Abstract
Movement-related brain activation patterns after subcortical stroke are characterized by relative overactivations in cortical motor areas compared with controls. In patients able to perform a motor task, overactivations are greater in those with more motor impairment. We hypothesized that recruitment of motor regions would shift from primary to secondary motor networks in response to impaired functional integrity of the corticospinal system (CSS). We measured the magnitude of brain activation using functional MRI during a motor task in eight chronic subcortical stroke patients. CSS functional integrity was assessed using transcranial magnetic stimulation to obtain stimulus/response curves for the affected first dorsal interosseus muscle, with a shallower gradient representing increasing disruption of CSS functional integrity. A negative correlation between the gradient of stimulus/response curve and magnitude of task-related brain activation was found in several motor-related regions, including ipsilesional posterior primary motor cortex [Brodmann area (BA) 4p], contralesional anterior primary motor cortex (BA 4a), bilateral premotor cortex, supplementary motor area, intraparietal sulcus, dorsolateral prefrontal cortex and contralesional superior cingulate sulcus. There were no significant positive correlations in any brain region. These results suggest that impaired functional integrity of the CSS is associated with recruitment of secondary motor networks in both hemispheres in an attempt to generate motor output to spinal cord motoneurons. Secondary motor regions are less efficient at generating motor output so this reorganization can only be considered partially successful in reducing motor impairment after stroke.
Keywords: functional brain imaging, motor recovery, motor system, stroke
Introduction
Functionally relevant cerebral reorganization in response to focal ischaemic damage has been demonstrated in humans using functional MRI (fMRI) and transcranial magnetic stimulation (TMS) (Ward and Cohen, 2004). Using fMRI, we have previously demonstrated relative increases in task-related brain activation in a number of motor related regions in patients with chronic subcortical hemiparetic stroke compared to a control group (Ward et al., 2003). These over-activations are typically seen in secondary motor regions such as premotor cortex, supplementary motor area (SMA) and cingulate motor areas (CMA), but vary in magnitude between patients. We have previously characterized this variability as a function of the clinical outcome, demonstrating greater increases in the magnitude of brain activation during hand grip in more impaired patients (Ward et al., 2003). Furthermore, the activation pattern in some patients with excellent recovery was not statistically different from that in normal controls (Ward et al., 2003). However, clinical outcome after stroke is in part related to the residual functional integrity of the corticospinal system (CSS) as assessed by TMS (Heald et al., 1993; Cruz et al., 1999; Pennisi et al., 1999) and diffusion tensor imaging (Thomalla et al., 2004). Foltys et al. (2003) performed fMRI and TMS in a group of stroke patients and demonstrated reduced amplitude motor evoked potentials (MEPs) and less cortical activation from the affected compared to unaffected hemispheres. All these patients had made a rapid recovery, so variability within this group was limited. Thus, the relationship between CSS disruption and patterns of motor system recruitment after stroke has yet to be formally explored.
Motor cortical stimulus/response curves plot the size of the MEP evoked by magnetic stimulation of a fixed site on the scalp across a range of intensities (Ridding and Rothwell, 1997). For an intrinsic hand muscle such as the first dorsal interosseus (FDI) the relationship is sigmoidal (Devanne et al., 1997) and most probably reflects increasing recruitment of the elements comprising the CSS, namely cortical circuitry, the motoneuron pool and spinal inter-neuronal relays (Burke et al., 1994). Although the exact contribution of each of these elements is not well understood, the resulting stimulus/response gradient is a sensitive reflection of the functional integrity of the CSS (Devanne et al., 1997; Ridding and Rothwell, 1997; Boroojerdi et al., 2001).
We therefore performed an experiment in a group of chronic subcortical stroke patients in order to compare the magnitude of brain activation during performance of a motor task with the functional integrity of the CSS. We hypothesized that greater CSS disruption would lead to a shift from the damaged primary to intact secondary motor networks suggesting that the widely observed recruitment of these regions after stroke is indeed related to impaired functional integrity of the CSS. We also recorded scores relating to motor impairment in our patients, expecting to see greater CSS damage and secondary motor network recruitment in patients with more impairment. Such a result would suggest that recovery is limited by anatomical damage, and can be only partially compensated by cerebral reorganization.
Methods
Subjects
Patients were recruited from the National Hospital for Neurology and Neurosurgery, Queen Square, London. All patients had suffered from first-ever stroke resulting in weakness of at least wrist and finger extensors and hand interossei (to ≤4+ on the Medical Research Council scale) for at least 48 h after onset of symptoms. Exclusion criteria consisted of (i) involvement of the cortex in infarcted brain tissue (ii) carotid artery occlusion or stenosis ≥70%; (iii) language or cognitive deficits sufficient to impair co-operation in the study; (iv) inability to perform the motor task due to complete paralysis of hand grip; (v) previous seizures. All patients received post-stroke therapy appropriate to their clinical needs.
All patients were right handed according to the Edinburgh handedness scale (Oldfield, 1971). Full written consent was obtained from all subjects in accordance to the Declaration of Helsinki. The study was approved by the Joint Ethics Committee of the Institute of Neurology, UCL and National Hospital for Neurology and Neurosurgery, UCL Hospitals NHS Foundation Trust, London.
Behavioural evaluation
Patients were evaluated on the same day as scanning with nine hole peg test (NHPT) and grip strength (GRIP). Maximum grip strength was measured using the same manipulandum as for MRI scanning. GRIP is calculated by the maximum hand grip strength for the affected hand divided by that of the unaffected hand, expressed as a percentage. NHPT score represents pegs per second for the affected hand divided by the pegs per second for the unaffected hand, expressed as a percentage.
TMS
TMS was performed using a MAGSTIM 200 stimulator connected to a figure-of-eight-shaped coil with an internal wing diameter of 7 cm. The handle of the coil was held pointing postero-laterally and the position was identified at which stimulation produced optimal MEPs in the contralateral paretic FDI. Surface EMG recordings were made using a belly-to-tendon montage from FDI of the paretic hand. The raw signal was amplified and filtered with a band-pass filter of 30 Hz to 1 kHz (Digitimer Ltd). Signals were digitized at 2 kHz (CED Power1401, Cambridge Electronic Design, Cambridge, UK) and stored on a laboratory computer for off-line analysis. The resting motor threshold (rMT) was determined, defined as the lowest stimulation intensity required to evoke an MEP in the relaxed FDI of >50 μV in 5 out of 10 trials. Subsequent testing was performed at rest, because the slope of the recruitment curve is altered by voluntary muscle activation. The degree of voluntary muscle activation would have been difficult to standardize across patients. Resting MEPs were obtained at four stimulus intensities (90, 110, 130 and 150% of rMT, or up to a maximum of 100% of stimulator output) with at least 10 trials at each intensity. After TMS maximum amplitude M-waves were elicited from FDI by supramaximal electrical stimulation of the ulnar nerve at the wrist.
