Abstract
The renal handling of immunoreactive insulin was studied in the isolated perfused normothermic rat kidney to determine (a) the relative contributions of glomerular clearance and peritubular clearance to the renal clearance of insulin under different conditions, (b) what metabolic factors influence the ability of tubular cells to remove insulin from the glomerular filtrate and the peritubular circulation, and (c) whether the same factors influence the luminal and contraluminal uptake of insulin.
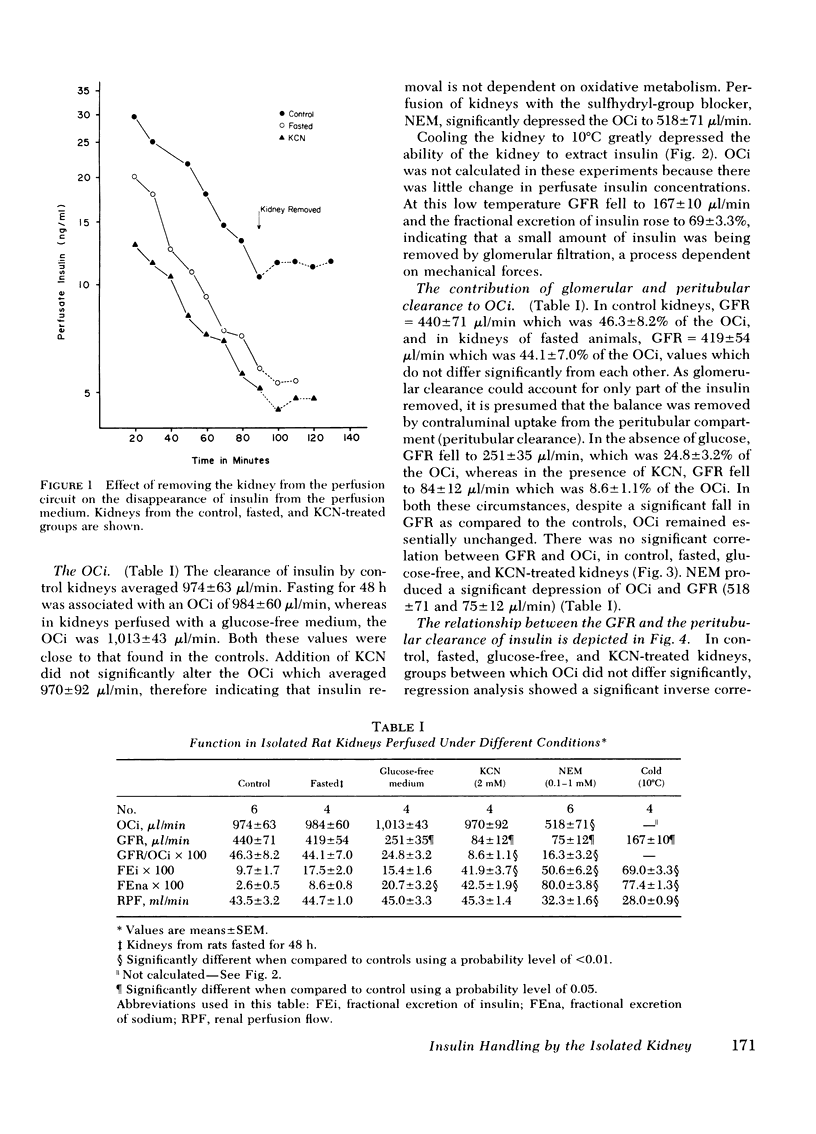
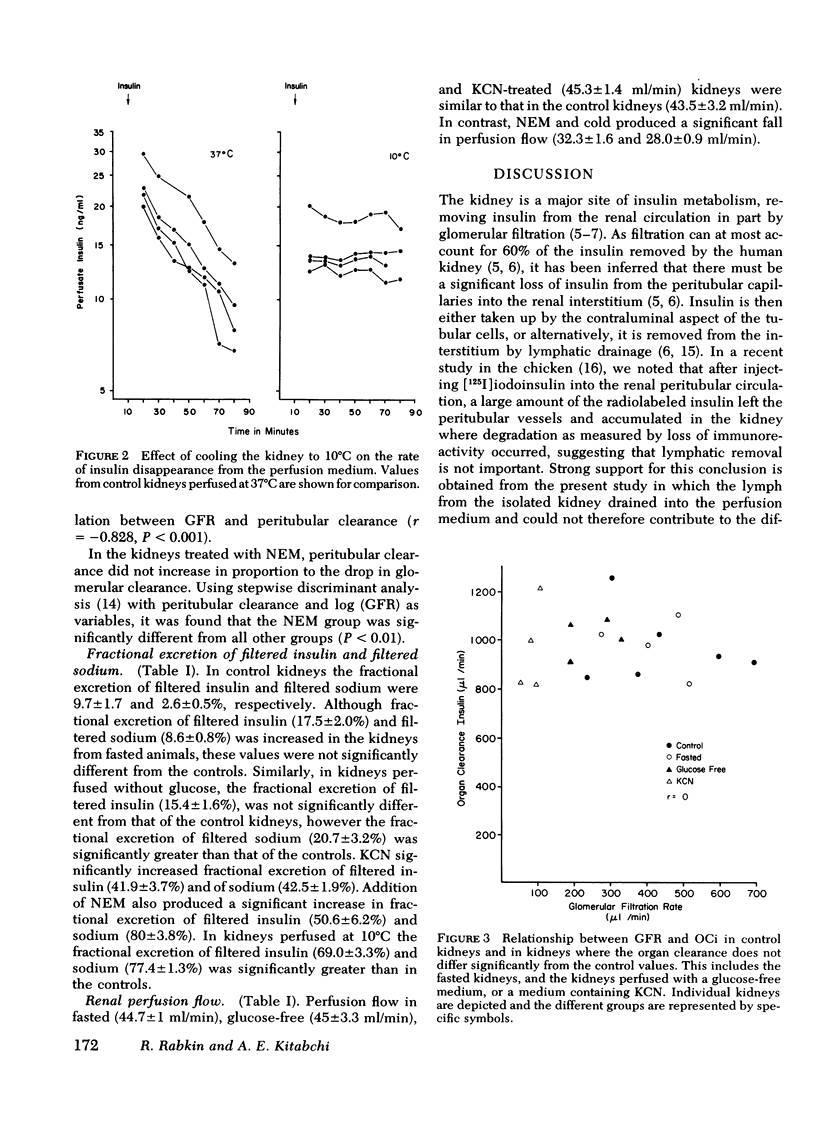
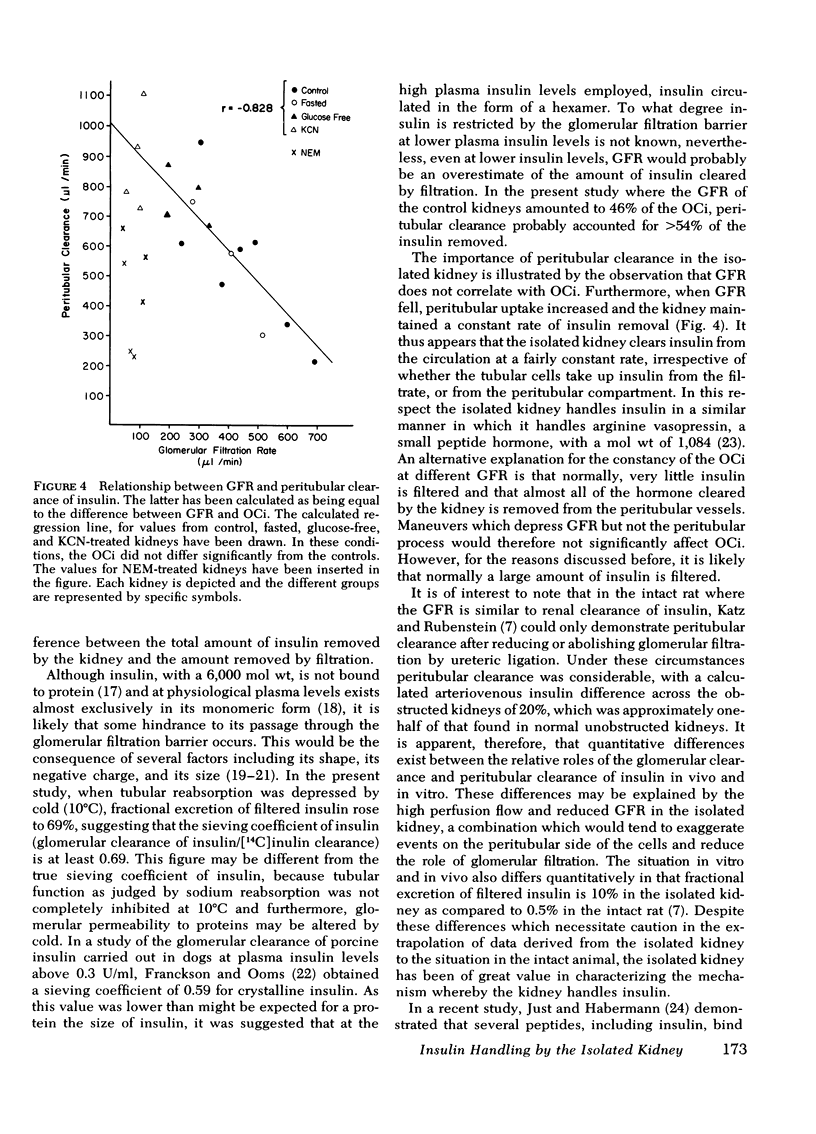
In control kidneys the organ clearance of insulin (OCi) was 974±63 μl/min (SEM), of which a maximum of 46% could theoretically be accounted for by filtration. OCi was not altered by fasting, lack of exogenous fuel (glucose), or the addition of cyanide. The glomerular filtration rate did not correlate with the OCi, but there was a significant (P < 0.001) negative correlation (r = −0.828) between the peritubular clearance and glomerular filtration rate. Both N-ethylmaleimide and cold (10°C) reduced the rate of insulin removal. Fractional excretion of filtered insulin (9.7±1.7% in controls) was not significantly altered by fasting or perfusing without glucose. In contrast, KCN increased fractional excretion of insulin to 41.9±3.7% whereas cold increased fractional excretion to 69.0±3.3%.
