Abstract
Striatal dopamine (DA) is increased by virtually all drugs of abuse, including alcohol. However, drug-associated cues are also known to provoke striatal DA transmission- a phenomenon linked to the motivated behaviors associated with addiction. To our knowledge, no one has tested if alcohol's classically conditioned flavor cues, in the absence of a significant pharmacologic effect, are capable of eliciting striatal DA release in humans. Employing positron emission tomography (PET), we hypothesized that beer's flavor alone can reduce the binding potential (BP) of [11C]raclopride (RAC; a reflection of striatal DA release) in the ventral striatum, relative to an appetitive flavor control. Forty-nine men, ranging from social to heavy drinking, mean age 25, with a varied family history of alcoholism underwent two [11C]RAC PET scans: one while tasting beer, and one while tasting Gatorade. Relative to the control flavor of Gatorade, beer flavor significantly increased self-reported desire to drink, and reduced [11C]RAC BP, indicating that the alcohol-associated flavor cues induced DA release. BP reductions were strongest in subjects with first-degree alcoholic relatives. These results demonstrate that alcohol-conditioned flavor cues can provoke ventral striatal DA release, absent significant pharmacologic effects, and that the response is strongest in subjects with a greater genetic risk for alcoholism. Striatal DA responses to salient alcohol cues may thus be an inherited risk factor for alcoholism.
Keywords: alcohol abuse, ethanol, taste, cue reactivity, classical conditioning, heritable risk
INTRODUCTION
Sensory stimuli associated with drug intoxication are studied for their capacity to elicit the urge to become intoxicated and to induce treatment relapse (Cooney et al, 1997; Litt et al, 2000; Grüsser et al, 2004). Thus, a large body of literature has examined the human brain structures that respond to such stimuli (for meta-analysis, see Schacht et al, 2012). The taste of an alcoholic beverage is arguably the most proximal conditioned stimulus to the ensuing intoxication. As such, it is capable of activating the mesocorticolimbic system (Filbey et al, 2008)— the network thought to encode the motivational significance of learned associations between such cues and the reward that follows (Berridge, 2007).
Compelling evidence suggests that drug-conditioned stimuli provoke ventral striatal dopamine (DA) transmission, which is believed to drive drug-seeking behavior (Salamone and Correa, 2002; Berridge, 2007). For example, increased ventral striatal DA corresponds with drug wanting in humans (Leyton et al, 2002; Evans et al, 2006), and artificial stimulation of DA neurons in the ventral tegmental area elicits drug seeking in rats (Phillips et al, 2003). An alternate view, however, specifies a role of DA in stimulus-outcome learning, without necessarily implicating motivation (Schultz, 2007). Many human brain imaging studies utilize functional magnetic resonance imaging (fMRI) to examine the neurobiology of cue responses, but fMRI is not specific to any neurotransmitter system; thus, observed ventral striatal activation in fMRI cannot be assumed to be DA release. However, in vivo changes in human striatal DA can be observed with positron emission tomography (PET) and [11C]raclopride (RAC) (Dewey et al, 1993).
Two previous human RAC PET studies suggest that orally consumed alcoholic drinks provoke ventral striatal DA release (Boileau et al, 2003; Urban et al, 2010). In both, however, the orosensory properties of alcohol could have been detectable in the mixed drink. While the DA effects in these studies were attributed to alcohol intoxication, animal studies indicate that alcohol-related taste cues may alone elicit DA release. Two studies demonstrate that ventral striatal DA peaks upon oral contact with ethanol, before the rise of alcohol concentration in the brain (Doyon et al, 2005; Doyon et al, 2003). This suggests that alcohol's taste or other orosensory cues are potent triggers for ventral striatal DA release, and that the DA release from oral alcohol in human studies could be attributed (at least in part) to a beverage's intraoral sensory cues.