Individual trials were examined off-line and those showing voluntary EMG activity were discarded. Peak-to-peak MEP amplitudes were measured in the remaining trials. These amplitudes were expressed as a fraction of the M-wave for that subject and plotted against stimulus intensity, giving a corrected MEP recruitment curve from the affected hemisphere of each patient. The gradient of the line of best fit for the recruitment curve (RCAH) was obtained using the least squares method. RCAH was calculated for each patient.
fMRI scanning
Motor paradigm
Scanning was performed within 2 days of TMS examination. During scanning, patients performed a dynamic isometric hand grip task using the impaired hand. Hand grips were performed using a MRI compatible manipulandum consisting of two force transducers (Honeywell FSG15N1A; Honeywell, NJ, USA) situated between two moulded plastic bars (width 6 cm). Compression of the two bars by isometric handgrip resulted in the generation of a differential voltage signal, linearly proportional to force exerted, which was fed into a signal conditioner (CED 1902; Cambridge Electronic Design, Cambridge, UK). This signal was digitized (CED 1401; Cambridge Electronic Design, Cambridge, UK) and fed into a computer running Cogent 2000 (Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/Cogent2000.html). The dynamic change in recorded signal was projected in real time onto a screen as a column whose height varied linearly with change in voltage and hence force. Prior to scanning, but whilst lying in the scanner, subjects were asked to grip the manipulandum with maximum force to generate a maximum voluntary contraction (MVC). The onset of a hand grip was indicated visually by an arrow pointing to the side of the affected hand displayed at the bottom of the screen for 3 s. The appearance of the arrow indicated that the subject was to perform a single brief handgrip with the affected hand, to be continued until the column representing force applied came into contact with a horizontal bar on the screen (indicating the target force of 15, 30 or 45% of the affected hand MVC on the day of scanning), at which point the grip could be released. A continuous scanning session (lasting 6 min 14 s) comprised 30 cued events (10 hand grips at each target force) and 30 null events in a randomized and counterbalanced order (SOA = 5.72 s). Prior to scanning, subjects were pre-trained until comfortable with the task. All patients performed the motor task outside the scanner in order that they might be observed for the presence of associated movements or mirror movements. To aid this assessment during scanning, patients held two identical hand grip manipulanda, one in each hand, during the performance of repetitive hand grip with the affected hand. These simultaneous recordings from both hands enabled us to detect true mirror movements (Nelles et al., 1998). After scanning a 100 mm visual analogue scale (VAS) (where 0 = ‘no effort’ and 100 = ‘maximum effort’) was used to assess the perceived effortfulness of the task.
Data acquisition
A 3T Siemens ALLEGRA system (Siemens, Erlangen, Germany) was used to acquire both T1-weighted anatomical images and -weighted MRI transverse echoplanar images (EPI) (64 × 64 mm, 3 × 3 mm pixels, echo time TE = 30 ms) with blood oxygenation level dependent (BOLD) contrast. The site of cerebral infarction was determined from the T1-weighted anatomical images. Each EPI comprised forty-eight 2 mm thick contiguous axial slices taken every 3 mm, positioned to cover the whole cerebrum, with an effective repetition time (TR) of 3.12 s per volume. In total, 120 volumes were acquired during each scanning session. The first six volumes were discarded to allow for T1 equilibration effects.
Image analysis
Imaging data were analysed using Statistical Parametric Mapping (SPM2, Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spm/) implemented in Matlab 6 (The Math-works Inc., USA) (Friston et al., 1995b; Worsley and Friston, 1995). All volumes were realigned to the first volume in order to correct for interscan movement. No subject moved >2 mm in any direction, but some of this movement was task-related. In order to remove some of this unwanted movement-related variance without removing variance attributable to the motor task, realigned images were processed using the ‘unwarp’ toolbox in SPM2 (Andersson et al., 2001) which is predicated on the assumption that susceptibility-by-movement interaction is responsible for a sizeable part of residual movement related variance. Given the observed variance (after realignment) and the realignment parameters, estimates of how deformations changed with subject movement were made, which were subsequently used to minimize movement related variance.
To correct for their different acquisition times, the signal measured in each slice was shifted relative to the acquisition of the middle slice using sinc interpolation in time. The resulting volumes were then normalized to a standard EPI template based on the Montreal Neurological Institute (MNI) reference brain in Talairach space (Talairach, 1998) and resampled to 3 ×3 × 3 mm3 voxels. All normalized images were then smoothed with an isotropic 8 mm full-width half-maximum Gaussian kernel to account for intersubject differences and allow valid statistical inference according to Gaussian random field theory (Friston et al., 1995a). The time-series in each voxel were high-pass filtered at 1/128 Hz to remove low frequency confounds and scaled to a grand mean of 100 over voxels and scans within each session.
Images from patients with left sided lesions were flipped about the midsagittal plane, so that all patients were assumed to have right hemispheric lesions, consistent with our previous published work (Ward et al., 2003).
Statistical analysis was performed in two stages. In the first stage, a single subject fixed effects model was used. All handgrips were defined as a single event type and modelled as delta functions. The resulting covariate was convolved with a canonical synthetic haemodynamic response function and then used in a general linear model (Friston et al., 1995b) together with a single covariate representing the mean (constant) term over scans. The parameter estimates for the covariate representing handgrips resulting from the least mean squares fit of the model to the data were calculated, and statistical parametric maps of the t statistic (SPM{t}) resulting from a linear contrast of the covariate were generated and stored as separate images for each subject.
A second stage of analysis was used to assess where in the brain task-related changes in BOLD signal are dependent on functional integrity of the CSS, as assessed using RCAH, in this group of patients. A multi-subject fixed effects model employing the same covariates as described for single session analysis was therefore used to determine voxels in which there is a correlation between the parameter estimates for the covariate representing the main effects of hand grip and RCAH. The subject specific contrast weightings were determined from the mean corrected RCAH value for each patient (such that the sum of all corrected RCAH values was equal to zero) and when applied to the model identified voxels in which there is a correlation (positive or negative) between the magnitude of activation and RCAH across the eight patients (the ‘task by RCAH’ interaction). The resulting SPM{t}s (for positive and negative correlation) were thresholded at P < 0.05 (family wise error), corrected for multiple comparisons across whole brain. For significant voxels, the correlation coefficient for the plot of parameter estimate against RCAH for each subject was calculated.
