Abstract
Objectives
This study sought to evaluate the impact of frailty in older adults undergoing transcatheter aortic valve replacement (TAVR) for symptomatic aortic stenosis.
Background
Frailty status impacts prognosis in older adults with heart disease; however, the impact of frailty on prognosis after TAVR is unknown.
Methods
Gait speed, grip strength, serum albumin, and activities of daily living status were collected at baseline and used to derive a frailty score among patients who underwent TAVR procedures at a single large-volume institution. The cohort was dichotomized on the basis of median frailty score into frail and not frail groups. The impact of frailty on procedural outcomes (stroke, bleeding, vascular complications, acute kidney injury, and mortality at 30 days) and 1-year mortality was evaluated.
Results
Frailty status was assessed in 159 subjects who underwent TAVR (age 86 ± 8 years, Society of Thoracic Surgery Risk Score 12 ± 4). Baseline frailty score was not associated with conventionally ascertained clinical variables or Society of Thoracic Surgery score. Although high frailty score was associated with a longer post-TAVR hospital stay when compared with lower frailty score (9 ± 6 days vs. 6 ± 5 days, respectively, p = 0.004), there were no significant crude associations between frailty status and procedural outcomes, suggesting adequacy of the standard selection process for identifying patients at risk for periprocedural complications after TAVR. Frailty status was independently associated with increased 1-year mortality (hazard ratio: 3.5, 95% confidence interval: 1.4 to 8.5, p = 0.007) after TAVR.
Conclusions
Frailty was not associated with increased periprocedural complications in patients selected as candidates to undergo TAVR but was associated with increased 1-year mortality after TAVR. Further studies will evaluate the independent value of this frailty composite in older adults with aortic stenosis.
Keywords: aortic stenosis, frailty, transcatheter valve
The phenotype of frailty has emerged in the published data as an important estimate of overall health status that is associated with morbidity and mortality in the general population (1), outcomes in older adults with coronary artery disease (2,3), and recovery after general (4) and cardiac surgery (5,6). Severe aortic stenosis (AS) in elderly persons is a condition in which frailty has been emerging as an important arbiter of clinical decision-making. Until recently, the only option for the treatment of severe AS was surgical aortic valve replacement (SAVR). Many older adults with AS do not receive operative intervention, as a result of the morbidity of SAVR (7). Although transcatheter aortic valve replacement (TAVR) provides a less invasive alternative to SAVR for high-risk patients (8,9), decisions with regard to the appropriateness of either TAVR or SAVR hinge on a surgical risk assessment, some of which depends upon an assessment of patient frailty. However, frailty is often not objectively measured in the clinical setting; rather, clinicians often rely on clinical judgment and risk scores such as the Society of Thoracic Surgery Score (10) and others (11–13) to determine preoperative risk and to guide treatment decisions.
Frailty, defined as a syndrome of impaired physiologic reserve and decreased resistance to stressors (14), is captured by the core domains of wasting and malnutrition, weakness, slowness, and inactivity (1) and is closely linked to the development of subsequent disability (15). We employed a multi-component frailty assessment in older adults before TAVR, to evaluate the prognostic implications of baseline frailty on procedural outcomes and survival after TAVR.
Methods
Participants
A prospective cohort design was used, evaluating 159 high-risk patients presenting to the inpatient or outpatient Valve Center at Columbia University Medical Center/New York-Presbyterian Hospital for severe AS who received TAVR as part of the PARTNER (Placement of AoRTic TraNscathetER Valve Trial, NCT00530894). Subjects were 60 years of age and older and had severe, calcific AS (aortic valve area <0.8 cm2 and mean gradient >40 mm Hg or jet velocity >4.0 m/s). All subjects had cardiac symptoms of advanced aortic valve disease but underwent TAVR after a careful selection process assessing their overall candidacy for the procedure. The Columbia University Medical Center Institutional Review Board approved this protocol, and all participants signed informed consent.
Study measurements
Baseline demographic, clinical, and echocardiographic information was collected for all patients. With these data, the STS risk score for an isolated SAVR was computed for each subject and reported as predicted mortality at 30 days. Markers of frailty were chosen to loosely parallel those operationalized by Fried et al. (1). Gait speed was assessed according to time in seconds to walk 15 ft (4.57 m). Participants were instructed to “walk at your comfortable pace” until a few steps past the 15-ft line. The timer was started with the first footfall after the 0-ft line and was stopped at the first footfall after the 15-ft line. The usual assist devices of subjects (e.g., walkers, canes) were permitted (16). If able, each subject completed 1 15-ft walk. Gait speed was calculated by dividing 4.57 m by time to walk this distance in seconds and reported in meters/second, as has been previously recommended (17). Those subjects unable to walk 15 ft were considered to have a gait speed of 0 m/s. Dominant hand grip strength was assessed as the average of 3 trials of maximal isometric grip measured in kilograms with a Jamar dynamometer (Sammons Preston, Chicago, Illinois). Instead of self-reported physical activity, independence in activities in daily living (ADLs) was assessed by the Katz ADL survey (18). The need for assistance with any 1 of the 6 ADLs resulted in the subject being considered dependent, and performing all activities independently was required to be considered independent. Serum albumin was measured on the day before TAVR and used as a marker of malnutrition and wasting.