Other factors may have a role in DA transmission. In particular, Weiss et al, 1993 found that rats selectively bred for high alcohol drinking showed a larger ventral striatal DA response to alcohol taste and intoxication than nonselected rats, suggesting that the genetic propensity for alcohol use disorders (AUD) is linked to differential striatal DA function. Two human studies found that, in subjects with a family history (FH) of alcoholism, the medial prefrontal cortex (which sends axonal projections to the striatum; Haber and Knutson, 2010), is activated more strongly in response to alcohol cues relative to family history-negative controls (Tapert et al, 2003; Kareken et al, 2010). Cue-elicited DA transmission may also relate to alcohol preference, as nonselected rats with the highest ethanol preference showed the largest increase in ventral striatal DA release in response to oral contact with alcohol (Doyon et al, 2005).
In this study, we used RAC PET to determine if a preferred beer's flavor (absent intoxication) could alone provoke changes in ventral striatal DA D2/D3 receptor availability in a large sample of beer drinkers. Beer's low alcohol concentration makes an ideal choice for an alcohol-paired flavor without risk of significant brain exposure to alcohol. Our primary hypothesis was that a preferred alcoholic drink's (beer) flavor, absent a significant dose of alcohol, would alone be sufficient to elicit DA release in the ventral striatum (VST) of adult male drinkers, relative to an appetitive control of similar flavor intensity (Gatorade). Furthermore, we examined other factors (FH, recent drinking, craving, and flavor perception) that could mediate this response.
MATERIALS AND METHODS
Subjects
All procedures were approved by the Indiana University School of Medicine Institutional Review Board, and all subjects signed informed consent agreements before completing study procedures. The 49 subjects were recruited from the community via flyers and classified ads. All were right-handed male beer drinkers in good self-reported physical and mental health. Although initially open to both sexes, heavy-drinking females who preferred beer and had no comorbid tobacco or drug use proved too rare. Recent drinking ranged from social to heavy (Table 1). The Semi-Structured Assessment for the Genetics of Alcoholism (Bucholz et al, 1994) assessed for past or current AUD. Four of the 49 subjects met DSM-IV criteria for alcohol dependence; however, these subjects did not differ from the rest of the group in drinks per week (P>0.1). Subjects were excluded if they failed a urine screen for illicit drugs, reported current illicit drug use, reported past treatment for substance dependence, were currently seeking treatment for substance use, did not consume beer more often than other alcoholic drinks, reported disliking Gatorade, or reported any symptoms, diagnoses, or treatment consistent with a current or past major Axis I psychiatric disorder (aside from alcohol dependence). Two subjects reported marijuana use of 30 times, and >30 times in the past year, with all other subjects' past use being lower. Regular cigarette smoking was exclusionary; although two subjects reported smoking up to two cigarettes per week (see Supplementary Materials and Methods for details).
Table 1. Subject Characteristics.
| Mean ± (SD) | Range | n (%) | |
|---|---|---|---|
| Age | 24.7 (3.5) | 21–35 | |
| Caucasian | — | — | 44 (90%) |
| Education | 15.5 (1.6) | 11–19 | |
| AUD relativesa | 1.0 (1.1) | 0–5 | |
| Drinks per weekb | 16.5 (11.3) | 2–45 | |
| Drinks per drinking dayb | 5.5 (3.4) | 1–13 | |
| Heavy drinking days per weekb,c | 1.5 (1.3) | 0–6 | |
| AUDITd | 10.2 (5.7) | 1–26 |
Number of first or second degree relatives with alcohol use disorder by self-report.
From the Timeline Followback Interview.
Greater than five drinks per day.
Alcohol Use Disorder Identification Test.
Gustatory Stimulus Delivery
Flavors were delivered using a computer controlled ‘gustometer' (modeled after Marciani et al, 2006), and targeted to 15 ml over 15 min. Beer, Gatorade, and water were delivered via PTFE (Teflon) tubing into a spray nozzle, which was positioned between subject's incisors and aimed onto the tongue. A computer monitor suspended from the scanner gantry displayed a visual ‘countdown' to the spray occurrence and questions about the stimuli.
