Abstract
In view of recent findings on the anatomic heterogeneity of rapid vasodilation via estrogen receptor-dependent mechanisms it is obvious that with regard to human physiology and disease much of it is still unknown, and research in this area is urgently needed. This is also important since chronic drug therapy with estrogens in women systemically affects the circulation and may affect certain arterial beds but not others. It is conceivable that the presence of any vascular disease (as was the case for coronary and carotid atherosclerosis in many of the patients in the large RCTs HERS and WHI) is likely to affect vascular responses to estrogens as well, and that any beneficial effects may be attenuated or even completely lost. Further work is required to decipher the mechanisms of vasodilation brought about by estrogens in humans and experimental animals, whether anatomic heterogeneity exists with regard to vascular beds and individual estrogen receptors, and how vascular disease - atherosclerosis in particular - affects responsiveness. Pharmacological tools for newly identified ERs are now available. The hypothesis that disease may modify or even abrogate estrogen-dependent or ER-selective vasodilation should also be tested. Finally, given that certain clinically approved drugs such as SERM or SERDs (thought only to block or downregulate nuclear ERs) actually cause vasodilation through GPER and have been shown in recent clinical studies to provide cardiovascular protection in postmenopausal women, we may have to re-think our current understanding, concepts, and strategies of how to interfere with the increased risk of vascular disease in women with estrogen deficiency or after menopause.
Keywords: estrogen, vasodilation, vascular tone, artery, estrogen receptor, GPER, heterogeneity, anatomic, atherosclerosis, randomized controlled trial
It has been 20 years since we first reported acute, endothelium-independent relaxation of precontracted human coronary arteries to 17β-estradiol, the biologicall most relevant sex steroid [1]. The vasodilating effect showed a heterogeneity being more pronounced in arteries obtained from women than those obtained from men [1]. In 1993, only estrogen receptor α (ERα) was known to exist [2], and we thus speculated that this estrogen receptor might be involved in these acute, “non-genomic” responses occurring within minutes. We also suggested it might also play a role in the acute increases in vascular cyclic nucleotide content observed after short-term exposure to 17β-estradiol [1,3], previously observed in uterine tissue [4]. We concluded that the acute vasodilating effects of endogenous estrogen may contribute to the protection from atherosclerosis [1]. Indeed, women with intact ovarian function are largely protected from atherosclerosis in their premenopausal years, and this protection has been closely linked to ovarian estrogen production. Accordingly, ovariectomy or ovarian dysfunction markedly increases the risk of coronary artery disease [5]. In agreement with our in vitro findings [1], vasodilator effects after intracoronary application of 17β-estradiol have been demonstrated in atherosclerotic coronary arteries of postmenopausal women [6].
Although acute vasodilating effects of estrogens, particularly those of 17β-estradiol, were first described close to 120 years ago [7] and subsequently have also been demonstrated in men [8], the complexity of the mechanisms through which estrogens mediate vasodilation is not fully understood [9,10]. Vascular effects of estrogen have been observed in many parts of the body, including reproductive organs, kidney, skin, heart, brain, and lung [9], and are particularly evident during pregancy [9]. Aside from the phenolic chemical properties of estrogens, which make them potent antioxidants [11], these sex steroids bind to specific targets in the vascular wall [12,13]. Acute effects of estrogens were first observed in the 1960s by Szego and colleagues, showing rapid increases in cyclic nucleotides in uterine cells [4,14]. In 1977, estrogen binding sites in endometrial cells were reported by Szego and Pietras [14], and estrogen binding sites were first detected in vascular endothelial cells by Colburn and Buonassisi [15]. The same group shortly before reported release of cyclic guanosine monophosphate (cGMP) from endothelial cells in response to acetylcholine [16]. Cyclic GMP would later be shown to be a critical mediator of endothelium-dependent relaxation by NO discovered by Robert Furchgott [17,18], which in turn, is also regulated by estrogen [19].