Outcome measures
All-cause mortality and procedural outcomes were prospectively assessed. Procedural outcomes included in-hospital life-threatening or major bleeding, major vascular complications, in-hospital major stroke, in-hospital acute kidney injury, and 30-day mortality. Life-threatening or major bleeding, major vascular complications, and major stroke were assessed according to the Valve Academic Research Consortium criteria (19). Acute kidney injury was defined as stage 2 or 3 kidney injury according to the Valve Academic Research Consortium recommended modified RIFLE Criteria (20), defined as an increase in serum creatinine to 200% or increase >0.3 mg/dl compared with baseline or the need for new renal replacement therapy. All-cause mortality at 30 days was assessed prospectively. One-year mortality was assessed at semiannual follow-up visits or by phone calls by trained research personnel when follow-up visits were not feasible.
Statistical analysis
The primary predictor variable was a frailty score derived with the markers of frailty. Gait speed and serum albumin were divided into quartiles. Grip strength was divided into quartiles stratified by sex. Given that more than 75% of subjects were independent in all 6 Katz ADLs, ADL status was dichotomized into a group with dependence in any ADL versus those with no ADL dependence. With these quartiles, a frailty score was operationalized in the following manner: 1) quartiles of albumin, gait speed, and grip strength were assigned values of 0 to 3 in descending order; and 2) a score of 0 for ADLs was assigned for ADL independence and 3 for any ADL dependence. These components were then summed to derive a frailty score for each subject (possible range 0 to 12), with the highest score representing the most frail, and the lowest score being the least frail (Table 1).
Table 1.
Components of Frailty Score
| Frailty Domain | Measure | Frailty Score |
|---|---|---|
| Slowness | 15-ft walk gait speed (m/s) |
Quartiles (0–3) |
| Weakness | Grip strength (kg) | Sex-based quartiles (0–3) |
| Wasting and malnutrition | Serum albumin (g/dl) | Quartiles (0–3) |
| Inactivity | Katz activities of daily living |
Any dependence = 3, Independent = 0 |
The cohort was dichotomized at the median frailty score, and baseline characteristics were compared between groups with the Student t test or Wilcoxon rank sum for continuous variables and the chi-squared or Fisher exact test for dichotomous variables as appropriate. Multiple logistic regression was used to evaluate the association of frailty score with procedural outcomes; the association of frailty markers and frailty score with long-term survival after TAVR was assessed with Cox proportional hazards modeling. In sensitivity analyses, frailty score was additionally modeled as a continuous variable and divided in tertiles. Receiver-operating curves were used to compare the accuracy of adding the composite frailty score that included all 4 markers of frailty and scores based on fewer markers of frailty to a model that included the best clinical variables at predicting 1-year mortality. Areas under the curve were compared with the method of DeLong and Delong. Reclassification analysis was performed according to the method of Pencina (21). Multivariable models adjusted for age, access route, and STS score, as well as differences in baseline characteristics between frailty groups. Due to the overall low number of clinical events, adjustment covariates were modeled in separate models to avoid over-fitting and in a fully adjusted model. Because results were similar, only results of the unadjusted and fully adjusted models are presented. Finally, to compare frailty with comorbidity, a comorbidity score was derived that assigned 1 point for each of the following conditions: diabetes, atrial fibrillation, prior coronary revascularization, prior stroke, peripheral vascular disease, chronic lung disease, glomerular filtration rate <40 ml/min, hemoglobin <10 g/dl, and ejection fraction <40%. Pearson’s correlation coefficients were used to estimate the association between frailty score and comorbidity score and frailty score and STS score. All analyses were performed with SAS (version 9.2, SAS, Cary, North Carolina). A p value of <0.05 was considered to be statistically significant.
Results
Frailty assessment was performed in 159 older adult patients before TAVR (transfemoral n = 89 or transapical n = 70). The mean age was 86 years, and 50% were men. Most subjects were admitted from the community (92% were community-dwelling, 8% were from a skilled care facility). Thirty subjects were unable to walk 15 ft due to shortness of breath at rest or extreme deconditioning. Those subjects were assigned a gait speed of 0 m/s. Gait speed, grip strength, serum albumin level, and activity of daily living status is listed in Table 2.