This study indicates that renal tubular cells remove insulin from the tubular lumen and the peritubular compartment. Furthermore, the data suggest that insulin removal by tubular cells is a temperature-sensitive process consisting of two different systems. The system associated with the luminal aspect of the cell appears to be dependent on oxidative metabolism, whereas the system associated with the contraluminal aspects of the cell appears to be independent thereof. Under several circumstances when the glomerular clearance of insulin falls thereby reducing the amount of insulin absorbed by the luminal aspect of the cell, contraluminal uptake increases, and a constant rate of insulin removal is maintained by the kidney.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourdeau J. E., Chen E. R., Carone F. A. Insulin uptake in the renal proximal tubule. Am J Physiol. 1973 Dec;225(6):1399–1404. doi: 10.1152/ajplegacy.1973.225.6.1399. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Baylis C., Deen W. M. Transport of molecules across renal glomerular capillaries. Physiol Rev. 1976 Jul;56(3):502–534. doi: 10.1152/physrev.1976.56.3.502. [DOI] [PubMed] [Google Scholar]
- Chamberlain M. J., Stimmler L. The renal handling of insulin. J Clin Invest. 1967 Jun;46(6):911–919. doi: 10.1172/JCI105597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortney M. A., Sawin L. L., Weiss D. D. Renal tubular protein absorption in the rat. J Clin Invest. 1970 Jan;49(1):1–4. doi: 10.1172/JCI106208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Goldberg M., Agus Z. S. The effects of glucose and insulin on renal electrolyte transport. J Clin Invest. 1976 Jul;58(1):83–90. doi: 10.1172/JCI108463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth W. C. Insulin and glucagon degradation by the kidney. II. Characterization of the mechanisms at neutral pH. Biochim Biophys Acta. 1976 Jul 21;437(2):531–542. doi: 10.1016/0304-4165(76)90021-0. [DOI] [PubMed] [Google Scholar]
- Franckson J. R., Ooms H. A. The glomerular clearance of exogenous insulin. Horm Metab Res. 1973 Mar;5(2):75–79. doi: 10.1055/s-0028-1093986. [DOI] [PubMed] [Google Scholar]
- Freychet P., Kahn R., Roth J., Neville D. M., Jr Insulin interactions with liver plasma membranes. Independence of binding of the hormone and its degradation. J Biol Chem. 1972 Jun 25;247(12):3953–3961. [PubMed] [Google Scholar]
- Fussganger R. D., Kahn C. R., Roth J., De Meyts P. Binding and degradation of insulin by human peripheral granulocytes. Demonstration of specific receptors with high affinity. J Biol Chem. 1976 May 10;251(9):2761–2769. [PubMed] [Google Scholar]
- Katz A. I., Rubenstein A. H. Metabolism of proinsulin, insulin, and C-peptide in the rat. J Clin Invest. 1973 May;52(5):1113–1121. doi: 10.1172/JCI107277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabchi A. E., Duckworth W. C., Brush J. S., Heinemann M. Direct measurement of proinsulin in human plasma by the use of an insulin-degrading enzyme. J Clin Invest. 1971 Sep;50(9):1792–1799. doi: 10.1172/JCI106669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabchi A. E., Stentz F. B. Degradation of insulin and proinsulin by various organ homogenates of rat. Diabetes. 1972 Nov;21(11):1091–1101. doi: 10.2337/diab.21.11.1091. [DOI] [PubMed] [Google Scholar]
- MORTIMORE G. E., TIETZE F., STETTEN D., Jr Metabolism of insulin-I 131; studies in isolated, perfused rat liver and hindlimb preparations. Diabetes. 1959 Jul-Aug;8(4):307–314. doi: 10.2337/diab.8.4.307. [DOI] [PubMed] [Google Scholar]
- MORTIMORE G. E., TIETZE F. Studies on the mechanism of capture and degradation of insulin-1131 by the cyclically perfused rat liver. Ann N Y Acad Sci. 1959 Sep 25;82:329–337. doi: 10.1111/j.1749-6632.1959.tb44913.x. [DOI] [PubMed] [Google Scholar]