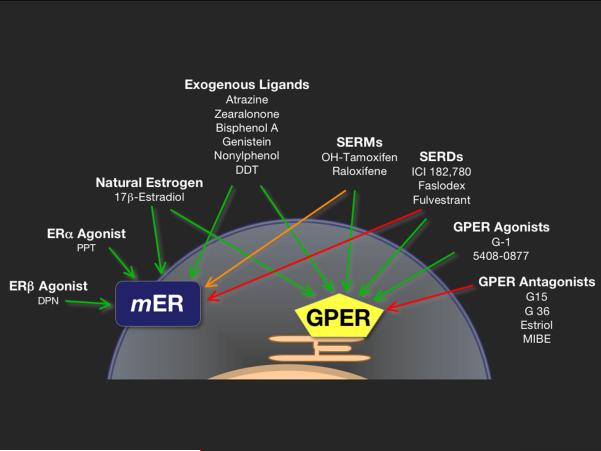
Today, we know that the vasodilating effects of estrogens and 17β-estradiol in particular are mediated by at least three known estrogen receptors, the “classical” nuclear receptors ERα, ERβ, and a membrane G protein-coupled estrogen receptor (GPER, formerly known as GPR30 which was originally cloned from human endothelial cells [20]. Estrogen receptors have been functionally demonstrated close to fifty years ago [21,22], however ERα was cloned only in the mid-1980s, while ERβ and GPER were cloned in the mid-1990s [23]. Research from the past decade has yielded evidence that membrane subpopulations of both ERα and ERβ exist, and that they are involved in rapid responses to estrogens (Fig. 1) [12,13,24,49]. The mechanisms identified so far mediating acute (“non-genomic”) vasodilatory effects of ER activation include direct effects on vascular smooth muscle [25], endothelial cell-derived release of NO, endothelium-dependent hyperpolarization, and modulation of ion channels [26], among others.
Figure 1.
Agonists and antagonists of membrane-associated subpopulations (mER) of ERα and ERβ, as opposed to GPER with intrinsic activity mediating or inhibiting rapid estrogen signaling. Green arrows: activation, red arrows: inhibition, and the orange arrow: tissue-dependent activation or inhibition. Effects can be achieved by natural (endogenous estrogen such as 17β-estradiol) as well as by synthetic drugs (selective agonists for ERα, ERβ, or GPER), SERMS, SERDs, plant-derived substances (genistein), or highly stable environmental pollutants and xenoestrogens (atrazine, zearalonone, bisphenol A, nonylphenol, or DDT). SERM, selective estrogen receptor modulator; SERD, selective estrogen receptor downregulator. Figure modified from Steroids 2012; 77:935-942. M. Barton: Position paper: The membrane estrogen receptor GPER--Clues and questions, with permission of Elsevier Publishers.
In 2007 we published a comparative analyses of vascular estrogen receptor expression in human arteries and veins, as well as vasodilator responses to 17β-estradiol, also describing mRNA expression of GPER in intact human arteries [27]. Nevertheless, studies on vascular estrogen receptor expression and function are scarce, both with regard to the different estrogen receptors as well as to the different vascular beds in which these receptors have been detected [28]. So far, vasodilating effects of estrogens have been described in arteries from females and males, both in humans and in experimental animals [26], but so far no thorough and systematic investigation has been performed – neither with regard to individual estrogen receptors nor regarding different vascular beds.
In the present issue of the Journal, Reslan and colleagues [29] now present results of a comprehensive analysis of the vascular effects of the non-selective estrogen receptor agonist 17β-estradiol and selective estrogen receptor agonists of ERα, ERβ, and GPER, extending and integrating previous studies in rats in which 17β-estradiol or GPER-selective agonists caused vasodilation of precontracted carotid arteries [30], aorta and mesenteric arteries [31,32], and renal arteries [33]. Vasodilating effects of such compounds are also present in murine and human arteries [31]. Using conduit and resistance arteries as well as pulmonary arteries from ovary-intact female rats, the investigators also studied endothelium-dependent, NO-mediated relaxation as well as α1-adrenoceptor-mediated vascular contraction. Finally, Restan et al. also determined protein expression of estrogen receptors in the six different arterial beds, and functionally studied and determined the effects of acute exposure to ER agonists on vascular production of stable metabolites of the endothelial vasodilator NO.
The results presented are remarkable as they allow, for the first time, direct and simultaneous comparison of vascular reactivity as well as acute ER-selective vasodilation between a number of arterial beds, including the pulmonary artery. The data indicate that – except for the mesenteric artery – acetylcholine-mediated relaxation in the ovary-intact rat is strictly NO-dependent in the aorta, carotid artery, renal artery and pulmonary circulation. By contrast, relaxations to 17β-estradiol as a non-selective estrogen receptor agonist at concentrations of or below 1 micromolar are markedly different between these vascular beds causing no relaxation in the carotid and pulmonary artery compared to relaxation in the other four arteries studied. Selective agonists such as 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (PPT, ERα), 2,3-bis(4-hydroxyphenyl) propionitrile (DPN, ERβ), and (±)-1-[(3aR*,4S*,9bS*)-4-(6-Bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]- ethanone (G-1, GPER) were similarly without effect in the pulmonary and carotid artery, while they caused relaxation in the other arteries studied at subnanomolar - i.e. physiological – estrogen concentrations. The findings in the carotid artery contrast data by Broughton et al. [30] and Murata et al. [34] in male and female rats using G-1, where relaxation in response to activation of GPER was reported. The reason(s) for the different results are currently unclear. With regard to any study investigating acute effects to estrogen receptor agonists, however, it should be noted that the vasodilating effects reported for supra-micromolar concentrations shown here are likely to be only of pharmacological but not physiological relevance. In addition, since high concentrations of ER selective agonists such as PPT, DPN and G-1 may lose their receptor selectivity [35], any data obtained at concentration of 10 μmolar and above are likely not due to selective ER subtype activation. For example, the EC50 values of DPN for ERα and ERβ are ~70 nM and 1 nM, respectively, suggesting that at concentrations greater than ~100 nM, selectivity is mostly lost.