Table 2.
Markers of Frailty
| Frailty score | 5 (4) |
| Albumin | 3.8 (0.6) |
| Gait speed | |
| Subjects able to walk 15 feet | 0.57 (0.35) |
| All subjects with walk attempted | 0.46 (0.41) |
| Grip strength | |
| Men | 22.0 (11.3) |
| Women | 12.8 (5.8) |
| Number of independent ADLs | |
| 0–1 | 2 (1%) |
| 2–3 | 12 (8%) |
| 4–5 | 23 (14%) |
| 6 | 122 (77%) |
| Any ADL impairment | 37 (23%) |
Values are median (interquartile range) or n (%).
ADL = activities of daily living; IQR = interquartile range
Overall, the prevalence of comorbid illnesses was high, because 50% of subjects had 3 or more comorbid conditions (interquartile range 1). Frailty score was not associated with a number of comorbid illnesses (R2 = 0.003, p = 0.5) or STS score (R2 = 0.002, p = 0.6). Demographic and clinical characteristics did not differ between the groups with high and low frailty score (Table 3). Specifically, age, sex, body size, cardiac function, severity of AS, and number of comorbid conditions did not differ between the groups with high and low frailty scores. There was a higher prevalence of hyperlipidemia and percutaneous coronary intervention (PCI) among subjects in the group with the lower frailty score. Hemoglobin was lower among subjects with a higher frailty score. However, B-type natriuretic peptide, leucocyte count, and renal function did not differ between groups. There was no difference in frailty score among those subjects who underwent TAVR via the transapical compared with the transfemoral approach (mean frail score 5.3 ± 3.0 vs. 5.4 ± 3.0, respectively, p = 0.83).
Table 3.
Baseline Characteristics
| Overall (n = 159) |
Frail (Score >5) (n = 76) |
Not Frail (Score ≤5) (n = 83) |
p Value | |
|---|---|---|---|---|
| Age (yrs) | 86.2 (7.7) | 87.1 (6.6) | 85.4 (8.4) | 0.15 |
| Female | 80 (50%) | 40 (53%) | 40 (48%) | 0.58 |
| Body mass index (kg/m2) | 24.7 (5.6) | 25.0 (6.4) | 24.5 (4.9) | 0.58 |
| Community dwelling | 134 (92%) | 61 (90%) | 73 (94%) | 0.39 |
| Transapical access | 70 (44%) | 30 (39%) | 40 (48%) | 0.27 |
| 23-mm valve | 62 (40%) | 28 (37%) | 34 (41%) | 0.69 |
| Diabetes | 37 (23%) | 19 (25%) | 18 (22%) | 0.65 |
| Hypertension | 95 (79%) | 58 (76%) | 67 (81%) | 0.50 |
| Hyperlipidemia | 95 (60%) | 38 (50%) | 57 (69%) | 0.02 |
| Atrial fibrillation or flutter | 67 (42%) | 34 (45%) | 83 (40%) | 0.53 |
| Previous percutaneous coronary angioplasty | 67 (42%) | 22 (29%) | 45 (54%) | 0.001 |
| Previous coronary artery bypass | 64 (40%) | 27 (36%) | 37 (45%) | 0.25 |
| Previous pacemaker | 50 (31%) | 24 (32%) | 26 (31%) | 0.97 |
| Previous stroke | 14 (9%) | 9 (12%) | 5 (6%) | 0.20 |
| Peripheral vascular disease | 41 (26%) | 16 (21%) | 25 (30%) | 0.19 |
| Pulmonary disease | 47 (30%) | 22 (29%) | 25 (30%) | 0.39 |
| Mean gradient (mm Hg) | 45 (15) | 45 (15) | 45 (15) | 0.94 |
| Aortic valve area (cm2) | 0.6 (0.2) | 0.6 (0.2) | 0.6 (0.2) | 0.33 |
| Ejection fraction (%) | 48 (16) | 47 (15) | 49 (17) | 0.48 |
| Glomerular filtration rate (ml/min) | 56 (23) | 54 (21) | 57 (25) | 0.45 |
| Platelet count × 109/l | 202 (67) | 201 (71) | 203 (64) | 0.82 |
| B-natriuretic peptide pg/ml | 1,411 (1,421) | 1,498 (1,245) | 1,333 (1,564) | 0.06 |
| Hemoglobin g/dl | 11.3 (1.5) | 11.1 (1.6) | 11.6 (1.5) | 0.055 |
| Leukocyte count × 109/l | 7.5 (2.8) | 7.7 (3.1) | 7.3 (2.4) | 0.46 |
| STS score (%) | 11.9 (3.9) | 11.9 (4.0) | 12.1 (3.9) | 0.72 |
Values are n(%).
