Abstract
The subjective measures used to study mood disorders in humans cannot be replicated in animals; however, the increasing application of objective neuropsychological methods provides opportunities to develop translational animal tasks. Here we describe a novel behavioral approach, which has enabled us to investigate similar affective biases in rodents. In our affective bias test (ABT), rats encounter two independent positive experiences—the association between food reward and specific digging substrate—during discrimination learning sessions. These are performed on separate days under either neutral conditions or during a pharmacological or affective state manipulation. Affective bias is then quantified using a preference test where both previously rewarded substrates are presented together and the rat's choices recorded. The absolute value of the experience is kept consistent and all other factors are counterbalanced so that any bias at recall can be attributed to treatment. Replicating previous findings from studies in healthy volunteers, we observe significant positive affective biases following acute treatment with typical (fluoxetine, citalopram, reboxetine, venlafaxine, clomipramine) and atypical antidepressants (agomelatine, mirtazapine), and significant negative affective biases following treatment with drugs associated with inducing negative affective states in humans (FG7142, rimonabant, 13-cis retinoic acid). We also observed that acute psychosocial stress and environmental enrichment induce significant negative and positive affective biases, respectively, and provide evidence that these affective biases involve memory consolidation. The positive and negative affective biases induced in our test also mirror the antidepressant and pro-depressant effects of these drugs in patients suggesting our test has both translational and predictive validity. Our results suggest that cognitive affective biases could contribute to drug- or stress-induced mood changes in people and support the hypothesis that a cognitive neuropsychological mechanism contributes to antidepressant drug efficacy.
Keywords: depression, antidepressant, pro-depressant, affective bias, animal model
INTRODUCTION
The lack of good translational animal tests for psychiatry research is a major obstacle to the development of new treatments, and limits the interpretation of animal experiments investigating disease biology (Cryan and Holmes, 2005; Cryan and Slattery, 2007; Nestler and Hyman, 2010; Keeler and Robbins, 2011, Berton et al, 2012). Affective disorders such as depression represent a major social and economic cost (Moussavi et al, 2007) yet researching their basic biology, identifying novel drug targets, and evaluating the efficacy of new treatments are severely restricted by the animal tests currently available. Studies in animals have also failed to predict clinical outcomes in relation to safety and pro-depressive side effects. Current methods used to assay depression-related behaviors, such as the forced swim test, work well for drugs with mechanisms of action similar to current ones but do not offer a good approach for investigating novel drug mechanisms (Berton et al, 2012). These limitations may underlie the lack of development of new, improved treatments and are known to have led to very costly failures within the drug industry. For example, aprepitant, the neurokinin receptor antagonist, lacked efficacy in patients despite animal studies predicting an antidepressant profile (Hickie and Rogers, 2011). The anti-obesity drug, rimonabant, was withdrawn from the market following evidence that its use was associated with an increased risk of suicidal tendencies and depression, something which the animal safety studies undertaken failed to predict (Rumsfeld and Nallamothu, 2008).
Recent advances in the use of cognitive neuropsychological testing in human depression research have provided a new opportunity for developing translational methods in non-human species. A cognitive model of depression was first proposed by Beck, (1967) and has received renewed interest in recent years. Cognitive neuropsychological research has revealed that affective disorders are associated with negative biases in emotional processing and cognition (Beck, 1967; Gur et al, 1992; Watkins et al, 1996; Surguladze et al, 2005; Leppänen, 2006; Ressler and Mayberg, 2007; Mathews and MacLeod, 2005; Gotlib and Joormann, 2010; Elliott et al, 2011; Roiser et al, 2012). Studies investigating emotional recognition, categorization, and memory have shown that both depressed patients and those with a vulnerability to depression exhibit negative cognitive biases (Gur et al, 1992; Watkins et al, 1996; Surguladze et al, 2005; Leppänen, 2006; Ressler and Mayberg, 2007; Mathews and MacLeod, 2005; Gotlib and Joormann, 2010; Elliott et al, 2011; Roiser et al, 2012). One of the most exciting observations using these approaches has been consistent data showing that acute and short-term treatments with antidepressant drugs induce a positive shift in emotional processing (Harmer et al, 2008; Harmer et al, 2009; Pringle et al, 2011; Roiser et al, 2012). Recent findings also suggest that acute administration of an antidepressant modifies autobiographical memories associated with personal experiences (Papadatou-Pastou et al, 2012). Neuroimaging studies broadly confirm relevant functional effects of antidepressants in regions such as the amygdala and orbitofronal cortex, whereas hyperactivity in the subgenual cingulated cortex has been linked to depressed mood (Leppänen, 2006; Ressler and Mayberg, 2007; Pringle et al, 2011). Taken together, these observations suggest that a specific cognitive mechanism has an important role not only just in the development and perpetuation of depressive illness (Elliott et al, 2011; Roiser et al, 2012) but also its treatment with antidepressant therapies (Harmer et al, 2009; Pringle et al, 2011; Roiser et al, 2012). Further, it provides a new paradigm for screening novel antidepressant drugs in humans. It could also provide a bridging mechanism for back-translation of such screening tests into rodents.
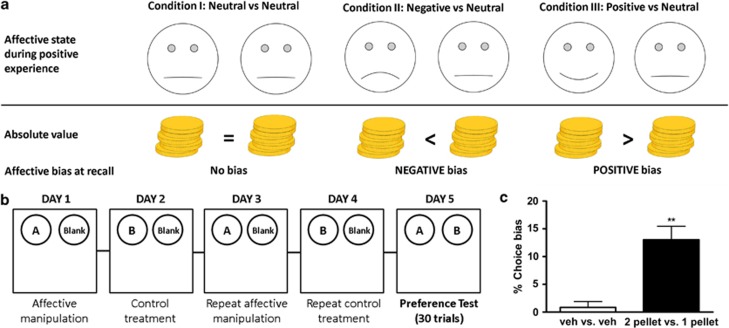
Developments in the animal welfare field provided the first evidence that non-human species exhibit cognitive affective biases (Harding et al, 2004; Mendl et al, 2010), although pharmacological validation of these approaches has been limited (Anderson et al, 2013). In this study, we describe the evaluation of an affective bias test (ABT) for rats designed to test the hypothesis that drugs with acute effects on affective bias in human will have the same effects in rodents (Figure 1a). In our ABT, rats encounter two independent positive experiences—the association between food reward and specific digging substrate—during discrimination learning sessions. These are performed on separate days under either neutral conditions or during a pharmacological or affective state manipulation (Figure 1b). Affective bias is then quantified using a preference test where both previously rewarded substrates are presented together and the rat's choices recorded. The absolute value of the experience is kept consistent and all other factors are counterbalanced so that any bias at recall can be attributed to treatment, as predicted by our hypothesis (Figure 1a). To test the predictive validity of the task, we investigated both typical and atypical antidepressants, drugs known to induce negative affective states in human, and a number of control treatments including drugs of abuse. We further investigated affective bias in this task using psychosocial manipulations of affective state and tested whether the mechanism involved memory consolidation.