Anatomical identification was carefully performed by superimposing the maxima of activation foci both on the MNI brain and on the normalized structural images of each subject, and labelling with the aid of the atlas of Duvernoy (Duvernoy, 1991).
Results
Clinical data
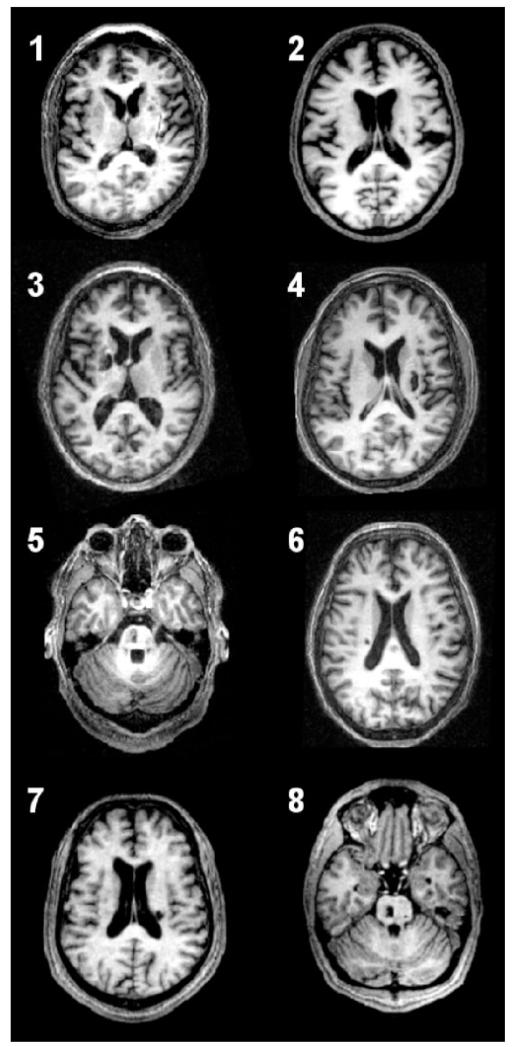
Patient characteristics are listed in Table 1. The group comprised eight patients (range 22–69 years, mean 48.1 years). Structural brain images are shown in Fig. 1. All patients were able to perform the task adequately. No patient displayed mirror movements or synergistic flexor movements in more proximal joints as assessed outside the scanner by direct observation, and during scanning by inspection of the force recordings from the unaffected hand during movement of the affected hand.
Table 1.
Patient characteristics
| Patient | Age | Sex | Site of lesion | Time since stroke (months) |
Initial severity |
Grip strength (%)* |
NHPT (%)* |
PMH | Medication | VAS ratings (0–100 mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M | R striatocapsular | 5 | 0 | 24.90 | 0 | Hypertension | Aspirin, bendrofluazide, ramipril |
14 |
| 2 | 46 | M | R internal capsule | 8 | 0 | 87.30 | 100.5 | Nil | Aspirin | 13 |
| 3 | 69 | M | L striatocapsular | 9 | 4 | 60.5 | 69.7 | Hypertension | Aspirin, ramipril, simvastatin |
28 |
| 4 | 44 | M | R striatocapsular | 8 | 0 | 43.6 | 5.1 | Nil | Aspirin | 23 |
| 5 | 33 | M | L pons | 45 | 0 | 68.7 | 48.9 | Nil | Aspirin | 27 |
| 6 | 51 | M | L corona radiata | 6 | 4 | 85.7 | 89.6 | Hypertension | Aspirin, ramipril, simvastatin |
19 |
| 7 | 58 | M | R internal capsule | 7 | 0 | 93.3 | 94.5 | Hypertension | Aspirin, bendrofluazide, simvastatin |
24 |
| 8 | 22 | M | L pons | 4 | 0 | 86.6 | 85.8 | Nil | Nil | 25 |
M = male; R = right; L = left; NHPT = nine hole peg test; PMH = past medical history; VAS = visual analogue scale (where 0 mm = ‘no effort’ and 100 mm = ‘maximum effort’). Initial severity represents strength of finger extension (MRC scale) at time of stroke as recorded in medical notes.
Values as measured at the time of study.
Fig. 1.
Axial T1-weighted MRI scans at the level of maximum infarct volume for each patient performed at the time of the functional MRI.
TMS results
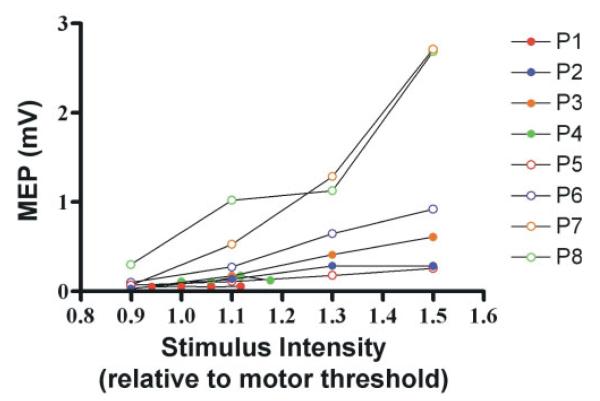
M-wave, rMT and RCAH values for each patient are given in Table 2. The recruitment curve slopes are shown in Fig. 2.
Table 2.
TMS results
| Patient | M-wave (mV) | rMT | RCAH |
|---|---|---|---|
| 1 | 6.86 | 80 | 0.003 |
| 2 | 13.34 | 41 | 0.048 |
| 3 | 18.2 | 39 | 0.055 |
| 4 | 11.04 | 85 | 0.016 |
| 5 | 10.81 | 52 | 0.031 |
| 6 | 18.04 | 65 | 0.078 |
| 7 | 13.42 | 44 | 0.324 |
| 8 | 16.79 | 54 | 0.216 |
M-waves are from the paretic hand first dorsal interosseus muscle (FDI). RCAH = affected hemisphere stimulus/response curve gradient from FDI (corrected for M-waves). rMT = resting motor threshold for FDI from affected hemisphere, given as percentage of maximum stimulator output.
Fig. 2.
MEP recruitment curves from the affected hemisphere of each patient. The values shown are the mean MEP amplitudes (mV) elicited at each stimulus intensity (relative to the rMT) prior to correction for M-wave amplitude.