STS = Society of Thoracic Surgery.
Procedural and 30-day outcomes
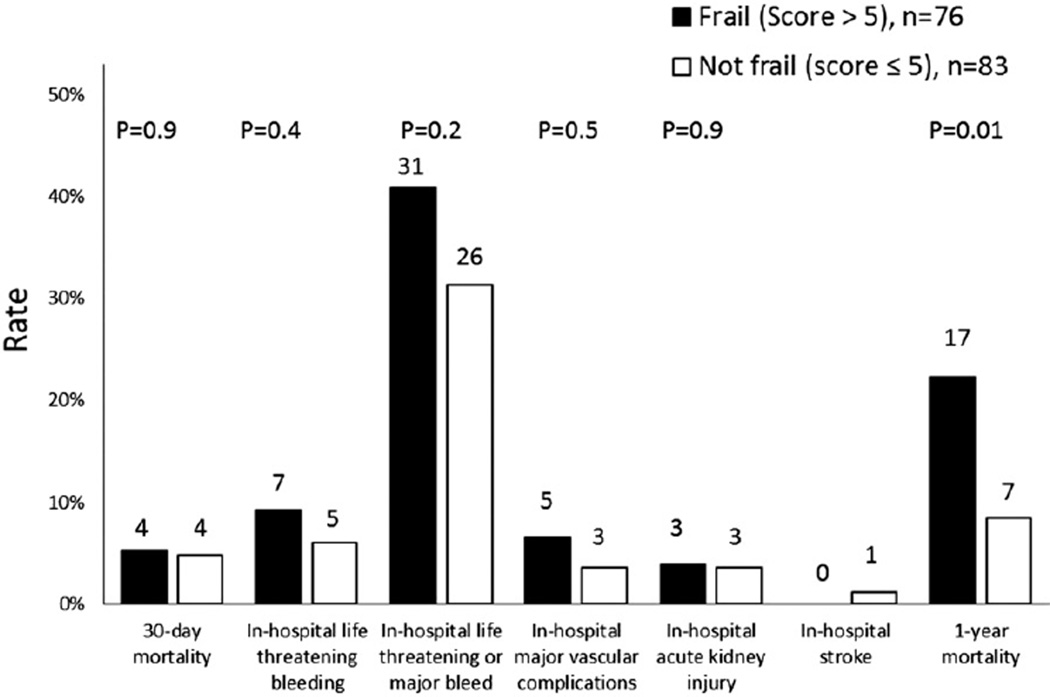
During the transcatheter valve procedure there was no difference in the amount of contrast used among subjects with a frailty score >5 compared with those with a lower frailty score (99 ± 64 ml vs. 86 ± 36 ml, respectively, p = 0.7). A high frailty score was associated with a longer post-TAVR hospital stay when compared with a lower frailty score (9 ± 6 days vs. 6 ± 5 days, respectively, p = 0.004). Overall, at 30 days, among 159 subjects there were 8 (5%) deaths, 1 (1%) in-hospital stroke, 8 (5%) in-hospital major vascular complications, and 57 (36%) in-hospital life-threatening or major bleeding events, and 6 (4%) subjects developed acute kidney injury (Fig. 1). There was no crude association between frailty status and procedural outcomes. After adjustment for age, access route, STS score, prior PCI, hyperlipidemia, and hemoglobin, a frailty score of >5 was associated with in-hospital life-threatening or major bleeding events (odds ratio: 2.2, 95% confidence interval [CI]: 1.02 to 4.6, p = 0.04). There was a higher frequency of blood transfusions after TAVR in the group with a frailty score >5 (24 (32.9%) versus 15 (18.1%), p = 0.03. There was no statistically significant interaction between access route and frailty score category for the life-threatening or major bleeding endpoint.
Figure 1.