Figure 1.
Hypothesis, method overview, and results from experiment design validation. The task is designed to test the hypothesis that the manipulations used during learning will induce a bias at recall, which predicts their effects on mood and emotional processing seen in human (a). Animals undergo a 5-day training and testing procedure (b). This involves the acquisition of two independent positive experiences (substrate-reward association learning in a bowl digging, discrimination task) over four counterbalanced pairing sessions under control or treatment conditions. Treatment-induced affective bias is then assayed by recording the number of choices made for each substrate-reward association. An example of the experimental procedure for one animal is shown in b. All conditions are fully counterbalanced and the positions of the bowls are pseudorandom to ensure rats do not use spatial cues. The results shown in c illustrate control experiments undertaken to test the validity of the experiment design. Vehicle vs vehicle treatment confirmed that the assay design does not result in a significant bias (one sample t15=0.56, p=0.58, n=16). We also show that rats will bias their responding toward the substrate they previously learnt to associate with the higher value reward (c, one sample t-test, t15=4.2, **p<0.001, n=16). Data shown as mean % choice bias±SEM (Veh, vehicle control).
MATERIALS AND METHODS
Apparatus
The animals were tested in a Perspex arena, 40 cm2. The substrates, eg, paper bedding, sawdust, sand, cloth, perlite, etc, were placed in glazed pottery bowls and presented in a pseudo-random order in the left or right position to prevent the rats using spatial cues.
Subjects
The animals used were 6 cohorts of 16 male Lister-hooded rats weighing approximately 300–350 g at the start of dosing (Harlan, UK), housed in groups of two to four under temperature-controlled conditions and a 12 : 12 h light–dark cycle (lights off at 0700 h). They were maintained at approximately 90% of their free-feeding weight by restricting access to laboratory chow (Purina, UK) to ∼18 g per rat per day. Water was provided ad libitum. All procedures were conducted in accordance with the requirements of the UK Animals (Scientific Procedures) Act 1986 and in accordance with local institutional guidelines. All behavioral testing was carried out between 0900 h and 1700 h during the animals' active phase.
Training
On the first day of training, one pellet (45 mg sucrose tablet, Sandown Scientific, UK) was buried within the sawdust of two bowls, and another three placed on top to encourage digging. Each rat was individually placed in the test arena and given 10 min to explore or until all pellets had been consumed and the rat had left the second bowl. On the consecutive training day, a single sugar pellet was buried in each bowl and the rats were allowed 5 min to explore both bowls. Training was complete when each rat was able to find the pellet in both bowls on 12 consecutive trials.
ABT: general protocol
Each study followed a standard protocol of four pairing sessions followed by a choice test session on the fifth day. All pharmacological studies were carried out blinded to treatment. Control experiments with two vs one reward pellet and psychosocial manipulations of affective state were blind for the preference test session only. Animals were randomly assigned to one of four counterbalanced groups to prevent bias associated with substrate, drug, or treatment day. A summary is given in Supplementary Table 1.
Reward pairing
Each pairing session consisted of individual trials in which the rat was required to choose between one of the two bowls to locate a sugar pellet reward. In each of these trials, one of the bowls contained a ‘reward-paired' substrate and the other contained a different, ‘blank' substrate. The blank substrate was the same for all pairing sessions. In the blank substrate, the equivalent number of sugar pellets was crushed into the bowl to avoid discrimination from reward-paired substrate (A or B) based on odor. The rat was placed in front of the two bowls and allowed to dig in one of the two bowls. Once the animal began to dig, the other bowl was removed from the test arena. Digging in the reward-paired substrate was recorded as a correct trial, and digging in the blank substrate was recorded as an incorrect trial. The latency to dig was also recorded for each trial and the session was completed once the rat reached a criterion of six consecutive correct trials (the probability of making a six consecutive correct choices by chance being 0.015). The second pairing session followed the same protocol, but the rats were presented with the second reward-paired substrate. The two pairing sessions were repeated to give a total of four sessions on consecutive days.
Preference testing
On the fifth day, the rats were presented with both reward-paired substrates for a total of 30 trials. A single-pellet reward was placed using a random reinforcement protocol such that there was a one in three reward probability for each substrate.
Effect of absolute reward value (1 or 2) on affective bias
Reward-pairing sessions were as described except one reward-paired substrate was paired with a single sugar pellet, whereas the other was paired with two sugar pellets. During choice testing, both substrates were rewarded equally with a single sugar pellet using the random reinforcement protocol.
Systemic pharmacological treatments
One substrate was paired following drug pre-treatment vs vehicle treatment. The absolute value of the reward (one pellet) was the same for each session. All dose-response drug studies used a within-subject fully counterbalanced drug treatment schedule across 4 weeks (eg, three doses of drug plus vehicle) so that on any given week, all treatments were equally represented. Single-dose experiments followed the same protocol but using only one dose of the drug.
Drugs
Fluoxetine1 (0.3, 1.0, 3.0 mg/kg, i.p., t=−30 min), citalopram1 (0.1, 0.3, 1.0, 3.0 mg/kg, i.p., t=−30 min), reboxetine1 (0.1, 0.3, 1.0 mg/kg, i.p., t=−30 min), venlafaxine1 (1.0, 3.0, 10.0 mg/kg, i.p., t=−30 min), clomipramine1 (1.0 mg/kg, i.p., t=−60 min), diazepam2 (0.3, 1.0, 3.0 mg/kg, i.p., t=−30 min), and morphine sulfate1 (5.0 mg/kg, t=−30 min) were purchased from Tocris Bioscience, UK. 13-cis-Retinoic acid2 (1.0, 3.0, 10.0 mg/kg, i.p., t=−60 min), FG71422 (1.0, 3.0, 5.0 mg/kg, i.p., t=−30 min), nicotine1 (0.06 mg/kg, i.p., t=−30 min), cocaine1 (3.0 mg/kg, i.p., t=−10 min), amphetamine (1.0 mg/kg, i.p., t=−15 min), and alcohol1 (800 mg/kg, i.p., t=−30 min) were purchased from Sigma-Aldrich, UK. Rimonabant2 (1.0, 3.0, 10.0 mg/kg, i.p., t=−30 min), agomelatine2 (0.1, 0.3, 1.0 mg/kg, i.p., t=−30 min), and aprepitant2 (0.3, 1.0, 3.0, 10.0, 30.0 mg/kg, i.p., t=−30 min) were kindly provided by Pfizer. Drugs were dissolved in 0.9% sterile saline1 or 10% DMSO, 20% cremophor, 80% saline vehicle2 in a dose volume of 1 ml/kg.