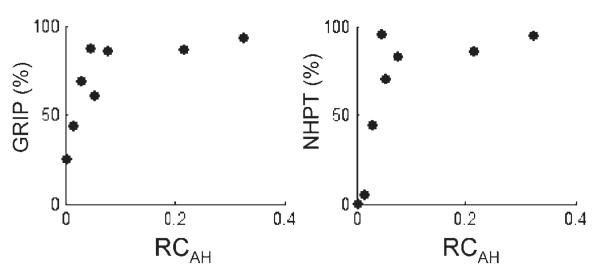
A non-significant positive correlation was found between RCAH and GRIP (r2 = 0.45, P = 0.07) and between RCAH and NHPT (r2 = 0.40, P = 0.09) (Fig. 3). No correlation was found between RCAH and age (r2 = 0.02, P = ns), or between RCAH and the VAS rating for effort by each patient (r2 = 0.09, P = ns).
Fig. 3.
GRIP and NHPT plotted against RCAH.
Imaging results
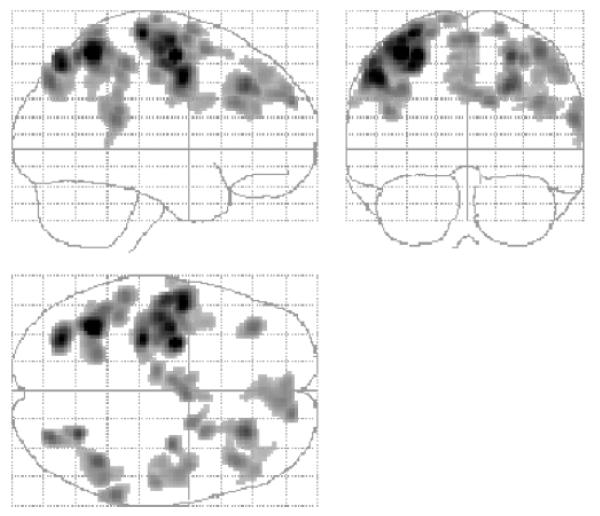
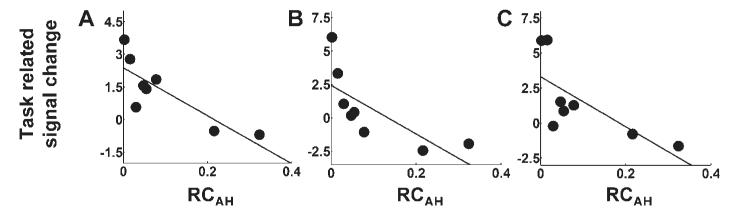
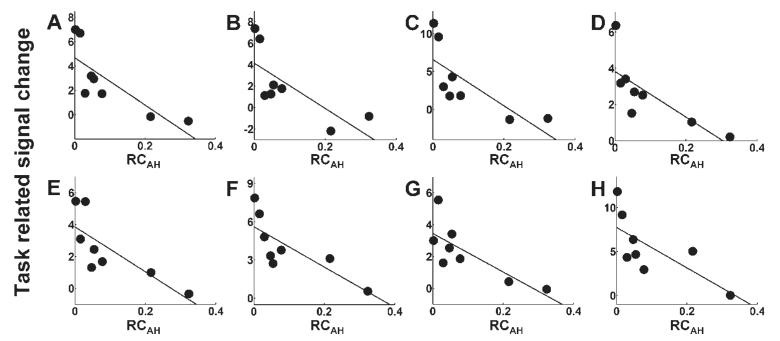
The correlation analysis identified several brain regions in which there was a negative correlation between task-related signal change (parameter estimate for the main effects of hand grip) and RCAH (Table 3 and Fig. 4). These included contralesional anterior primary motor cortex [Brodmann area (BA) 4a], ipsilesional posterior primary motor cortex (BA 4p), and contralesional primary sensory cortex (BA 1). The location of these peak voxels in the relevant BA was determined (P > 0.4) using the cytoarchitectonic maps provided at http://www.bic.mni.mcgill.ca/cytoarchitectonics/ (Geyer et al., 1996, 1999). In addition, a negative correlation between task-related signal change and RCAH was seen in bilateral premotor cortex, SMA, intraparietal sulcus, dorsolateral prefrontal cortex, and contralesional superior cingulate sulcus. The cluster of significant voxels located in contralesional premotor cortex extended in the ventral direction as far as z = 36, although the most ventral premotor peak was situated at x = −52, y = −4, z = 44. This observation suggests that the negative correlation between task-related signal change and RCAH might be present in both dorsolateral and ventrolateral contralesional premotor cortices. Plots of task-related signal change versus RCAH are shown in Figs 5, 6 and 7.
Table 3.
Imaging results
| Region | Side | Talairach coordinates in MNI space |
Z-value | Correlation analysis (r2) |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Primary motor cortex (BA 4a) | C | −32 | −26 | 68 | 6.35 | 0.54 |
| Primary motor cortex (BA 4p) | I | 42 | −14 | 44 | 4.72 | 0.68 |
| Primary sensory cortex (BA 1) | C | −52 | −14 | 52 | 5.68 | 0.51 |
| Dorsolateral premotor cortex | C | −28 | −8 | 56 | 6.69 | 0.62 |
| C | −52 | −4 | 44 | 6.21 | 0.54 | |
| C | −36 | −10 | 64 | 6.02 | 0.56 | |
| C | −42 | −16 | 60 | 5.88 | 0.56 | |
| I | 32 | −10 | 54 | 4.82 | 0.58 | |
| I | 50 | −6 | 50 | 4.71 | 0.59 | |
| SMA | C | −6 | −8 | 64 | 4.73 | 0.61 |
| I | 2 | −2 | 66 | 4.74 | 0.51 | |
| Cingulate sulcus | C | −2 | −2 | 50 | 4.79 | 0.61 |
| Intraparietal sulcus | C | −38 | −54 | 58 | 7.28 | 0.57 |
| C | −30 | −72 | 50 | 6.21 | 0.59 | |
| I | 26 | −62 | 56 | 5.39 | 0.56 | |
| Dorsolateral prefrontal cortex | C | −36 | 36 | 36 | 4.73 | 0.84 |
| I | 24 | 32 | 38 | 5.34 | 0.64 | |
Regions in which there is a negative correlation between affected hemisphere TMS recruitment curves and task-related brain activation across all stroke patients. Images for patients with left sided lesions were flipped about the mid-sagittal line, so that all patients were assumed to have a lesion on the right side. Voxels are significant at P < 0.05, corrected for multiple comparisons across whole brain volume. Correlation analysis was performed at all significant voxels and the coefficient of determination (r2) is given. I = ipsilesional; C = contralesional; BA = Brodmann area.