Unadjusted Clinical Outcomes
Long-term survival
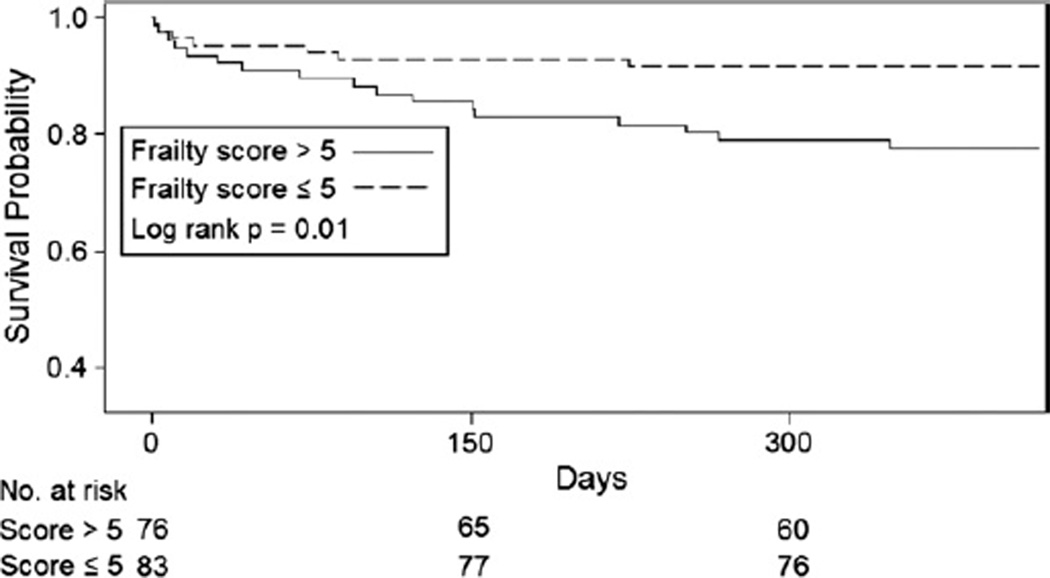
Follow-up time of 348 days or more was available for 85% of the cohort. In univariate analysis, quartiles of gait speed and grip strength were not associated with survival after TAVR (p = 0.3 and p = 0.3, respectively). Subjects with ADL limitation experienced increased mortality compared with those without any ADL limitation (hazard ratio [HR]: 2.13, 95% CI: 0.97 to 4.71, p = 0.07); however, these results did not meet statistical significance. Quartiles of serum albumin were associated with increased mortality after TAVR (HR: 1.51, 95% CI: 1.03 to 2.21, per increase in quartile, p = 0.03). Frailty score was associated with increased mortality after TAVR when modeled continuously (HR: 1.15, 95% CI: 1.02 to 1.30, per 1 U increase in frailty score, p = 0.02), divided in tertiles (HR: 1.71, 95% CI: 1.01 to 2.89, per increase in tertile, p = 0.04), and when dichotomized at the median (Fig. 2). Even after adjustment for age, access route, STS score, prior PCI, hyperlipidemia, and hemoglobin, a frailty score of >5 was associated with a 3–fold increase in mortality after TAVR (adjusted HR: 3.51, 95% CI: 1.43 to 8.62, p = 0.006), (Table 4). Comorbidity score was not associated with long-term mortality in this population (p = 0.3). Furthermore, in a model controlling for comorbidity score, frailty score remained significantly associated with mortality (HR: 1.15, 95% CI: 1.01 to 1.37, for each unit increase in frailty score, p = 0.03). There was no statistically significant interaction between access route and frailty score category when examining the association between frailty and long-term mortality. Adding the frailty score to clinical predictors of 1-year mortality increased the area under the receiver-operating curve; however, the change in area under the curve was not statistically significant. Similarly, the net reclassification improvement of 0.24 did not meet statistical significance (p = 0.19) (Table 5).
Figure 2.
Kaplan-Meier Survival Estimates Stratified by Frailty Score
Table 4.
Association of Markers of Frailty and Frailty Score With Long-Term Survival After TAVR
| Model | HR (95% CI) | p Value |
|---|---|---|
| Quartile of grip strength | 1.18 (0.84–1.66) | 0.3 |
| Quartile of gait speed | 1.19 (0.82–1.72) | 0.3 |
| Any ADL limitation | 2.13 (0.97–4.71) | 0.07 |
| Quartile of serum albumin | 1.51 (1.03–2.21) | 0.03 |
| Score | 1.15 (1.02–1.30) | 0.02 |
| Adjusted score* | 1.15 (1.02–1.30) | 0.03 |
| Score >5 | 3.16 (1.33–7.51) | 0.009 |
| Adjusted score >5* | 3.51 (1.43–8.62) | 0.006 |
Adjusted for age, access route, Society of Thoracic Surgery score, prior percutaneous coronary intervention, hyperlipidemia, and hemoglobin.
ADL = activities of daily living; CI => confidence interval; HR = hazard ratio; TAVR = transcatheter aortic valve replacement.
Table 5.