All drug doses were selected based on previous studies and in-house data kindly provided by Pfizer. For the antidepressant drugs, dose were based on ED50 data from in vivo displacement of [3H]citalopram (fluoxetine=0.75 mg/kg, citalopram=0.1 mg/kg; venlafaxine=3.6 mg/kg, s.c., Anne Schmidt, personal communication) or [3H]nisoxetine (reboxetine, 3 mg/kg s.c., ∼70% NET occupancy, Gray et al, personal communication). The doses used were also in a similar range to those published in our previous behavioral studies using monoamine reuptake inhibitors (Robinson, 2012; Humpston et al, 2013). Doses for agomelatine (Bourin et al, 2004), aprepitant (Wallace-Boone et al, 2007), FG7142 (Rogers et al, 1995), rimonabant (Tzavara et al, 2003), and 13-cis-retinoic acid (Ferguson et al, 2005; O'Reilly et al, 2006) were based on previous studies in rodents. Doses used for diazepam were based on previous studies showing anxiolytic effects in other animal models (Wieland and Lucki, 1990). The drugs of abuse were tested at doses previously shown to be effective in conditioned place preference experiments (Bardo et al, 1995; Stewart et al, 1996).
Restraint stress and isolation housing
One substrate was paired during a 24-h period of isolation housing (1700–1700 h) and immediately following 10 min in a restraint tube (∼09.00 h). Isolated rats were housed in unenriched cages separated with paper partitions to prevent visual contact between animals. The other substrate was paired during control conditions (normal group housing and without restraint stress). Isolated animals were returned to their home cages at 1700 h on day 4, before choice testing on day 5.
Social play
One substrate was paired during an 8-h period of ‘social play' (0900–1700 h). During this period, animals were placed in groups of eight in a highly enriched arena (100 × 100 × 50 cm3) with access to water ad libitum. All rats had been habituated to the arena for ∼2 h per day for 5 consecutive days before the start of the study.
Effects of treatments before and immediately after pairing sessions
One substrate was paired with the venlafaxine treatment (3 mg/kg, i.p.) or restraint stress and isolation housing, administered before or immediately following the pairing session.
Statistical analysis
Graphs were constructed using Graphad Prism 4.0 (Graphpad Software, USA). Choice bias was calculated based on the number of choices made for the treatment-paired substrate vs the total number of trials (30/animal). Latency and trials to criterion were recorded during pairing sessions and analyzed to determine if the drug had any nonspecific effects, eg, sedation, anorexia. Statistical analyses were performed using SPSS ver 16. For the dose response experiments, choice bias data were analyzed using a repeated-measures ANOVA with TREATMENT as factor. Post hoc analysis for each drug dose used a one-sample t-test against a theoretical mean of 0% choice bias. Analysis of the choice latency and trials to criterion was made using a paired t-test comparing drug vs vehicle for the pairing sessions. Choice data for single-dose studies were made using a one-sample t-test against a theoretical mean of 0% choice bias. Where appropriate, between treatments comparisons were made using a paired t-test.
RESULTS
Evaluation of Experiment Design Validity
Experiments performed using two neutral treatments confirmed the experiment design with no significant bias observed (one sample t-test, t15=0.56, p=0.58, n=16/grp) and a mean choice bias close to zero percent (Figure 1c). In our second control experiment, we doubled the absolute value of the reward during one of the pairing sessions to determine whether rats would bias their subsequent choices toward the previously encountered ‘more positive' experience. Consistent with our prediction, animals made significantly more choices (one sample t-test, t15=4.2, p<0.001, n=16) for the substrate previously paired with the higher amount of reward (Figure 1c). Paired t-tests revealed that during the two-pellet pairing sessions animals were faster to respond (test group 2) or faster to learn the association (test group 1). A summary of the results from the pairing session are given in Supplementary Table 2.
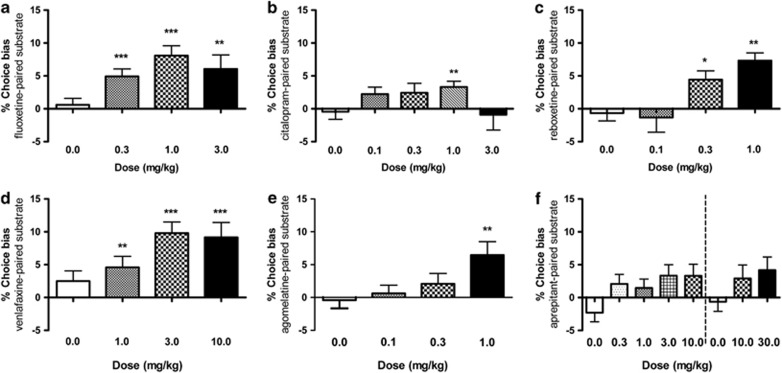
Acute Treatment with Typical and Atypical Antidepressants Induce Positive Affective Biases in Rats
Acute treatment with the serotonin-specific reuptake inhibitor (SSRI), fluoxetine, induced a highly significant positive bias in rats (0.3–3.0 mg/kg, RM ANOVA, F3,45=6.10, p=0.0014, Figure 2a). A similar effect was also observed for the SSRI, citalopram (0.1–1.0 mg/kg, RM ANOVA, F3,45=1.9, p=0.046, Figure 2b). We observed that results for both SSRIs, although more clearly seen with citalopram, exhibited bell-shaped dose-response curves over a narrow dose range. We observed that individual animals showed different levels of sensitivity to the SSRIs, suggesting their ability to induce a positive affective bias was highly dose dependent. The noradrenaline-specific reuptake inhibitor, reboxetine (0.1–1.0 mg/kg, RM ANOVA, F3,45=7.15, p=0.0005, Figure 2c), and mixed serotonin and noradrenaline reuptake inhibitor, venlafaxine (0.3–10.0 mg/kg, RM ANOVA, F3,45=4.43, p=0.0082, Figure 2d), induced a significant positive affective biases in rats.
Figure 2.
Acute treatment with typical and atypical antidepressants induce a positive affective bias in rats. Acute treatment with antidepressant drugs induced significant, dose-dependent positive affective bias in rats. The results shown in panel a illustrate induction of positive bias following acute treatment with the serotonin-specific reuptake inhibitors fluoxetine (RM ANOVA, F3,45=6.10, p=0.0014, n=16) and citalopram (RM ANOVA, F3,45=1.9, p=0.046, b). The noradrenaline reuptake inhibitor, reboxetine (RM ANOVA, F3,45=7.15, p=0.0005, n=16, c), and mixed noradrenaline and serotonin reuptake inhibitor, venlafaxine (RM ANOVA, F3,45=4.43, p=0.0082, d), both induced a significant dose-dependent positive affective bias. The atypical antidepressant, agomelatine induced a significant positive bias (RM ANOVA, F3,45=4.1, p=0.01, e), whereas aprepitant showed only weak efficacy (RM ANOVA, 0–10 mg/kg, F3,45=2.9, p=0.029, RM ANOVA 0–30 mg/kg, F3,45=2.32, p=0.11, f) with no post-hoc significant difference observed. Data shown as mean % choice bias±SEM, *p<0.05, **p<0.01, ***p<0.001 (one sample t-test against the theoretical mean of 0%). Dotted line indicates different experiments.