- Maack T. Renal handling of low molecular weight proteins. Am J Med. 1975 Jan;58(1):57–64. doi: 10.1016/0002-9343(75)90533-1. [DOI] [PubMed] [Google Scholar]
- Miller A. T., Jr, Hale D. M., Alexander K. D. Histochemical studies on the uptake of horseradish peroxidase by rat kidney slices. J Cell Biol. 1965 Nov;27(2):305–312. doi: 10.1083/jcb.27.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHARA H. T., EVERETT N. B., SIMMONS B. S., WILLIAMS R. H. Metabolism of insulin-I 131 and glucagon-I 131 in the kidney of the rat. Am J Physiol. 1958 Feb;192(2):227–231. doi: 10.1152/ajplegacy.1958.192.2.227. [DOI] [PubMed] [Google Scholar]
- Nishiitsutsuji-Uwo J. M., Ross B. D., Krebs H. A. Metabolic activities of the isolated perfused rat kidney. Biochem J. 1967 Jun;103(3):852–862. doi: 10.1042/bj1030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizet A., Lefebvre P., Crabbé J. Control by insulin of sodium potassium and water excretion by the isolated dog kidney. Pflugers Arch. 1971;323(1):11–20. doi: 10.1007/BF00586561. [DOI] [PubMed] [Google Scholar]
- Pekar A. H., Frank B. H. Conformation of proinsulin. A comparison of insulin and proinsulin self-association at neutral pH. Biochemistry. 1972 Oct 24;11(22):4013–4016. doi: 10.1021/bi00772a001. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Shiu R. P., Friesen H. G. Studies of insulin, growth hormone and prolactin binding: tissue distribution, species variation and characterization. Endocrinology. 1974 Aug;95(2):521–531. doi: 10.1210/endo-95-2-521. [DOI] [PubMed] [Google Scholar]
- Rabkin R., Jones J., Kitabchi A. E. Insulin extraction from the renal peritubular circulation in the chicken. Endocrinology. 1977 Dec;101(6):1828–1833. doi: 10.1210/endo-101-6-1828. [DOI] [PubMed] [Google Scholar]
- Rabkin R., Rubenstein A. H., Colwell J. A. Glomerular filtration and proximal tubular absorption of insulin 125 I. Am J Physiol. 1972 Nov;223(5):1093–1096. doi: 10.1152/ajplegacy.1972.223.5.1093. [DOI] [PubMed] [Google Scholar]
- Rabkin R., Simon N. M., Steiner S., Colwell J. A. Effect of renal disease on renal uptake and excretion of insulin in man. N Engl J Med. 1970 Jan 22;282(4):182–187. doi: 10.1056/NEJM197001222820402. [DOI] [PubMed] [Google Scholar]
- Rennke H. G., Venkatachalam M. A. Glomerular permeability: in vivo tracer studies with polyanionic and polycationic ferritins. Kidney Int. 1977 Jan;11(1):44–53. doi: 10.1038/ki.1977.6. [DOI] [PubMed] [Google Scholar]
- Ross B. D., Epstein F. H., Leaf A. Sodium reabsorption in the perfused rat kidney. Am J Physiol. 1973 Nov;225(5):1165–1171. doi: 10.1152/ajplegacy.1973.225.5.1165. [DOI] [PubMed] [Google Scholar]
- Steiner D. F. The Banting Memorial Lecture 1976. Insulin today. Diabetes. 1977 Apr;26(4):322–340. doi: 10.2337/diab.26.4.322. [DOI] [PubMed] [Google Scholar]
- Strober W., Waldmann T. A. The role of the kidney in the metabolism of plasma proteins. Nephron. 1974;13(1):35–66. doi: 10.1159/000180368. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Ohsawa N., Kosaka K. Radioreceptor assay for insulin. J Clin Endocrinol Metab. 1976 Feb;42(2):399–402. doi: 10.1210/jcem-42-2-399. [DOI] [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Binding and degradation of 125I-insulin by rat hepatocytes. J Biol Chem. 1975 Nov 10;250(21):8389–8398. [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Retention and degradation of 125I-insulin by perfused livers from diabetic rats. J Clin Invest. 1976 Apr;57(4):885–896. doi: 10.1172/JCI108365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uete T., Tsuchikura H. Autoregulatory system of insulin degradation in liver. I. Decreased 131 I-insulin degradation in the liver of insulin deficient rats. J Biochem. 1972 Jul;72(1):157–163. doi: 10.1093/oxfordjournals.jbchem.a129881. [DOI] [PubMed] [Google Scholar]


