotional lability. Of note, this finding of reduced connectivity in between the orbitofrontal cortex and VS may not be specific to ADHD; rather, it may underlie emotional lability across other diagnostic categories. Such a hypothesis could readily be tested in follow-up studies by including patients with mood and anxiety disorders in addition to children with ADHD.
Of note, in both the EA and ER circuits, we found specific, independent behavioral correlates—that is, reduced connectivity in EA circuits correlated with executive attention deficits but not with emotional lability; and conversely, reduced connectivity in ER circuits correlated with emotional lability but not with executive attention deficits. Demonstrating this double dissociation is critical in testing the dual pathway model and its suggestion that each circuit contributes separately to the clinical phenotype of ADHD. That is, the findings imply that ADHD pathophysiology cannot be subsumed under one discrete neural system or one neurocognitive domain; rather, at least two neurocognitive domains must be considered (Sonuga-Barke 2005).
Several limitations of our study are worth considering. First, whereas we used an objective measure of executive attention (ie, the Continuous Performance Task), no objective measure of emotional lability with established reliability and validity has been developed, so we relied upon a subjective measure (ie, parent report). Follow-up studies, however, could corroborate this subjective measure of emotional lability with existing behavioral measures that assess related neuropsychological domains such as the Delay Frustration and Choice Delay Tasks (Bitsakou et al 2006). A second study limitation is that several of the participants with ADHD had a comorbid oppositional defiance disorder or conduct disorder (ODD/CD). Though the connectivity findings remained statistically significant after controlling for comorbid disorders (Supplemental Table 1), the magnitude of the statistical differences between children with ADHD and healthy controls was reduced. For children with ADHD, an additional diagnosis of ODD/CD is often indicative of more severe clinical impairment. Covarying for the ODD/CD comorbidity may therefore reduce the statistical significance of connectivity findings because this reduces the influence of the most severely affected ADHD children (i.e. the ADHD participants who differ most markedly from healthy control participants). Third, head motion during MRI scanning can distort connectivity measures (Power et al 2011). We found no group differences in head motion during scanning and we took several steps to limit motion as a potential confound (Supplemental Material S2); nonetheless, the confounding effects of head motion cannot be entirely excluded. Fourth, emotional lability is a multidimensional psychological construct and arguably emerges from several component parts including altered arousal and appraisals of emotional stimuli (Gross 1998), as well as deficits in motivation and reward processing. Our study was not designed to parse out the constituent elements that make up emotional lability and thus cannot discern which dimension of emotional lability is most closely associated with reduced connectivity in the ER network. Fifth, we found more widespread areas of reduced connectivity in the EA vs ER network. Arguably, this suggest that children with ADHD have greater dysfunction in the EA vs EA network, yet some caution is needed in this making this interpretation. Proximity to the frontal and maxillary sinuses renders the mesial OFC, a core component of the ER network, susceptible to imaging artifact. This imaging artifact in a portion of the ER network may to some extant account for why the spatial extent of reduced connectivity in the ER network was less than that of the EA network. Finally, our study included a limited number of participants and thus follow-up studies with larger cohorts are needed to establish the stability of the study’s findings.
In conclusion, this is the first rs-fcMRI study to demonstrate neurobiological correlates for the dual pathway model in a cohort of medication-naïve children with ADHD. Our findings suggest that attentional and affective brain circuits each contribute independently to the clinical presentation of ADHD. Future research could explore how treatment affects these two circuits and whether greater disturbances in one circuit vs the other has prognostic significance.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported in part by NIMH grants K23-MH091249 (JP), K02-74677 (BSP), NIEHS grants R01ES015579, R01ES015579-01A2S1 (VR), and NIDA grant R01DA027100 (VR).