Receiver-Operating Curve for 1-Year Mortality
| Model | Area | 95% CI |
|---|---|---|
| Clinical model* | 0.727 | 0.62–0.83 |
| Clinical model + score that includes albumin | 0.734 | 0.63–0.83 |
| Clinical model + score that includes albumin and ADL status |
0.749 | 0.64–0.85 |
| Clinical model + score that includes albumin, ADL status, and gait speed |
0.767 | 0.68–0.85 |
| Clinical model + score that includes albumin, ADL status, gait speed, and grip strength |
0.772 | 0.68–0.86 |
Clinical model includes access route, sex, history of stroke, and hyperlipidemia.
ADL = activities of daily living; CI = confidence interval.
Discussion
We found that in older adults with AS undergoing TAVR: 1) frailty status as estimated by a composite of grip strength, gait speed, ADL status, and serum albumin was not associated with clinical factors or risk scores used in traditional risk assessment in this population; 2) although after adjustment for important baseline differences frailty status was associated with bleeding outcomes, it did not seem to be associated with other adverse periprocedural events, including vascular complications, acute kidney injury, stroke, or procedural mortality; and 3) frail status was independently associated with reduced long-term survival after TAVR. Before considering the implications of these findings, however, some methodological issues are addressed.
First, all patients included in this study were carefully evaluated and deemed to be appropriate candidates for TAVR and met the strict inclusion criteria for the PARTNER Trial. Furthermore, these subjects represent the earliest U.S. TAVR experience characterized by advanced age, a high burden of comorbid illness, and a high prevalence of frailty. Therefore, the generalizability of these findings to unselected or lower-risk populations or to a population undergoing traditional SAVR is unknown. For these reasons, it is important to continue to evaluate these markers of frailty and others in larger cohorts of older adults undergoing TAVR and SAVR.
Further methodological concerns relate to the components of the frailty score. Although we believe the components are reasonable, they do represent a departure from previously validated frailty assessment tools (1). Specifically, low albumin and disability might be the result of frailty and/or chronic disease and not frailty per se. However, the prognostic value of a score that contains ADL status and serum albumin suggests that assessing the domains of malnutrition and disability does predict outcomes in this high-risk population. Furthermore, our measure of disability was an assessment of ADL. Because ADL dependence develops late and is present among the most vulnerable subset of subjects, this finding might not prove to be generalizable to lower-risk cohorts. An assessment of instrumental ADL captures earlier manifestation of dependence and disability and might prove to be complementary; however, these data were unavailable. Despite evidence for sarcopenic obesity and a high prevalence of frailty in obese older adults (22), clinicians often associate low body mass index (BMI) with the frailty phenotype. Therefore we included BMI in an exploratory frailty model. However, the model that included BMI was not superior to the frailty index alone.
An additional statistical concern relates to the value of the composite compared with single-item measures. The area under the receiver-operating curve was highest when the frailty score that included albumin, ADLs, gait speed, and grip strength was added to selected clinical variables. This suggests that in this population a multi-item composite outperforms single-item assessment tools. However, due to the small sample size and the limited number of clinical events, the CIs for the areas under the curve were wide, and these results did not meet statistical significance. Therefore, larger studies will be needed to definitively determine the superiority of this composite compared with more limited models.
Finally, although this study is an important first step in establishing the prognostic importance of frailty in older adults with AS who receive TAVR, many questions remain unanswered. What is the impact of comorbidity on the development of frailty in older adults with AS? In this cohort, frail subjects received fewer PCIs. However, this dataset cannot establish that incomplete revascularization and residual ischemia contribute to the vulnerability associated with the frailty phenotype. Despite the only partial overlap of frailty and comorbidity in epidemiological studies (23), further studies should be designed to explore the association of cardiac and noncardiac comorbid illnesses with the development of frailty in older adults with AS. Furthermore, the impact of social support, access to health care, and socioeconomic status on frailty or on outcomes in frail older adults with cardiac disease remains unexplored.
Cognitive impairment is closely linked to cardiovascular disease, adverse cognitive and clinical outcomes after cardiac surgery, early mortality, and permanent institutionalization in older adults (24–27). The impact of cognitive impairment on clinical outcomes after TAVR is an important area that requires further study, because older adults might benefit from a formal cognitive evaluation to optimize patient selection and complement formal cardiovascular risk assessment. The association with frailty and cognitive status in this population remains unknown.
Additionally, a better understanding of the clinical outcomes related to frailty is needed. We did not identify associations with frailty status and vascular complications, stroke, acute kidney injury, or procedural mortality, albeit we lacked statistical power due to the low overall rate of these events. Alternatively, this might suggest that the rigorous screening process successfully selected for patients at low risk for peri-procedural events. The association between frailty markers and increased bleeding has been previously identified (28); however, the mechanism underlying the higher bleeding rate in the frail group requires further investigation. The higher transfusion rate seen in the frail group might be related to more bleeding events seen or a lower baseline hemoglobin. The impact of the higher bleeding rate on outcome in frail older adults will require larger multicenter evaluation. Further studies will be needed to address the impact of frailty on additional important endpoints, including quality of life, repeat hospital stay rates, and causes of death.