Fig. 4.
SPM {Z}s representing regions in which there is a negative correlation between RCAH and task-related signal change.
Results are displayed on a ‘glass brain’ shown from the right side (top left image), from behind (top right image), and from above (bottom left image). Voxels are significant at P < 0.001 (uncorrected), and clusters are significant at P < 0.05 (corrected), for the purposes of display.
Fig. 5.
Plots of task-related signal change (parameter estimate for the main effect of handgrip) versus RCAH for (A) ipsilesional primary motor cortex (BA 4p, x = 42, y = −14, z = 44), (B) contralesional primary motor cortex (BA 4a, x = 32, y = −26, z = 68), (C) contralesional primary sensory cortex (BA 1, x = 52, y = 14, z = 52). Corresponding correlation coefficients are given in Table 3.
Fig. 6.
Plots of task-related signal change (parameter estimate for the main effect of handgrip) versus RCAH for (A) contralesional premotor cortex (x = −28, y = −8, z = 56), (B) contralesional premotor cortex (x = 52, y = −4, z = 44), (C) contralesional premotor cortex (x = −36, y = −10, z = 64). (D) ipsilesional premotor cortex (x = 32, y = −10, z = 54), (E) ipsilesional premotor cortex (x = 50, y = −6, z = 50), (F) contralesional cingulate sulcus (x = −2, y = −2, z = 50), (G) contralesional SMA (x = −6, y = −8, z = 64), (H) ipsilesional SMA (x = 2, y = −2, z = 66). Corresponding correlation coefficients are given in Table 3.
Fig. 7.
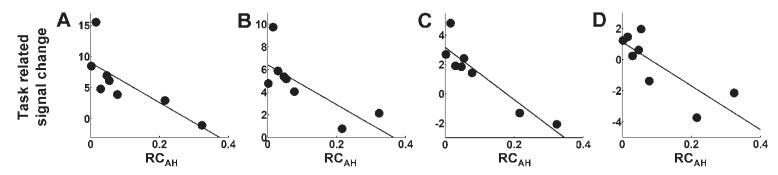
Plots of task-related signal change (parameter estimate for the main effect of handgrip) versus RCAH for (A) contralesional intraparietal sulcus (x = −38, y = −54, z = 58), (B) ipsilesional intraparietal sulcus (x = 26, y = −62, z = 56), (C) contralesional dorsolateral prefrontal cortex (x = −36, y = 36, z = 36), (D) ipsilesional dorsolateral prefrontal cortex (x = 24, y = 32, z = 38). Corresponding correlation coefficients are given in Table 3.
We report a trend towards a significant negative correlation in ipsilateral medial superior frontal gyrus for descriptive purposes (x = 14, y = 58, z = 28, Z-score = 4.65, P = 0.054, corrected for multiple comparisons).
There were no regions in which a significant positive correlation between task-related brain activation and RCAH was seen, although we had hypothesized that a positive correlation would be seen in the ipsilesional hand region of M1 (BA 4a). We examined the data at the M1 peak for the main effect of hand grip across the patient group (x = 40, y = −24, z = 58). At this peak voxel a non-significant positive correlation between task-related brain activation and RCAH was found (r2 = 0.36, P = 0.11, see Supplementary figure).
Discussion
These results demonstrate a clear relationship between task-related brain activation within the distributed motor system and the functional integrity of the surviving CSS in a cohort of chronic subcortical stroke patients. Task-related brain activation shifts from primary to secondary motor networks in patients with greater functional disruption to the CSS. Secondary motor networks are less efficient at generating motor output so that this type of reorganization may be only partly successful in reversing motor impairment after stroke. The best outcome is likely to be seen in those with intact or partially intact primary motor networks. fMRI and TMS parameters in our patients with good outcome appear to be closer to those seen in control subjects.
Measurement of the functional integrity of the CSS
TMS has been used in many studies to assess the relationship between MEP of the affected hand and functional outcome after stroke (Heald et al., 1993; Cicinelli et al., 1997; D’Olhaberriague et al., 1997; Turton et al., 1999; Traversa et al., 2000; Byrnes et al., 1999, 2001). In general, these studies suggest that lower thresholds and greater amplitudes are associated with better outcome. Thickbroom et al. (2002) demonstrated a positive correlation between MEP amplitude and grip strength, and a negative correlation between MEP threshold and grip strength in a group of stroke patients. MEP parameters did not correlate with dexterity of the affected hand, despite the fact that grip strength and dexterity were correlated. Skilled hand movements are largely dependent on the cortico-motoneuronal system (Porter and Lemon, 1993) and thus it appears that MEP amplitude and threshold may not provide the optimal index of the functional status of the corticomotor projection (Thickbroom et al., 2002). Furthermore, MEP thresholds show a high degree of variability between healthy subjects and can change with levels of general alertness. Thus although thresholds are generally raised in patients with severe corticospinal tract impairment, a single MEP amplitude (for example, at 120% of rMT) represents only a single point on the recruitment curve. Measurement of the recruitment curve gradient generated by plotting MEPs at four different stimulus intensities is less likely to be susceptible to this variability. For this reason we chose to use motor cortical stimulus/response curves as a more reliable index of the functional integrity of the CSS (Devanne et al., 1997; Ridding and Rothwell, 1997; Boroojerdi et al., 2001).
Brain activation in ipsilesional M1
Damage to the CSS fibres from M1 will render this region less useful in the production of direct motor output signal to spinal cord motoneurons. We found only a trend (r2 = 0.36, P = 0.11, see Supplementary figure) towards decreasing task-related signal change in the hand region of ipsilesional anterior M1 (BA 4a) with increasing CSS disruption in our patients with subcortical lesions. However, ipsilesional M1 is clearly an important substrate for recovery and our patients with lower RCAH and less ipsilesional anterior M1 (BA 4a) activity had a tendency towards poorer outcomes. Werhahn et al. (2003) have previously demonstrated that subcortical stroke patients with poorer recovery had an elevated rMT. Furthermore, disruption of ipsilesional M1 with TMS was less likely to impair a motor task in patients with poorer recovery, suggesting that it was less useful in generating motor output than in well recovered patients. These findings confirm that preservation of ipsilesional anterior M1 (BA 4a) and its cortico-motoneuronal projections results in less motor impairment after stroke.