Acute treatment with agomelatine, a melatonergic agonist and 5-HT2C antagonist, induced a significant positive affective bias (0.1–1.0 mg/kg, RM ANOVA, F3,45=4.1, p=0.01, Figure 2e), suggesting the assay is also sensitive to drugs acting through a non-monoamine reuptake mechanism. In contrast, the neurokinin antagonist, aprepitant exhibited only weak efficacy in our test. Using low doses, a significant main effect of treatment was observed (0.3–10.0 mg/kg, RM ANOVA, F3,45=2.9, p=0.029, Figure 2f), although no individual dose was significantly different from 0% choice bias. In a second dose response experiment, using higher doses, no significant main effect was seen (10–30 mg/kg, RM ANOVA, F2,30=1.89, p=0.11), suggesting aprepitant exhibits limited efficacy in this test. In this second experiment, aprepitant also significantly slowed the choice latency during pairing sessions at both 10 and 30 mg/kg (Supplementary Table 3). None of the other antidepressant drug treatments altered discrimination learning or motivation during the pairing sessions (Supplementary Table 3).
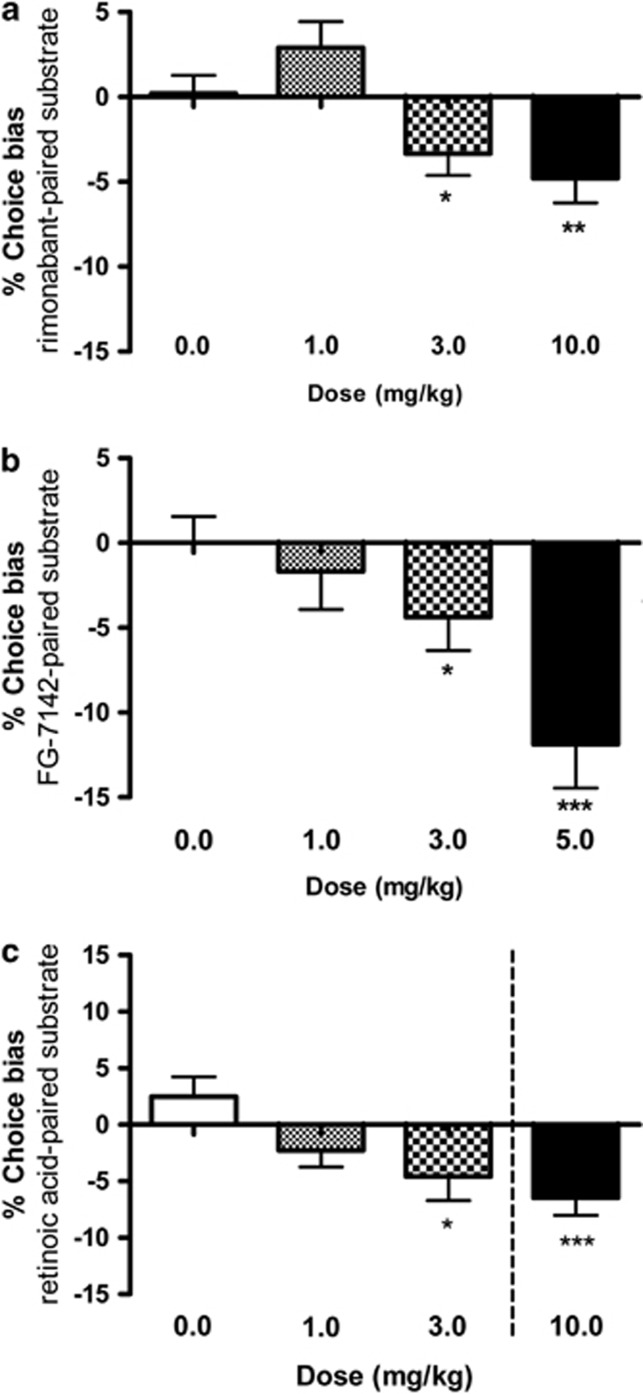
Pharmacological Induction of an Acute Negative Affective State Induces a Negative Affective Bias
Animals treated with the cannabionoid1 receptor antagonist/inverse agonist, rimonabant made significantly fewer choices for the substrate-reward experience encountered following treatment indicating a negative affective bias (1.0–10.0 mg/kg, RM ANOVA, F3,45=2.04, p=0.03, Figure 3a). Although at lower doses rimonabant tended to induce a positive affective bias, the higher doses induced a significant negative bias. Animals made significantly fewer choices for the substrate-reward experience encountered following treatment with FG7142 indicating induction of a negative affective bias (1.0–5.0 mg/kg, RM ANOVA, F3,45=5.23, p=0.004, Figure 3b). Treatment with the active ingredient of Roaccutane, 13-cis-retinoic acid tended to induce a negative affective bias (1.0–3.0 mg/kg, RM ANOVA F=3.18, p=0.056) and a 10 mg/kg dose induced a significant negative bias when administered using the single dose method (t15=4.1, p=0.0009). Treatment with rimonabant, FG7142, or 13-cis-retinoic acid had no significant effects during discrimination learning (Supplementary Table 4).
Figure 3.
Drugs known to induce negative affective states in human induce negative affective biases in rats following acute treatment. The results shown in a illustrate induction of negative affective bias following acute treatment with the CB1-antagonist/inverse agonist, rimonabant (RM ANOVA, F3,45=2.04, p=0.003, n=16, a). A similar, significant negative affective bias was observed for the anxiogenic benzodiazepine inverse agonist, FG7142 (RM ANOVA, F3,45=5.23, p=0.004, n=16, b). Treatment with 13-cis-retinoic acid tended to induce a negative affective bias (RM ANOVA 0–3 mg/kg, F2,30=3.18, p=0.056) with post-hoc t-test against the theoretical mean suggesting an effect at 3 mg/kg (p<0.05 in parenthesis, c). A further, single-dose study using 10 mg/kg retinoic acid revealed a significant negative affective bias (t15=4.1, p=0.0009, c). Data shown as mean % choice bias±SEM, *p<0.05, **p<0.01, ***p<0.001 (one sample t-test against the theoretical mean of 0% or paired t-test). Dotted line indicates different experiments.