Despite these methodological limitations, our findings suggest that assessment of these 4 frailty markers could enhance the preoperative evaluation of high-risk older adults with severe AS. This study is not the first to study the impact of frailty in older adults with cardiac disease. With a standardized global clinical measure of fitness and frailty according to the methodology of Rockwood (29), Ekerstad et al. (3) identified approximately one-half of older adults hospitalized with a non-ST-segment elevation myocardial infarction as frail. In that cohort frailty was associated with a 5-fold increase in 1-month mortality (3). Sundermann et al. (6) employed a frailty assessment that incorporates Fried’s criteria (1) with an additional 5-item physical performance evaluation, 3-item laboratory evaluation, and respiratory function testing in a population of patients undergoing high-risk cardiac surgery. They found that the severely frail had a markedly increased surgical mortality, compared with moderately frail and not frail (22% vs. 8% and 4%, respectively) (6). In a cohort of TAVR subjects, Ewe et al. (30) demonstrated that baseline left ventricular ejection fraction and frailty (with Fried criteria [1]) were independent predictors of short-term survival after TAVR, a finding consistent with ours.
Other investigators have moved toward using gait speed as a single-item performance measure to identify the frailty phenotype, because gait speeding is a robust independent predictor of morbidity and mortality in the general population and in adults with cardiovascular disease (17,31,32). Purser (2) performed frailty assessment in older adults hospitalized for severe coronary artery disease with 2 separate composite indexes based on the work of Fried (1) and Rockwood (29). With Fried’s index, the overall prevalence of frailty in older adults with CAD was 27% (2). They found that the utility of single-item measures for identifying frailty was greatest for gait speed and that impaired gait speed predicted mortality at 6 months (2). Afilalo et al. (5) evaluated gait speed as a predictor of outcomes in elderly patients undergoing cardiac surgery and found that 46% of adults evaluated were frail (gait speed <0.76 m/s) and that frailty was associated with a 2- to 3-fold increase in risk of operative morbidity (stroke, acute renal failure, reoperation, prolonged ventilation, sternal wound infection) and mortality. On the basis of these results, gait speed determined by 5-m walking time is being incorporated into STS and American College of Cardiology registries and risk prediction tools (33).
In contrast, gait speed as a single item was not associated with survival after TAVR in the cohort we studied. This might be due to the high prevalence of extremely slow gait speed in this population. Indeed, with their cut points (0.7 to 0.76 m/s) 80% of the TAVR population would be classified as frail. However, there are significant differences between the cohorts. Among the cardiac surgery subjects mean age was 76 years, all were considered surgical candidates with a mean STS score of 3% for mortality, and there was a low prevalence of ADL disability (5). Among the cohort of hospitalized patients with coronary disease, mean age was 77 years, and mean gait speed was 0.71 ± 0.29 m/s, representing a cohort that is younger and more functional. In contrast, our study subjects were older (mean age 86 years) and had a median gait speed of 0.57 m/s and a mean STS score for mortality of 12%. Epidemiological data suggest that slow gait speed is the first manifestation of the frailty phenotype to develop, whereas shrinking occurs at a more advanced stage (34). An ADL dependence, often a consequence of frailty and comorbidity, is also a later phenomenon (23). In lower-risk populations with a wider range of gait speed, gait speed seems to capture the frailty phenotype and is a useful prognostic tool. This study demonstrates that in a sample of older and frailer patients, gait speed did not have independent risk prediction; instead, a composite index that includes ADL status and an index of malnutrition was better than gait speed for identifying frailty-related risk after TAVR. This highlights one potential advantage to using a composite index that includes early and late manifestations of frailty, namely the ability to discriminate risk across the spectrum of frailty.
Conclusions
A frailty score based on grip strength, gait speed, ADL, and serum albumin provides independent prognostic information in a cohort of older adults undergoing TAVR for severe symptomatic AS. Further studies must continue to evaluate the impact of frailty in older adults with severe AS and other cardiovascular disease and explore how to facilitate routine frailty assessment before major cardiac interventions.