The relationship between RCAH and magnitude of task-related activation in ipsilesional posterior M1 (BA 4p) was the opposite of that seen in ipsilesional anterior M1 (BA 4a). Increased activation was seen in ipsilesional BA 4p as a function of greater CSS damage. This finding is in keeping with a previously reported increase in ipsilesional BA 4p recruitment in stroke patients with moderate or poor recovery (Ward et al., 2003). This posterior part of M1, situated deep in central sulcus, is increasingly recruited during simple motor tasks requiring greater attention (Binkofski et al., 2002; Johansen-Berg and Matthews, 2002). Furthermore, task related activity in BA 4p has been demonstrated to increase (non-linearly) with increasing age in subjects performing a motor task (Ward and Frackowiak, 2003). It is also of interest that after 30 min of 1 Hz repetitive TMS to reduce the excitability of superficial left M1 (BA 4a) to afferent inputs, an increase in recruitment of left BA 4p during a right hand motor task can be demonstrated (Lee et al., 2003). Analysis of effective connectivity revealed that the stimulated part of M1 became less responsive to input from premotor cortex and SMA, but that connectivity between BA 4p and anterior motor areas in the left hemisphere increased. These examples clearly do not represent models of subcortical stroke, but taken together, the increase in activity in posterior M1 in all these different situations is suggestive of ‘spare capacity’ within the motor system. Nevertheless, our result in itself does not help to determine whether this change in BA 4p recruitment is functionally useful, in the way that increased activity in dorsolateral premotor cortex (PMd) appears to be (see Discussion below).
Brain activation in secondary motor regions
Can other cortical motor regions compensate for CSS damage? In the macaque, it has been proposed that the arrangement of primary motor cortex (M1), arcuate (lateral) premotor area, and SMA into parallel, independent motor networks might allow damage in one of these networks to be compensated for by activity in another (Strick, 1988). Our results support the idea that increased task related recruitment in secondary motor areas occurs in response to CSS disruption (and consequent reduced utility of ipsilesional anterior M1) in humans, but not that this recruitment can sustain full recovery. There is evidence however, that increasing recruitment of both ipsilesional and contralesional PMd is partially successful in compensating for CSS damage. Disruption of activity in either region using TMS can impair the performance of simple motor tasks in patients with subcortical stroke but not in controls (Johansen-Berg et al., 2002; Fridman et al., 2004). The effect of TMS to contralesional PMd is more pronounced in patients with poorer outcome, suggesting greater functional reliance on this region compared to well recovered patients or controls (Johansen-Berg et al., 2002). Patients with poorer outcome are likely to have greater disruption to the CSS. Thus the increased task related recruitment that we observed in contralesional PMd in response to increasing CSS disruption is likely to be functionally useful.
The inability of secondary motor areas to support complete recovery is likely to reflect the pathways through which they generate motor output. In the macaque, there is evidence of similarity between the corticospinal projections from the hand regions of M1, arcuate (lateral) premotor area, SMA and CMA (Dum and Strick, 1996; Rouiller et al., 1996; Strick, 1988). However, it appears that direct projections from SMA to spinal cord motoneurons for example, are less numerous and less able to generate an excitatory response than those from M1 (Maier et al., 2002). These direct projections from secondary motor areas to spinal cord motoneurons are thus unlikely to completely substitute for those from M1. Alternatively, non-monosynaptic pathways may play an increasingly prominent role if direct pathways are damaged. There is evidence to suggest that in stroke patients with poorer recovery (and greater damage to direct pathways) a greater proportion of the descending motor command is mediated through propriospinal projections (Mazevet et al., 2003; Stinear and Byblow, 2004). Premotor cortex is known to have projections to the reticular formation, which in turn, gives rise to bilateral reticulospinal projections to propriospinal premotoneurons (Benecke et al., 1991). Thus reorganized cortical motor regions could act through either monosynaptic or oligosynaptic pathways after stroke, although our results are not able to distinguish between these mechanisms.
We have also observed negative correlations between functional integrity of CSS and task-related activation in intraparietal sulcus and dorsolateral prefrontal cortex. This is likely to reflect the greater activation in fronto-parietal networks seen in patients with poorer outcome (Ward et al., 2003, 2004). Although the results of the VAS ratings for effort suggest no difference in perceived effortfulness of the task across subjects, it is likely that patients with greatest damage to CSS integrity and poor outcome are more attentive to the performance of the visuomotor task. It is interesting to speculate that this increased attention to task and associated increase in frontoparietal activity may be the factor that facilitates increased recruitment of components of the motor system which are capable of increasing output to the spinal cord motor neurons via existing anatomical connections (Lu et al., 1994; Fang et al., 2005).
Brain activation in contralesional M1
The role of contralesional M1 in motor recovery after stroke remains controversial (Ward, 2004). Our results demonstrate that when the functional integrity of the CSS in one hemisphere is impaired, recruitment of contralesional anterior M1 (BA 4a) is greater during the performance of a motor task. Mirror movements are often considered to confound the interpretation of contralesional M1 activation after stroke, although it is likely that they are a product of the reorganized system under investigation and are therefore of interest and are not solely confounds. We did not observe mirror movements during task practice outside the scanner in any patient, and neither did we detect mirror gripping during scanning. However, we cannot rule out a very small level of mirror activity not picked up by our methods, which might be detectable only by careful electromyography. Thus, contralesional anterior M1 (BA 4a) appears to be active in the post-stroke reorganized motor system of some patients, but it is not clear whether it is contributing to motor performance or impairing it. There is evidence from several studies that contralesional M1 may in fact be inhibiting ipsilesional M1 in certain types of subcortical stroke patients (Murase et al., 2004). Furthermore, minimizing the effect of this inhibition, by either reducing excitability of contralesional M1 or increasing excitability of ipsilesional M1 has led to improvements in motor performance in some subcortical stroke patients (Fregni et al., 2005; Hummel et al., 2005; Khedr et al., 2005; Mansur et al., 2005). However, there is as yet no information on the variability of responses between patients to such interventions. Our data clearly show that the organization of the post-stroke motor system is related to functional integrity of the CSS. It is highly likely that response to these interventions depends on how the post-stroke motor system is configured. Thus in order to target patients appropriately, some measures of residual functional anatomy may be helpful.
Experimental design issues
The results of this study must be viewed in the context of the patients studied. First, the use of a fixed effects analysis to examine for ‘task by RCAH’ interactions allows inference to be made about only the group of patients studied. Stroke patients are intrinsically variable in the way their residual brain networks are configured because of the variability of the lesions involved. The use of random effects analyses in order to extrapolate results to all stroke patients is often therefore inappropriate, if used solely in order to generate inferences about all stroke patients. Secondly, because we are studying the neural correlates of the generation of motor output, only patients who can perform the motor task can be selected. The use of hand grip rather than finger tapping does allow recruitment of a relatively broad range of patients, as hand grip returns earlier compared to fractionated finger movements (Heller et al., 1987) and compares well with other measures of upper limb function (Heller et al., 1987; Sunderland et al., 1989).