This study by Reslan and colleagues confirms our previous observations in porcine epicardial coronary arteries which suggested functional, “inhibitory” ER “cross-talk” [36]. The data presented by Reslan et al., - comparing responses to 17β-estradiol versus the ERα-agonist PPT - , now similarly suggest inhibitory “functional” ER “cross-talk”, since simultaneous activation of all 3 ERs by 17β-estradiol causes less vasodilation than equimolar concentrations of PPT alone - an effect that is particularly evident in the mesenteric and renal arteries. Indeed, cross-talk between estrogen receptors has been demonstrated in non-vascular cells (reviewed in [37]) and is likely to affect molecular and physiological effects of estrogens. Equally surprising was the finding that while 17β-estradiol-mediated vasodilation was fully inhibited by blocking nitric oxide synthase in all 6 vascular beds, individual activation of either ERα, ERβ or GPER alone causes vasodilation that becomes largely NO-independent in the mesenteric and renal artery. this suggests other underlying mechanisms such as endothelium-dependent hyperpolarization or direct, endothelium-independent vasodilator effects on the vascular smooth muscle [1] as well as a possible co-activating effect of all three receptors that involves endothelial nitric oxide synthase.
Why are the data by Reslan et al. important? First, in view of the disappointing results of large RCTs in postmenopausal women such as the Heart Estrogen and Progestin Replacement Study (HERS) or the Women's Health Initiative (WHI) in which hormone mixtures from horse urine with unknown activity on human estrogen receptors were used as “hormone therapy”, the present study again tells us that our understanding of the mechanisms by which natural human estrogens activate estrogen receptors is still scarce - both for human as well as animal physiology. Second, the study by Reslan et al. confirms that it is important whether estrogen receptors are activated selectively or simultaneously [36], which appears to determine the activation of rather specific vasodilatory mechanisms. Third, the study provides evidence that at least in resistance arteries, endothelium-dependent hyperpolarization is an important contributor to ER-mediated vasodilation, previously only shown for ERβ in mouse mesenteric [38] and porcine epicardial coronary arteries [36]. Fourth, this study is the first to characterize responses of unselective and selective ER agonists in the pulmonary artery, a vascular bed that is different from all the other arteries investigated because of its high oxygen tension environment. Finally, the data suggest that certain arterial blood vessels – at least in ovary-intact female rats – may be entirely insensitive to ER-mediated vasodilation.
If one extrapolates these findings to human physiology and disease, it will become apparent that much is still unknown and research in this area is urgently needed [39]. This may be important since chronic drug therapy with estrogens in women systemically affects the circulation and may have effects on certain arterial beds but not others. It is conceivable that the presence of any vascular disease (as was the case for coronary and carotid atherosclerosis in many of the patients in the large RCTs Heart Estrogen and Progestin Replacement Study (HERS) and the Women's Health Initiative (WHI)) is likely to affect vascular responses to estrogens as well, and that any beneficial effects may be attenuated or even completely lost [40]. Further work is required to decipher the mechanisms of vasodilation brought about by estrogens in humans and experimental animals, whether anatomic heterogeneity exists with regard to vascular beds and individual estrogen receptors, and how vascular disease, atherosclerosis in particular, affects responsiveness [39,41], and pharmacologcial tools for newly identified ERs are now available [42–48]. The hypothesis that disease may modify or even abrogate estrogen-dependent or ER-selective vasodilation should also be tested [39,41]. Finally, given that certain clinically approved drugs such as SERM or SERDs (thought only to block or downregulate nuclear ERs) actually cause vasodilation through GPER [26,37] (Figure) and have been shown in recent clinical studies to provide cardiovascular protection in postmenopausal women (reviewed in [40]), we may have to re-think our current understanding, concepts, and strategies of how to interfere with vascular disease in women due to estrogen deficiency.