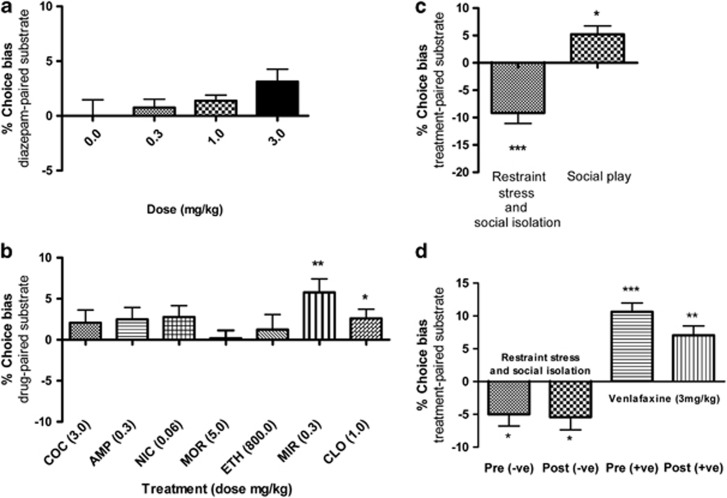
Specificity of the Rodent ABT
Acute treatment with the non-antidepressant anxiolytic, diazepam (0.3–3.0 mg/kg), did not induce a significant bias at recall (Figure 4a). However, at the highest dose, the drug induced a significant increase in choice latency during pairing sessions associated with drug treatment (t15=2.8, p<0.014; Supplementary Table 5). No significant affective bias, positive or negative, was observed when the assay was run using acute administrations of a either stimulant or non-stimulant drugs of abuse (Figure 4b). Each drug was tested at a dose previously shown to induce conditioned place preference in animals (Bardo et al, 1995; Stewart et al, 1996). Both morphine (5 mg/kg) and alcohol (800 mg/kg) induced a significant increase in choice latency during pairing sessions associated with drug treatment (Supplementary Table 5), probably due to sedative effects. Animals treated with a higher dose of amphetamine (1.0 mg/kg) or cocaine (10.0 mg/kg) failed to complete the pairing sessions (data not shown). We also tested two further antidepressants, mirtazapine (0.3 mg/kg) and clomipramine (1.0 mg/kg), and show that acute treatments resulted in a positive bias in the ABT (Figure 4b).
Figure 4.
Pharmacological and psychosocial manipulations reveal that affective bias is specific to manipulations associated with mood changes in human and may involve memory consolidation. Neither the anxiolytic, diazepam (panel a), nor any of the drugs of abuse tested (b) induce any significant affective bias (COC, cocaine; AMP, amphetamine; NIC, nicotine; MOR, morphine; ETH, ethanol). The antidepressants clomipramine (CLO) and mirtazapine (MIR) induced positive affective biases (b). The ABT is also sensitive to psychosocial manipulations of affective state in rats (c). Restraint stress and social isolation during substrate-reward learning induced a significant negative choice bias at recall (t15=4.8, p=0.0002). Conversely, animals undergoing a period of social play during learning showed a significant positive bias (t15=2.7, p=0.017). Results in d show that affective bias in the task involves memory consolidation. Restraint stress immediately following (t15=2.8, p=0.013), as well as before (t15=2.8, p=0.013) substrate-reward learning, induced a significant negative bias at recall. Similarly, venlafaxine induced a significant positive bias in animals treated before (t15=7.8, p<0.0001) or immediately following learning (t15=5.0, p=0.0002). Data shown as mean % choice bias±SEM, *p<0.05, **p<0.01, ***p<0.001 (one sample t-test against the theoretical mean of 0% or paired t-test).
The ABT is Sensitive to Affective State Changes Induced by Psychosocial Manipulations in Rats
Induction of an acute negative affective state, using restraint stress and isolation housing, resulted in animals making significantly fewer choices for the substrate-reward experience associated with the affective manipulation (t15=4.8, p=0.0002, Figure 4c). These effects were similar in magnitude to the results obtained following pharmacological manipulations (Figure 3a–c). Rats made significantly more choices for the substrate-reward associations learnt during exposure to the enriched environment, suggesting that social enrichment induces a positive affective bias similar to the effects seen following antidepressant drug treatment (t15=2.7, p=0.017, Figure 4c).
Role of Memory Consolidation in Affective Bias
Using restraint stress and isolation housing, we show that there is no significant effect of the timing of the affective manipulation, indicating that the negative affective bias observed is specific and probably involves effects on memory consolidation post-encoding (Figure 4d). Venlafaxine treatment before and immediately after learning resulted in a significant, positive affective bias (Figure 4d). Although there is a tendency for the effects post-learning to be lower, this is not significantly different from pre-learning administration.
DISCUSSION
Our assay was specifically developed to test whether pharmacological and non-pharmacological manipulations induce biases in rodent choice behavior and how these relate to affective state and mood changes in humans. Our initial experiments confirmed the design of the assay and its sensitivity to absolute changes in reward value. Results from the ABT, using acute antidepressant drug treatments, revealed significant positive affective biases in rats replicating the results in healthy volunteers (Table 1, Pringle et al, 2011). We observe similar positive affective biases following acute treatments with both typical and atypical antidepressants. Treatment with fluoxetine, citalopram, clomipramine, reboxetine, or venlafaxine induced a significant positive affective bias, although the effects of the SSRIs tended to be bell-shaped, an effect that may relate to their tendency to induce anxiogenic effects at higher doses. We also observed a relatively higher efficacy with fluoxetine vs citalopram, which may result from differences in their pharmacokinetic profile. Citalopram was found to have a relatively short half-life in rats (Arnt et al, 1984), whereas fluoxetine has a long half-life of about 24 h (Wong et al, 1990). In this study, we only tested the racemic mixture of citalopram and the S-isomer is known to be more effective, whereas the R-isomer has been proposed to reduce overall efficacy through competition for the binding site on the serotonin transporter (Casper et al, 2009). Further studies using escitalopram will be needed to address this. It is also interesting to note that our results found significant effects with the monoamine reuptake inhibitors at doses which were lower than those effective in animal models of depression such as the forced swim test (Table 1), although similar to doses previously shown to be effective in cognitive behavioral tests in rodents (Robinson, 2012; Humpston et al, 2013.
Table 1. Comparison Between Observations in Neuropsychological Studies of Emotional Processing in Healthy Volunteers, Rodent Forced Swim Data and Results from Our Rodent Affective Bias Test.
| Clinical effects/side effects | Facial emotion recognition (mg)a | Emotional memory (mg)a | Forced swim test (mg/kg)b | Affective bias test (mg/kg)b | References | |
|---|---|---|---|---|---|---|
| Citalopram | Antidepressant | Pos (20) | Pos (20) | Pos (5–20) | Pos (1.0–3.0) | Harmer et al, 2004; Browning et al, 2007 |
| Reboxetine | Antidepressant | Pos (4.0) | Pos (4.0) | Pos (10) | Pos (0.3–1.0) | Harmer et al, 2003; Harmer et al, 2004; Connor et al, 1999 |
| Mirtazapine | Antidepressant | Pos (15) | Pos (15) | Pos (20) | Pos (0.3) | Arnone et al, 2009; Reneric et al, 2002 |
| Agomelatine | Antidepressant | Pos (25) | Pos (25) | Pos (4–32) | Pos (1.0) | Harmer et al, 2011; Bourin et al, 2004 |
| Aprepitant | Failed antidepressant | Pos (125) | Neu (125) | Posc (1.0–30) | Neu (0.3–10) | Chandra et al, 2010; Wallace-Boonce et al, 2007 |
| Diazepam | Benzodiazepine anxiolytic | Neu (5.0) | Neu (5.0) | Neu (5.0) | Neu (3.0) | Murphy et al, 2008; Wieland and Lucki, 1990 |
| Amphetamine | Psychostimulant | Neud (20) | Neu (20) | Pos (10) | Neu (0.3) | Wardle et al, 2012; Porsolt et al, 1977; Wieland and Lucki, 1990 |
| Rimonabant | Anti-obesity, pro-depressant side effects | Neu (20) | Neg (20) | Pos (3.0) | Neg (1.0–3.0) | Horder et al, 2009; Tzavara et al, 2003 |
Facial emotion recognition: effect on perception and/or threshold of facial expression of emotion following acute or short-term treatment.