Abbreviations and Acronyms
- ADLs
activities of daily living
- AS
aortic stenosis
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
- PCI
percutaneous coronary intervention
- SAVR
surgical aortic valve replacement
- STS
Society of Thoracic Surgery
- TAVR
transcatheter aortic valve replacement
Footnotes
Drs. Genereux, Daneault, Paradis, Kodali and Williams have consulted for Edwards Lifesciences. Dr. Kodali has served on the advisory board of TAVI-St. Jude’s Medical and Thubrikar Aortic Valve. Drs. Smith and Moses received travel reimbursement from Edwards Lifesciences for their work on the PARTNER trial. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54:1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 3.Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction Circulation. 2011;124:2397–2404. doi: 10.1161/CIRCULATIONAHA.111.025452. [DOI] [PubMed] [Google Scholar]
- 4.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 6.Sundermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33–37. doi: 10.1016/j.ejcts.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26:2714–2720. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- 8.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 9.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 10.Shroyer AL, Coombs LP, Peterson ED, et al. The Society of Thoracic Surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg. 2003;75:1856–1864. doi: 10.1016/s0003-4975(03)00179-6. discussion 1864–5. [DOI] [PubMed] [Google Scholar]
- 11.Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816–822. doi: 10.1016/s1010-7940(99)00106-2. discussion 822-3. [DOI] [PubMed] [Google Scholar]
- 12.Tu JV, Jaglal SB, Naylor CD. Multicenter validation of a risk index for mortality, intensive care unit stay, and overall hospital length of stay after cardiac surgery. Steering Committee of the Provincial Adult Cardiac Care Network of Ontario. Circulation. 1995;91:677–684. doi: 10.1161/01.cir.91.3.677. [DOI] [PubMed] [Google Scholar]
- 13.Rahmanian PB, Adams DH, Castillo JG, Carpentier A, Filsoufi F. Predicting hospital mortality and analysis of long-term survival after major noncardiac complications in cardiac surgery patients. Ann Thorac Surg. 2010;90:1221–1229. doi: 10.1016/j.athoracsur.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:pe24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 15.Gallucci M, Ongaro F, Amici GP, Regini C. Frailty, disability and survival in the elderly over the age of seventy: evidence from “The Treviso Longeva (TRELONG) Study.”. Arch Gerontol Geriatr. 48:281–283. doi: 10.1016/j.archger.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Afilalo J. Frailty in patients with cardiovascular disease: why, when, and how to measure. Curr Cardiovasc Risk Rep. 2011;5:467–472. doi: 10.1007/s12170-011-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shelkey M, Wallace M. Katz index of independence in activities of daily living. J Gerontol Nurs. 1999;25:8–9. doi: 10.3928/0098-9134-19990301-05. [DOI] [PubMed] [Google Scholar]
- 19.Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–269. doi: 10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Neph. 2006;2:364–377. doi: 10.1038/ncpneph0218. [DOI] [PubMed] [Google Scholar]
- 21.Pencina MJ, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 22.Rockwood K. Frailty and its definition: a worthy challenge. J Am Geriatr Soc. 2005;53:1069–1070. doi: 10.1111/j.1532-5415.2005.53312.x. [DOI] [PubMed] [Google Scholar]
- 23.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 24.Millar K, Asbury AJ, Murray GD. Pre-existing cognitive impairment as a factor influencing outcome after cardiac surgery. Br J Anaesth. 2001;86:63–67. doi: 10.1093/bja/86.1.63. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. National Center for Health Statistics. [Accessed on April 18];Health Data Interactive. 2012 Available at: www.cdc.gov/nchs/hdi.htm.
- 26.Harrington MB, Kraft M, Grande LJ, Rudolph JL. Independent association between preoperative cognitive status and discharge location after cardiac surgery. Am J Crit Care. 2011;20:129–137. doi: 10.4037/ajcc2011275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larson EB, Shadlen MF, Wang L, et al. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004;140:501–509. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 28.Pahor M, Guralnik JM, Salive ME, Chrischilles EA, Brown SL, Wallace RB. Physical activity and risk of severe gastrointestinal hemorrhage in older persons. JAMA. 1994;272:595–599. [PubMed] [Google Scholar]
- 29.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52:1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 30.Ewe SH, Ajmone Marsan N, Pepi M, et al. Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am Heart J. 2010;160:1113–1120. doi: 10.1016/j.ahj.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Vermeulen J, Neyens JC, van Rossum E, Spreeuwenberg MD, de Witte LP. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr. 2011;11:33. doi: 10.1186/1471-2318-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green P, Woglom AE, Genereux P, et al. Gait speed and dependence in activities of daily living in older adults with severe aortic stenosis. Clin Cardiol. 2012;35:307–314. doi: 10.1002/clc.21974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.STS/ACC TVT Registry. [Accessed on July 19, 2012]; Available at: https://www.ncdr.com/TVT.
- 34.Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008;63:984–990. doi: 10.1093/gerona/63.9.984. [DOI] [PubMed] [Google Scholar]