When studying patients with different performance abilities it is inevitable that confounds will be present in the experimental design (Baron et al., 2004). We ensured that the target forces for each patient were set as a proportion of each patient’s own affected hand MVC. Furthermore in order to ensure that hand grips were performed at the same rate, and that the task was no more effortful across subjects, we used a sparse event-related design. It is unlikely that differences in activation patterns across the group were related to differences in the perceived effortfulness of the task. This is supported by the results of the VAS ratings for effort completed by each subject. However, patients with better recovery were able to exert greater forces during hand grip. Increased grip force in controls leads to linearly increasing magnitude of signal change in contralateral sensorimotor cortex (Ward and Frackowiak, 2003). Thus if differences in the activation patterns of subjects was primarily related to differences in forces exerted during hand grip, it is more likely a positive correlation would have been seen between signal change and CSS integrity. This makes the positive correlation in ipsilesional M1 more difficult to interpret (and may explain why the result was not significant). However, for all other brain regions, this was not the case and so it is unlikely our results were confounded either by differences in effort or differences in absolute grip force used.
In the absence of previous data, we have assumed a linear relationship between CSS integrity and the magnitude of task-related activation in secondary motor regions. Although this has proved a reasonable first pass approximation in our patient group, inspection of the graphs in Figs 5–7 suggests that in many cases the relationship may be non-linear. Furthermore, the relationship between outcome and CSS integrity also appears to be non-linear. Future studies should investigate the nature of this relationship in more detail. It is possible that only moderate CSS integrity is sufficient to sustain normal motor function.
Conclusions
Many new treatments are being targeted at motor impairment in stroke patients (Ward and Cohen, 2004; Hummel and Cohen, 2005). These often rely on promoting or facilitating activity driven changes within surviving motor networks. Although reduction of motor impairment may be limited by anatomical damage, an increase in the efficiency of any residual motor system is likely to lead to clinical improvement, possibly through the same mechanisms as motor learning in the normal brain (Hikosaka et al., 2002). However, it is likely that in any given patient a treatment will be successful only if that motor network is accessible and functionally intact. Our results support the idea that the functional organization of a residual distributed motor system is related to the degree of disruption to the CSS. Stroke patients are a heterogeneous group. By explaining this heterogeneity between stroke patients in terms of measurable parameters, it should be possible to predict the response to treatments with known mechanisms and therefore to target individuals appropriately. TMS and fMRI assessments of residual functional anatomy will therefore provide a useful adjunct in the assessment of potentially useful treatments of residual motor impairment in stroke patients.
Supplementary Material
Acknowledgements
N.S.W., L.L., and R.S.J.F. are supported by the Wellcome Trust. J.M.N. is supported by Action Medical Research. J.C.R. is supported by the Medical Research Council. A.J.T. holds the Garfield Weston Chair of Clinical Neurology and Neurological Rehabilitation. We would like to thank Peter Aston and Eric Featherstone (Wellcome Department of Imaging Neuroscience) for the design and programming involved in creating the hand grip manipulandum. We would also like to thank the staff of the Acute Brain Injury Unit and Neurorehabilitation Unit at the National Hospital for Neurology and Neurosurgery, Queen Square, London, for their assistance.
Abbreviations
- BA
Brodmann area
- CSS
corticospinal system
- FDI
first dorsal interosseus
- fMRI
functional MRI
- MEP
motor evoked potential
- NHPT
nine hole peg test
- rMT
resting motor threshold
- SMA
supplementary motor area
- SPM
Statistical Parametric Mapping
- TMS
transcranial magnetic stimulation
- VAS
visual analogue scale
Footnotes
Supplementary material Supplementary data are available at Brain Online.
References
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–19. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Baron JC, Cohen LG, Cramer SC, Dobkin BH, Johansen-Berg H, Loubinoux I, et al. Neuroimaging in stroke recovery: a position paper from the First International Workshop on Neuroimaging and Stroke Recovery. Cerebrovasc Dis. 2004;18:260–7. doi: 10.1159/000080293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benecke R, Meyer BU, Freund HJ. Reorganisation of descending motor pathways in patients after hemispherectomy and severe hemispheric lesions demonstrated by magnetic brain stimulation. Exp Brain Res. 1991;83:419–26. doi: 10.1007/BF00231167. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Fink GR, Geyer S, Buccino G, Gruber O, Shah NJ, et al. Neural activity in human primary motor cortex areas 4a and 4p is modulated differentially by attention to action. J Neurophysiol. 2002;88:514–9. doi: 10.1152/jn.2002.88.1.514. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Battaglia F, Muellbacher W, Cohen LG. Mechanisms influencing stimulus-response properties of the human corticospinal system. Clin Neurophysiol. 2001;112:931–7. doi: 10.1016/s1388-2457(01)00523-5. [DOI] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Mazevet D, Meunier S, Pierrot-Deseilligny E. Non-monosynaptic transmission of the cortical command for voluntary movement in man. J Physiol. 1994;480:191–202. doi: 10.1113/jphysiol.1994.sp020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes ML, Thickbroom GW, Phillips BA, Wilson SA, Mastaglia FL. Physiological studies of the corticomotor projection to the hand after subcortical stroke. Clin Neurophysiol. 1999;110:487–98. doi: 10.1016/s1388-2457(98)00044-3. [DOI] [PubMed] [Google Scholar]
- Byrnes ML, Thickbroom GW, Phillips BA, Mastaglia FL. Long-term changes in motor cortical organisation after recovery from subcortical stroke. Brain Res. 2001;889:278–87. doi: 10.1016/s0006-8993(00)03089-4. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Traversa R, Rossini PM. Post-stroke reorganization of brain motor output to the hand: a 2–4 month follow-up with focal magnetic transcranial stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:438–50. doi: 10.1016/s0924-980x(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Cruz MA, Tejada J, Diez TE. Motor hand recovery after stroke. Prognostic yield of early transcranial magnetic stimulation. Electromyogr Clin Neurophysiol. 1999;39:405–10. [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–38. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- D’Olhaberriague L, Espadaler Gamissans JM, Marrugat J, Valls A, Oliveras LC, Seoane JL. Transcranial magnetic stimulation as a prognostic tool in stroke. J Neurol Sci. 1997;147:73–80. doi: 10.1016/s0022-510x(96)05312-9. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16:6513–25. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: surface, blood supply, and three-dimensional anatomy. Springer-Verlag; New York: 1991. [Google Scholar]