Footnotes
Dr. Posner is a principal investigator on an investigator-initiated grant from Shire Pharmaceuticals. The remaining authors have no competing interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Rescorla LA. Manual for ASEBA school-age forms & profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VA: 2001. [Google Scholar]
- Aylward EH, Reiss AL, Reader MJ, Singer HS, Brown JE, Denckla MB. Basal ganglia volumes in children with attention-deficit hyperactivity disorder. Journal of Child Neurology. 1996;11:112–115. doi: 10.1177/088307389601100210. [DOI] [PubMed] [Google Scholar]
- Barkley R. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of adhd. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Major life activity and health outcomes associated with attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry. 2002;63(Suppl 12):10–15. [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (compcor) for bold and perfusion based fmri. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Fried R, Kaiser R, Dolan CR, Schoenfeld S, Doyle AE, Seidman LJ, Faraone SV. Educational and occupational underattainment in adults with attention-deficit/hyperactivity disorder: A controlled study. Journal of Clinical Psychiatry. 2008 doi: 10.4088/jcp.v69n0803. [DOI] [PubMed] [Google Scholar]
- Bitsakou P, Antrop I, Wiersema JR, Sonuga-Barke EJS. Probing the limits of delay intolerance: Preliminary young adult data from the delay frustration task (deft) Journal of Neuroscience Methods. 2006;151:38–44. doi: 10.1016/j.jneumeth.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience & Biobehavioral Reviews. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carmona S, Hoekzema E, Ramos Quiroga JA, Richarte V, Canals C, Bosch R, Rovira M, Carlos Soliva J, Bulbena A, Tobeña A. Response inhibition and reward anticipation in medication naïve adults with attention deficit/hyperactivity disorder: A within subject case control neuroimaging study. Human Brain Mapping. 2011 doi: 10.1002/hbm.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. Large-scale brain systems in adhd: Beyond the prefrontal–striatal model. Trends in Cognitive Sciences. 2011 doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C, Sitarenios G, Parker J, Epstein J. The revised conners’ parent rating scale (cprs-r): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Conners CK. In: The conners continuous performance test. Staff M, editor. Multi-Health Sysems; North Tonawanda, NY: 2000. [Google Scholar]
- Del Arco A, Mora F. Prefrontal cortex–nucleus accumbens interaction: In vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacology Biochemistry and Behavior. 2008;90:226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies D, Kelly A, Uddin L, Shehzad Z, Biswal B, Walters J, Castellanos F, Milham M. Functional connectivity of human striatum: A resting state fmri study. Cerebral Cortex. 2008;18:2735. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ. Parent and teacher ratings of adhd symptoms: Psychometric properties in a community-based sample. Journal of Clinical Child Psychology. 1991;20:245–253. [Google Scholar]
- Fitzgerald KD, Welsh RC, Stern ER, Angstadt M, Hanna GL, Abelson JL, Taylor SF. Developmental alterations of frontal-striatal-thalamic connectivity in obsessive-compulsive disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2011 doi: 10.1016/j.jaac.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner CD, Frith JB, Poline JB, Heather RS, Frackowiak RS. Spatial registration and normalization of images. Human Brain Mapping. 1995;3:165–189. [Google Scholar]
- Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Annals of the New York Academy of Sciences. 1995;769:71–84. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2:271. [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, Lopez-Sola M, Hernandez-Ribas R, Deus J, Alonso P, Yucel M, Pantelis C. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Archives of General Psychiatry. 2009;66:1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four factor index of social status. Yale University; New Haven, CT: 1975. Unpublished manuscript. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Kiddie-sads-present and lifetime version (k-sads-pl) version 1.0. 1996 [Google Scholar]
- Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. American Journal of Psychiatry. 2009;166:664. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaron M, Deniau J, Menetrey A, Glowinski J, Thierry A. Prefrontal cortex inputs of the nucleus accumbens-nigro-thalamic circuit. Neuroscience. 1996;71:371–382. doi: 10.1016/0306-4522(95)00455-6. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: Do we need neuropsychologically impaired subtypes? Biological Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, Martin L, Durkin K, Blair C, Royal J, Hugdahl K, Peterson BS. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63:795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Gorman D, Nagel BJ. Tyrosine supplements for adhd symptoms with comorbid phenylketonuria. Journal of Neuropsychiatry and Clinical Neurosciences. 2009;21:228–230. doi: 10.1176/jnp.2009.21.2.228. [DOI] [PubMed] [Google Scholar]
- Posner J, Maia TV, Fair D, Peterson BS, Sonuga-Barke EJ, Nagel BJ. The attenuation of dysfunctional emotional processing with stimulant medication: An fmri study of adolescents with adhd. Psychiatry Research: Neuroimaging. 2011a doi: 10.1016/j.pscychresns.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Nagel BJ, Maia TV, Mechling A, Oh M, Wang Z, Peterson BS. Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2011b;50:828–837. e823. doi: 10.1016/j.jaac.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity mri networks arise from subject motion. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Crocetti D, Adler M, Mahone EM, Denckla MB, Miller MI, Mostofsky SH. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. The American Journal of Psychiatry. 2009;166:74. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striata hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch J, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport J. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences. 2007;104:19649. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional mri in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences. 2010;107:11020. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, Hechtman L, Hinshaw S, Turkel E. The ecological validity of delay aversion and response inhibition as measures of impulsivity in ad/hd: A supplement to the nimh multimodal treatment study of ad/hd. Journal of Abnormal Child Psychology. 2001;29:215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E, Bitsakou P, Thompson M. Beyond the dual pathway model: Evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:345–355. doi: 10.1016/j.jaac.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in ad/hd—a dual pathway model of behaviour and cognition. Behavioural Brain Research. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: From common simple deficits to multiple developmental pathways. Biological Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Sergeant JA, Nigg J, Willcutt E. Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: Nosologic and diagnostic implications. Child and Adolescent Psychiatric Clinics of North America. 2008;17:367–384. ix. doi: 10.1016/j.chc.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Sonuga Barke E, Taylor E. The effect of delay on hyperactive and non hyperactive children’s response times: A research note. Journal of Child Psychology and Psychiatry. 2006;33:1091–1096. doi: 10.1111/j.1469-7610.1992.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Thorell LB. Do delay aversion and executive function deficits make distinct contributions to the functional impact of adhd symptoms? A study of early academic skill deficits. Journal of Child Psychology and Psychiatry. 2007;48:1061–1070. doi: 10.1111/j.1469-7610.2007.01777.x. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Dockstader C, Tannock R. Temporal information processing in adhd: Findings to date and new methods. Journal of Neuroscience Methods. 2006;151:15–29. doi: 10.1016/j.jneumeth.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.