Emotional memory: effect on recall or recognition of positive vs negative personality characteristics following acute or short-term treatment.
Affective bias test: effect on choice bias.
Forced swim test: effect on immobility time.
‘Pos' indicates a positive effect (enhanced positive and/or reduced negative), ‘Neg' indicates a negative effect (reduced positive and/or enhanced negative), ‘Neu' indicates no significant effect.
Total dose.
Effective dose (or full experimental dose range where no significant effect was observed).
Gerbil forced swim test.
Decreased threshold for detecting both positive and negative emotions.
A similar positive bias was also observed following treatment with agomelatine and mirtazapine but not aprepitant consistent with their antidepressant efficacy, or lack thereof, in depressed patients (Keller et al, 2006; Hickie and Rogers, 2011). Similar to the effects of antidepressant treatment, environmental enrichment induced a small but significant positive bias suggesting that non-pharmacological induction of a positive affective state induces a similar positive affective bias in this test. Although these studies used experience of a positive event rather than the processing of an emotional stimulus, as measured in human studies, a similar acute effect of antidepressant drugs was observed.
In our study, both pharmacological and non-pharmacological inductions of negative affective states were shown to induce negative affective bias in rats in this task. The pharmacological treatments used have previously been shown to induce negative affective symptoms in human (Evans and Lowry, 2007; Rumsfeld and Nallamothu, 2008; Horder et al, 2009; Bremner et al, 2012) and psychosocial stress is thought to have an important role in the development of depression (Heim et al, 2008; Vreeburg et al, 2009; Wingenfeld and Wolf, 2011). Pharmacological induction of negative affective states and their effects on cognition have not been widely studied in humans, however, acute treatment with rimonabant induces negative biases in emotional processing in healthy volunteers (Horder et al, 2009), and therapeutic use increases the risk of developing depressive symptoms and causes suicidal ideation in some patients (Rumsfeld and Nallamothu, 2008). Similarly, treatment with 13-cis-retinoic acid has been associated with an increased incidence of depression in patients (Bremner et al, 2012). These results mirror the findings in human suggesting this test could provide a method that predicts risk associated with drug-induced adverse psychiatric effects. The results from the psychosocial stress manipulation are particularly interesting as they suggest that positive experiences encountered during a negative affective state are likely to have reduced value compared with experiences encountered in a positive or neutral affective state. In depression, patients exhibit anhedonia and reduced motivation to engage in rewarding activities (DSM IV, 1994), an effect that may be related to the psychological effects observed in this animal paradigm.
Similar experiments performed with the anxiolytic drug, diazepam, and known drugs of abuse, did not induce affective biases in this task. One issue that has challenged the monoamine hypothesis of depression is the lack of efficacy of the stimulant drugs of abuse such as amphetamine and cocaine, which are also inhibitors of monoamine reuptake. In our studies, neither the stimulant nor non-stimulant drugs of abuse enhanced reinforcer value at recall despite being administered at doses known to induce conditioned place preference in animals (Bardo et al, 1995; Stewart et al, 1996). Recently, a study investigating emotional processing in human following amphetamine treatment found a similar lack of effect, although the authors observed that amphetamine reduced the threshold for detection of both positive and negative emotion (Wardle et al, 2012). Given our later findings suggesting that the effects observed in this assay involve memory consolidation, the relatively short half-lives of cocaine and amphetamine may also limit their effects on memory consolidation.
Studies in animals have shown that dopamine and opioid signaling, associated with mesolimbic pathways, are important in the experience of reward and reward value at the time they are encountered (Berridge and Kringelbach, 2008). The fact that the drugs of abuse tested failed to induce positive affective biases, suggests that hedonia, arising from direct effects on reward systems, is not involved in effects we observed. Studies using the sucrose preference test have also largely failed to show any hedonic or anhedonic effects following acute manipulations of affective state (Papp, 2012; unpublished observation), suggesting that changes in hedonic processes, at the time of ingestion, are not a key mediator of these effects. Emotional arousal and stress-induced activation of the hypothalamic-pituitary axis, leading to increased levels of glucocorticoids, has been shown to enhance memory consolidation but impair memory at retrieval (Kim and Diamond, 2002). In our study, the direction of the bias observed corresponds with affective valence suggesting the effects are not due to an enhancement or impairment in memory formation but a bias in the value attributed to the experience. Control variables recorded during the pairing sessions suggest that these biases did not develop as a result of direct effects on appetitive behavior or motivation at the time of learning. The latency to approach the bowl and dig was not significantly altered by drug treatments, which induced significant positive or negative affective bias. Drugs from a number of different classes and pharmacological sites of action were used and the results are consistent with their actions on emotional behavior rather than appetite or motivation. We also further investigated the effects of venlafaxine or psychosocial stress and memory by administering the treatments before or immediately after exposure to the positive experience (substrate-reward association). Both treatments induced similar positive and negative affective biases irrespective of the timing of treatment, suggesting these results arise from effects of treatment on consolidation of the memory of that experience.
In patients treated with antidepressants, their subjective reporting of mood does not improve immediately and clinical benefits are delayed. The neuropsychological hypothesis proposes that these delays are due to the fact that the subjective awareness of mood is related to a patient's prior experiences and memory (Harmer et al, 2009). Time for new, positively biased experiences and learning is required before the acute effects of treatment of cognitive function translate to a shift in memory and subjective experience of mood. Our findings add to the neuropsychological hypothesis to show that acute antidepressant treatments also positively bias reward learning to influence memory. These animal data suggest that drugs that can induce changes in mood state also have acute effects on memories for positive experiences biasing the way they are stored long term to influence the subsequent behavior.