- Fang PC, Stepniewska I, Kaas JH. Ipsilateral cortical connections of motor, premotor, frontal eye, and posterior parietal fields in a prosimian primate, Otolemur garnetti. J Comp Neurol. 2005;490:305–33. doi: 10.1002/cne.20665. [DOI] [PubMed] [Google Scholar]
- Foltys H, Krings T, Meister IG, Sparing R, Boroojerdi B, Thron A, et al. Motor representation in patients rapidly recovering after stroke: a functional magnetic resonance imaging and transcranial magnetic stimulation study. Clin Neurophysiol. 2003;114:2404–15. doi: 10.1016/s1388-2457(03)00263-3. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551–5. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–58. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner L, Poline JB, Poline JB, Frith CD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995a;2:165–89. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995b;2:189–210. [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, et al. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–7. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage. 1999;10:63–83. doi: 10.1006/nimg.1999.0440. [DOI] [PubMed] [Google Scholar]
- Heald A, Bates D, Cartlidge NE, French JM, Miller S. Longitudinal study of central motor conduction time following stroke. 2. Central motor conduction measured within 72 h after stroke as a predictor of functional outcome at 12 months. Brain. 1993;116:1371–85. doi: 10.1093/brain/116.6.1371. [DOI] [PubMed] [Google Scholar]
- Heller A, Wade DT, Wood VA, Sunderland A, Hewer RL, Ward E. Arm function after stroke: measurement and recovery over the first three months. J Neurol Neurosurg Psychiatry. 1987;50:714–9. doi: 10.1136/jnnp.50.6.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002;12:217–22. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Drivers of brain plasticity. Curr Opin Neurol. 2005;18:667–74. doi: 10.1097/01.wco.0000189876.37475.42. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–9. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–8. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Matthews PM. Attention to movement modulates activity in sensori-motor areas, including primary motor cortex. Exp Brain Res. 2002;142:13–24. doi: 10.1007/s00221-001-0905-8. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA. 2002;99:14518–23. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Siebner HR, Rowe JB, Rizzo V, Rothwell JC, Frackowiak RS, et al. Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:5308–18. doi: 10.1523/JNEUROSCI.23-12-05308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MT, Preston JB, Strick PL. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J Comp Neurol. 1994;341:375–92. doi: 10.1002/cne.903410308. [DOI] [PubMed] [Google Scholar]
- Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex. 2002;12:281–96. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]
- Mansur CG, Fregni F, Boggio PS, Riberto M, Gallucci-Neto J, Santos CM, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64:1802–4. doi: 10.1212/01.WNL.0000161839.38079.92. [DOI] [PubMed] [Google Scholar]
- Mazevet D, Meunier S, Pradat-Diehl P, Marchand-Pauvert V, Pierrot-Deseilligny E. Changes in propriospinally mediated excitation of upper limb motoneurons in stroke patients. Brain. 2003;126:988–1000. doi: 10.1093/brain/awg088. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–9. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nelles G, Cramer SC, Schaechter JD, Kaplan JD, Finklestein SP. Quantitative assessment of mirror movements after stroke. Stroke. 1998;29:1182–7. doi: 10.1161/01.str.29.6.1182. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pennisi G, Rapisarda G, Bella R, Calabrese V, Maertens De Noordhout A, Delwaide PJ. Absence of response to early transcranial magnetic stimulation in ischemic stroke patients: prognostic value for hand motor recovery. Stroke. 1999;30:2666–70. doi: 10.1161/01.str.30.12.2666. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal function and voluntary movement. Oxford University Press; Oxford, UK: 1993. [Google Scholar]
- Ridding MC, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr Clin Neurophysiol. 1997;105:340–4. doi: 10.1016/s0924-980x(97)00041-6. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Moret V, Tanne J, Boussaoud D. Evidence for direct connections between the hand region of the supplementary motor area and cervical motoneurons in the macaque monkey. Eur J Neurosci. 1996;8:1055–9. doi: 10.1111/j.1460-9568.1996.tb01592.x. [DOI] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. The contribution of cervical propriospinal premotoneurons in recovering hemiparetic stroke patients. J Clin Neurophysiol. 2004;21:426–34. doi: 10.1097/00004691-200411000-00006. [DOI] [PubMed] [Google Scholar]
- Strick PL. Anatomical organization of multiple motor areas in the frontal lobe: implications for recovery of function. Adv Neurol. 1988;47:293–312. [PubMed] [Google Scholar]
- Sunderland A, Tinson D, Bradley L, Hewer RL. Arm function after stroke. An evaluation of grip strength as a measure of recovery and a prognostic indicator. J Neurol Neurosurg Psychiatry. 1989;52:1267–72. doi: 10.1136/jnnp.52.11.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournaux P. Co-planar stereotaxic atlas of the human brain. Thieme; Stuttgart: 1998. [Google Scholar]
- Thickbroom GW, Byrnes ML, Archer SA, Mastaglia FL. Motor outcome after subcortical stroke: MEPs correlate with hand strength but not dexterity. Clin Neurophysiol. 2002;113:2025–9. doi: 10.1016/s1388-2457(02)00318-8. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage. 2004;22:1767–74. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Oliveri M, Giuseppina Palmieri M, Filippi MM, Pasqualetti P, et al. Neurophysiological follow-up of motor cortical output in stroke patients. Clin Neurophysiol. 2000;111:1695–703. doi: 10.1016/s1388-2457(00)00373-4. [DOI] [PubMed] [Google Scholar]
- Turton A, Lemon RN. The contribution of fast corticospinal input to the voluntary activation of proximal muscles in normal subjects and in stroke patients. Exp Brain Res. 1999;129:559–72. doi: 10.1007/s002210050926. [DOI] [PubMed] [Google Scholar]
- Ward NS. Functional reorganization of the cerebral motor system after stroke. Curr Opin Neurol. 2004;17:725–30. doi: 10.1097/00019052-200412000-00013. [DOI] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–8. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RSJ. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–88. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126:1430–48. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. The influence of time after stroke on brain activations during a motor task. Ann Neurol. 2004;55:829–34. doi: 10.1002/ana.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG. Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann Neurol. 2003;54:464–72. doi: 10.1002/ana.10686. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited—again. Neuroimage. 1995;2:173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.