Acknowledments
Supported by Swiss National Science Foundation (grants 108 258 and 122 504, to M.B., and 135 874 and 141 501 to M.R.M), and the National Institutes of Health (R01 CA127731 and CA163890 to E.R.P.).
References
- 1.Mügge A, Riedel M, Barton M, Kuhn M, Lichtlen PR. Endothelium-independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc Res. 1993;27:1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- 2.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v- erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 3.Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci U S A. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szego CM, Davis JS. Adenosine 3',5'-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci U S A. 1967;58:1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bittner V. Menopause and cardiovascular risk cause or consequence? J Am Coll Cardiol. 2006;47:1984–1986. doi: 10.1016/j.jacc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Rosano GM, Collins P, Gerbara O, Sheiban I, Silvestri A, Wajngarten M, Ramires JA, Fini M, Mercuro G. Effect of estradiol 17beta upon coronary artery vasoconstrictor response to methylergometrine maleate in female menopausal patients. Int J Cardiol. 2006;107:254–259. doi: 10.1016/j.ijcard.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 7.MacKenzie JN. Irritation of the sexual apparatus. Am J Med Sci. 1884;87:360. [Google Scholar]
- 8.Reynolds SRM, Foster FL. Vascular action of estrogen in the human male. J Clin Invest. 1939;18:649–655. doi: 10.1172/JCI101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54:11–18. doi: 10.1161/HYPERTENSIONAHA.108.120022. [DOI] [PubMed] [Google Scholar]
- 11.Sugioka K, Shimosegawa Y, Nakano M. Estrogens as natural antioxidants of membrane phospholipid peroxidation. FEBS Lett. 1987;210:37–39. doi: 10.1016/0014-5793(87)81293-0. [DOI] [PubMed] [Google Scholar]
- 12.Kim KH, Toomre D, Bender JR. Splice isoform estrogen receptors as integral transmembrane proteins. Mol Biol Cell. 2011;22:4415–4423. doi: 10.1091/mbc.E11-05-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2008;73:864–869. doi: 10.1016/j.steroids.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szego CM, Davis JS. Inhibition of estrogen-induced elevation of cyclic 3',5'-adenosine monophosphate in rat uterus. I. By beta-adrenergic receptor-blocking drugs. Mol Pharmacol. 1969;5:470–480. [PubMed] [Google Scholar]
- 15.Colburn P, Buonassisi V. Estrogen-binding sites in endothelial cell cultures. Science. 1978;201:817–819. doi: 10.1126/science.684408. [DOI] [PubMed] [Google Scholar]
- 16.Buonassisi V, Venter JC. Hormone and neurotransmitter receptors in an established vascular endothelial cell line. Proc Natl Acad Sci U S A. 1976;73:1612–1616. doi: 10.1073/pnas.73.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 18.Furchgott RF. A research trail over half a century. Annu Rev Pharmacol Toxicol. 1995;35:1–27. doi: 10.1146/annurev.pa.35.040195.000245. [DOI] [PubMed] [Google Scholar]
- 19.Caulin-Glaser T, Garcia-Cardena G, Sarrel P, Sessa WC, Bender JR. 17 beta-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ Res. 1997;81:885–892. doi: 10.1161/01.res.81.5.885. [DOI] [PubMed] [Google Scholar]
- 20.Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun. 1997;240:737–741. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- 21.Jensen EV, DeSombre ER. Estrogen-receptor interaction. Science. 1973;182:126–134. doi: 10.1126/science.182.4108.126. [DOI] [PubMed] [Google Scholar]
- 22.Jensen EV, Desombre ER, Hurst DJ, Kawashima T, Jungblut PW. Estrogen-receptor interactions in target tissues. Arch Anat Microsc Morphol Exp. 1967;56:547–569. [PubMed] [Google Scholar]
- 23.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 25.Meyer MR, Haas E, Prossnitz ER, Barton M. Non-genomic regulation of vascular cell function and growth by estrogen. 2009;308:9–16. doi: 10.1016/j.mce.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer MR, Prossnitz ER, Barton M. The G protein-coupled estrogen receptor GPER/GPR30 as a regulator of cardiovascular function. Vascul Pharmacol. 2011;55:17–25. doi: 10.1016/j.vph.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007;49:1358–1363. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- 28.Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–1026. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- 29.Reslan OM, Yin Z, do Nascimento GR, Khalil RA. Subtype-Specific Estrogen Receptor-Mediated Vasodilator Activity in the Cephalic, Thoracic and Abdominal Vasculature of Female Rat. J Cardiovasc Pharmacol. 2013 doi: 10.1097/FJC.0b013e31828bc88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broughton BR, Miller AA, Sobey CG. Endothelium-dependent relaxation by G protein-coupled receptor 30 agonists in rat carotid arteries. Am J Physiol Heart Circ Physiol. 2010;298:H1055–1061. doi: 10.1152/ajpheart.00878.2009. [DOI] [PubMed] [Google Scholar]