Our results suggest that affective state arises from a specific neurochemical environment, which also acts to modulate memory consolidation. This may involve similar processes to those suggested for emotional processing biases observed in people and would support the neuropsychological hypothesis of antidepressant drug action (Harmer et al, 2009). Considering the similarity of effects, we observed between pharmacological and psychosocial manipulations, the drugs used may induce neurochemical changes in the brain, which mimic the effects of naturally induced affective states resulting in drug-induced biases. The effects appear to involve actions during memory consolidation suggesting they require the affective state or drug to retain its actions beyond the initial acquisition phase. Drugs with shorter half-lives, eg, stimulants, may not have long enough pharmacological action or may not involve actions on key transmitter systems. The pharmacological profiles of the drugs exhibiting efficacy in this assay suggest that direct or indirect effects on the serotonin system are important.
Rather than the value of a particularly memory being formed on the basis of that specific transient experience, an evolutionary advantage may be gained by integrating the value attributed to individual experiences with the overall affective state of the organism. Importantly, affective state at the time of the experience does not appear to be required for the effects we observe, suggesting that a rewarding experience can be subsequently biased if a later positive or negative affective state is encountered. A biological process that integrates experiences encountered and affective state could offer an evolutionary advantage.
Overall, these findings suggest that our ABT provides a non-human assay of cognitive affective bias with both translational and predictive validity. We provide evidence that drugs which have acute effects on objective measures of emotional processing in human induce similar effects in our rodent test (Table 1, Pringle et al, 2011). Further work is needed to understand more about the mechanisms involved in the effects we have observed, although our initial studies suggest the affective bias observed results from effects on memory consolidation. Extending the validation of the assay using additional antidepressant treatments and manipulations known to induce negative affective states, eg, tryptophan depletion and CO2-inhalation are necessary. However, this work adds support to theories about cognitive neuropsychological mechanisms in depression (Roiser et al, 2012) involving a direct and acute relationship between affective state and cognitive processes associated with memory for positive experiences, which may have long-term effects on behavior. Although we are not able to measure affective biases in animals using stimuli directly equivalent to healthy volunteer and patient studies, the results obtained suggest a good correlation between affective bias associated with a positive experience, as measured in the ABT, and the biases in emotional processing, particularly with regard to memory, seen with similar treatments in humans (Harmer et al, 2008; Pringle et al, 2011; Roiser et al, 2012).
Acknowledgments
We thank Andy Mead, Pfizer, and the Pfizer Biostatistics Department (John Sherington, Foteini Strimenopoulou and John Parrott) for their input into the experiment design and statistical analysis of the rodent affective bias test. We also acknowledge Emma Mitchell, Nadia Crelin, Radhika Patel, Christian Wood, and Nicholas Donnelly who contributed to work developing the assay. SAS is funded by a BBSRC Industrial CASE studentship with Pfizer, UK. This work was also funded by an RCUK academic fellowship awarded to ESJR with additional financial support provided by the British Pharmacological Society Integrative Pharmacology Fund and the Wellcome Trust (Reference no. 084621/Z/08/Z).
MRM has consulted for Servier in relation to agomelatine. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Anderson MH, Munafò MR, Robinson ES. Investigating the psychopharmacology of cognitive affective bias in rats using an affective tone discrimination task. Psychopharmacology (Berl) 2013;226:601–613. doi: 10.1007/s00213-012-2932-5. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J, Fredricson-Overo K. Prolonged treatment with the specific 5-HT-uptake inhibitor citalopram: effect on dopaminergic and serotonergic functions. Pol J Pharmacol Pharm. 1984;36:221–230. [PubMed] [Google Scholar]
- Arnone D, Horder J, Cowen PJ, Harmer CJ. Early effects of mirtazapine on emotional processing. Psychopharmacology. 2009;203:685–691. doi: 10.1007/s00213-008-1410-6. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression: Clinical, Experimental, and Theoretical Aspects. Harper & Row: New York; 1967. [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology. 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, Hahn C, Thase ME. Are we getting closer to valid translational models for major depression. Science. 2012;338:75–79. doi: 10.1126/science.1222940. [DOI] [PubMed] [Google Scholar]
- Bourin M, Mocaër E, Porsolt R. Antidepressant-like activity of S 20098 (agomelatine) in the forced swimming test in rodents: involvement of melatonin and serotonin receptors. J Psychiatry Neurosci. 2004;29:126–133. [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Shearer KD, McCaffery PJ. Retinoic acid and affective disorders: the evidence for an association. J Clin Psychiatry. 2012;73:37–50. doi: 10.4088/JCP.10r05993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M, Reid C, Cowen PJ, Goodwin GM, Harmer CJ. A single dose of citalopram increases fear recognition in healthy subjects. J Psychopharmacology. 2007;21:684–690. doi: 10.1177/0269881106074062. [DOI] [PubMed] [Google Scholar]
- Chandra P, Hafizi S, Massey-Chase RM, Goodwin GM, Cowen PJ, Harmer CJ. NK1 receptor antagonism and emotional processing in healthy volunteers. J Psychopharmacol. 2010;24:481–487. doi: 10.1177/0269881109103101. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Kelliher P, Harkin A, Kelly JP, Leonard BE. Reboxetine attenuates forced swim test-induced behavioural and neurochemical alterations in the rat. Eur J Pharmacol. 1999;379:125–133. doi: 10.1016/s0014-2999(99)00492-6. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modeling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Slattery DF. Animal models of mood disorders: recent developments. Curr Opin Psychiatry. 2007;20:1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- DSM IV 1994American Psychiatric Association, Diagnostic and statistical manual of mental disorders4th ed.American Psychiatric Press: Washington DC [Google Scholar]
- Elliott R, Zahn R, Deakin JF, Anderson IM. Affective cognition and its disruption in mood disorders. Neuropsychopharmacology. 2011;36:153–182. doi: 10.1038/npp.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AK, Lowry CA. Pharmacology of the beta-carboline FG-7,142, a partial inverse agonist at the benzodiazepine allosteric site of the GABA A receptor: neurochemical, neurophysiological, and behavioral effects. CNS Drug Rev. 2007;13:475–501. doi: 10.1111/j.1527-3458.2007.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S, Cisneros F, Gough B, Hanig J, Berry K. Chronic oral treatment with 13-cis-retinoic acid (isotretinoin) or all-trans-retinoic acid does not alter depression-like behaviors in rats. Toxicol Sci. 2005;87:451–459. doi: 10.1093/toxsci/kfi262. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;27:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res. 1992;42:241–251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Harding EJ, Paul ES, Mendl M. Animal behaviour: cognitive bias and affective state. Nature. 2004;427:312. doi: 10.1038/427312a. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, de Bodinat C, Dawson GR, Dourish CT, Waldenmaier L, Adams S, et al. Agomelatine facilitates positive versus negative affective processing in healthy volunteer models. J Psychopharmacol. 2011;25:1159–1167. doi: 10.1177/0269881110376689. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry. 2008;195:102–108. doi: 10.1192/bjp.bp.108.051193. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Hill SA, Taylor MJ, Cowen PJ, Goodwin GM. Toward a neuropsychological theory of antidepressant drug action: increase in positive emotional bias after potentiation of norepinephrine activity. Am J Psychiatry. 2003;160:990–992. doi: 10.1176/appi.ajp.160.5.990. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Hill SA, Taylor MJ, Cowen PJ, Goodwin GM. Toward a neuropsychological theory of antidepressant drug action: increase in positive emotional bias after potentiation of norepinephrine activity. Am J Psychiatry. 2004;160:990–992. doi: 10.1176/appi.ajp.160.5.990. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, O'Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, et al. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166:1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley N, Cowen P, Goodwin G. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161:1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hickie IB, Rogers NL. Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet. 2011;378:621–631. doi: 10.1016/S0140-6736(11)60095-0. [DOI] [PubMed] [Google Scholar]
- Horder J, Cowen PJ, Di Simplicio M, Browning M, Harmer CJ. Acute administration of the cannabinoid CB1 antagonist rimonabant impairs positive affective memory in healthy volunteers. Psychopharmacology. 2009;205:85–91. doi: 10.1007/s00213-009-1517-4. [DOI] [PubMed] [Google Scholar]
- Humpston CS, Wood CM, Robinson ES. Investigating the roles of different monoamine transmitters and impulse control using the 5-choice serial reaction time task. J Psychopharmacol. 2013;27:213–221. doi: 10.1177/0269881112466182. [DOI] [PubMed] [Google Scholar]
- Keeler JF, Robbins TW. Translating cognition from animals to humans. Biochem Pharmacol. 2011;81:1356–1366. doi: 10.1016/j.bcp.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Kasper S, Sacher J, Klein N, Mossaheb N, Attarbaschi-Steiner T, Lanzenberger R, et al. Differences in the dynamics of serotonin reuptake transporter occupancy may explain superior clinical efficacy of escitalopram versus citalopram. Int Clin Psychopharmacol. 2009;24:119–125. doi: 10.1097/YIC.0b013e32832a8ec8. [DOI] [PubMed] [Google Scholar]
- Keller M, Montgomery S, Ball W, Morrison M, Snavely D, Liu G, et al. Lack of efficacy of the substance P (neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biol Psychiatry. 2006;59:216–223. doi: 10.1016/j.biopsych.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Lemogne C, Piolino P, Friszer S, Claret A, Girault N, Jouvent R, et al. Episodic autobiographical memory in depression: specificity, autonoetic consciousness, and self perspective. Conscious Cogn. 2006;15:258–268. doi: 10.1016/j.concog.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Leppänen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006;19:34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Mendl M, Burman OH, Paul ES. An integrative and functional framework for the study of animal emotion and mood. Proc Biol Sci. 2010;277:2895–2904. doi: 10.1098/rspb.2010.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Downham C, Cowen PJ, Harmer CJ. Direct effects of diazepam on emotional processing in healthy volunteers. Psychopharmacology. 2008;199:503–513. doi: 10.1007/s00213-008-1082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly K, Shumake J, Gonzalez-Lima F, Lane MA, Bailey SJ. Chronic administration of 13-cis-retinoic acid enhances depression-related behavior in mice. Neuropsychopharmacology. 2006;31:1919–1927. doi: 10.1038/sj.npp.1300998. [DOI] [PubMed] [Google Scholar]
- Papadatou-Pastou M, Miskowiak KW, Williams JM, Harmer CJ, Reinecke A. Acute antidepressant drug administration and autobiographical memory recall: a functional magnetic resonance imaging study. Exp Clin Psychopharmacol. 2012;20:364–372. doi: 10.1037/a0027969. [DOI] [PubMed] [Google Scholar]
- Papp M. Models of affective illness: chronic mild stress in the rat. Curr Protoc Pharmacol. 2012;57:5.9.1–5.9.11. doi: 10.1002/0471141755.ph0509s57. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Pringle A, Browning M, Cowen PJ, Harmer CJ. A cognitive neuropsychological model of antidepressant drug action. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1586–1592. doi: 10.1016/j.pnpbp.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Reneric JP, Bouvard M, Stinus L. In the rat forced swimming test, chronic but not subacute administration of dual 5-HT/Naantidepressant treatments may produce greater effects than selective drugs. Behav Brain Res. 2002;136:521–532. doi: 10.1016/s0166-4328(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ES. Blockade of noradrenaline re-uptake sites improves accuracy and impulse control in rats performing a five-choice serial reaction time tasks. Psychopharmacology (Berl) 2012;219:303–312. doi: 10.1007/s00213-011-2420-3. [DOI] [PubMed] [Google Scholar]
- Rogers RJ, Cole JC, Aboualfa K, Stephenson LH. Ethopharmacological analysis of the effects of putative ‘anxiogenic' agents in the mouse. Pharmacol Biochem Behav. 1995;52:805–813. doi: 10.1016/0091-3057(95)00190-8. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012;37:117–136. doi: 10.1038/npp.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsfeld JS, Nallamothu BK. The hope and fear of rimonabant. JAMA. 2008;299:1601–1602. doi: 10.1001/jama.299.13.1601. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Murphy JM, McBride WJ, Lumeng L, Li TK. Place conditioning with alcohol in alcohol-preferring and -nonpreferring rats. Pharmacol Biochem Behav. 1996;53:487–491. doi: 10.1016/0091-3057(95)02102-7. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Tzavara T, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, et al. The CB1 receptor antagonist SR141716A selectively increases monaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol. 2003;138:544–553. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66:617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- Wallace-Boone TL, Newton AE, Wright RN, Lodge NL, McElroy JF. Behavioral and pharmacological validation of the gerbil forced-swim test: effects of neurokinin-1 receptor antagonists. Neuropsychopharmacology. 2007;33:191–192. doi: 10.1038/sj.npp.1301586. [DOI] [PubMed] [Google Scholar]
- Wardle MC, Garner MJ, Munafò MR, de Wit H. Amphetamine as a social drug: effects of d-amphetamine on social processing and behavior. Psychopharmacology. 2012;223:199–210. doi: 10.1007/s00213-012-2708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins PC, Vache K, Vernay SP, Muller S, Mathews A. Unconscious mood-congruent memory bias in depression. J Abnormal Psychol. 1996;105:34–41. doi: 10.1037//0021-843x.105.1.34. [DOI] [PubMed] [Google Scholar]
- Wieland S, Lucki I. Antidepressant-like activity of 5-HT1A agonists measured with the forced swim test. Psychopharmacology. 1990;101:497–504. doi: 10.1007/BF02244228. [DOI] [PubMed] [Google Scholar]
- Wingenfeld K, Wolf OT. HPA axis alterations in mental disorders: impact on memory and its relevance for therapeutic interventions. CNS Neurosci Ther. 2011;17:714–722. doi: 10.1111/j.1755-5949.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DT, Fuller RW, Robertson DW. Fluoxetine and its two enantiomers as selective serotonin uptake inhibitors. Acta Pharm Nord. 1990;2:171–180. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.