- 31.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu C, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, et al. Regulatory role of G-protein coupled estrogen receptor for vascular function and obesity. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology. 2009;150:3753–3758. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurt AH, Buyukafsar K. Vasoconstriction induced by G1, a G-protein-coupled oestrogen receptor1 (GPER-1) agonist, in the isolated perfused rat kidney. Eur J Pharmacol. 2013;702:71–78. doi: 10.1016/j.ejphar.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Murata T, Dietrich HH, Xiang C, Dacey RG., Jr. G protein-coupled estrogen receptor agonist improves cerebral microvascular function after hypoxia/reoxygenation injury in male and female rats. Stroke. 2013;44:779–785. doi: 10.1161/STROKEAHA.112.678177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blasko E, Haskell CA, Leung S, Gualtieri G, Halks-Miller M, Mahmoudi M, Dennis MK, Prossnitz ER, Karpus WJ, Horuk R. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J Neuroimmunol. 2009;214:67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Traupe T, Stettler CD, Li H, Haas E, Bhattacharya I, Minotti R, Barton M. Distinct roles of estrogen receptors alpha and beta mediating acute vasodilation of epicardial coronary arteries. Hypertension. 2007;49:1364–1370. doi: 10.1161/HYPERTENSIONAHA.106.081554. [DOI] [PubMed] [Google Scholar]
- 37.Barton M. Position paper: The membrane estrogen receptor GPER--Clues and questions. Steroids. 2012;77:935–942. doi: 10.1016/j.steroids.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Luksha L, Poston L, Gustafsson JA, Hultenby K, Kublickiene K. The oestrogen receptor beta contributes to sex related differences in endothelial function of murine small arteries via EDHF. J Physiol. 2006;577:945–955. doi: 10.1113/jphysiol.2006.121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer MR, Haas E, Barton M. Need for research on estrogen receptor function: importance for postmenopausal hormone therapy and atherosclerosis. Gend Med. 2008 doi: 10.1016/j.genm.2008.03.004. In press. [DOI] [PubMed] [Google Scholar]
- 40.Barton M. Cholesterol and atherosclerosis: Modulation by estrogen. Curr Opin Lipidol. 2013 doi: 10.1097/MOL.0b013e3283613a94. in press. [DOI] [PubMed] [Google Scholar]
- 41.Meyer MR, Barton M. ERalpha, ERbeta, and gpER: novel aspects of oestrogen receptor signalling in atherosclerosis. Cardiovasc Res. 2009;83:605–610. doi: 10.1093/cvr/cvp187. [DOI] [PubMed] [Google Scholar]
- 42.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 43.Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, et al. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, Oprea TI, Yamaguchi Y, Hayashi S, Sklar LA, et al. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J Steroid Biochem Mol Biol. 2011;127:358–366. doi: 10.1016/j.jsbmb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lappano R, Rosano C, De Marco P, De Francesco EM, Pezzi V, Maggiolini M. Estriol acts as a GPR30 antagonist in estrogen receptor-negative breast cancer cells. Mol Cell Endocrinol. 2011;320:162–170. doi: 10.1016/j.mce.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Vivacqua A, Bonofiglio D, Albanito L, Madeo A, Rago V, Carpino A, Musti AM, Picard D, Ando S, Maggiolini M. 17beta-estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the G protein-coupled receptor GPR30. Mol Pharmacol. 2006;70:1414–1423. doi: 10.1124/mol.106.026344. [DOI] [PubMed] [Google Scholar]
- 47.Rosano C, Lappano R, Santolla MF, Ponassi M, Donadini A, Maggiolini M. Recent advances in the rationale design of GPER ligands. Curr Med Chem. 2012;19:6199–6206. [PubMed] [Google Scholar]
- 48.Lappano R, Santolla MF, Pupo M, Sinicropi MS, Caruso A, Rosano C, Maggiolini M. MIBE acts as antagonist ligand of both estrogen receptor alpha and GPER in breast cancer cells. Breast Cancer Res. 2012;14:R12. doi: 10.1186/bcr3096. [DOI] [PMC free article] [PubMed] [Google Scholar]