Subjective Ratings
Subjects responded to computerized rating scales at four time points—once to establish a pre-scan baseline (after two training water sprays to habituate subjects), and three times during scanning (Figure 1b). ‘Desire' to drink alcohol was assessed with four items (#11, #18, #21, and #32 from the Alcohol Craving Questionnaire: Singleton et al, 2000) on a seven-point visual analog scale (VAS; 1=strongly disagree, 7=strongly agree). Subjects were asked the number of beers they wanted at the moment, with responses in 0.5 beer increments (assuming a standard 12 oz. beer). Perceived flavor pleasantness was measured on a VAS (1=‘Least Pleasant Ever', 7=‘Most Pleasant Ever'). Perceived flavor intensity was evaluated using Green's Labeled Magnitude scale (Green et al, 1996), which ranged from ‘barely detectable' to ‘strongest imaginable'. Rating scales were presented via the computer monitor, and responses were recorded using a wireless mouse.
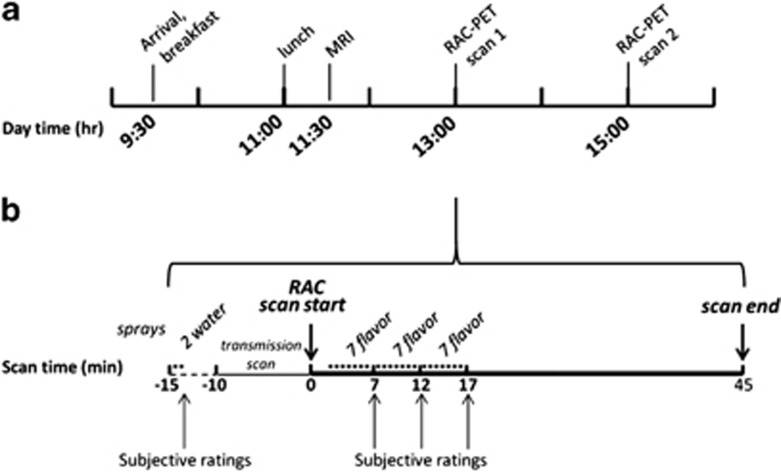
Figure 1.
(a) General study day timeline. (b) Within-scan timeline for tastants and ratings is identical for both scans except for flavor type (beer, Gatorade counterbalanced.) The two water spray trials comprise the water baseline condition. MRI=magnetic resonance imaging; RAC=[11C]raclopride; PET=positron emission tomography; *=spray (water or flavor), 0.7 ml vol.
Procedure
Subjects underwent two RAC PET scans, 2 h apart (six half-lives), counterbalanced for beer and Gatorade flavor (beer first, n=25). On the morning of the study day (Figure 1a), subjects arrived at the Indiana Clinical Research Center. They received breakfast, were rescreened for illicit drugs with a urine test, and assessed for alcohol intoxication using a breath alcohol concentration meter. An IV catheter was placed in an antecubital fossa vein for RAC injection.
MRI was acquired first, followed by two PET scans (Figure 1a for general timeline; Figure 1b for stimulus delivery/ratings). The beer brand used was that which each subject drank most frequently. Before imaging, subjects tasted 15 ml of each of the three tastants to insure flavor recognition. Immediately preceding PET, subjects were instructed that, ‘they may receive water, beer, or Gatorade' during any scan, and that they could, ‘receive the same liquid for both scans or different liquids in each.' Subjects were instructed that they would receive small amounts of tastants, but not enough for intoxication. While supine in the PET scanner, but before imaging, the gustometer delivered two water sprays to habituate subjects to stimulus delivery. Subjects then rated the water to assure that they understood the subjective rating scales. Gustatory stimulation (beer or Gatorade, delivered through a water-chilled jacket) began precisely 2 min after RAC administration/scan start to assure consistent timing across subjects (Yoder et al, 2004), and continued periodically during the next 15 min. Each spray was preceded by a visual 5 s countdown and the word ‘sip' upon spray delivery to avoid surprise/head motion. Flavor sprays were 750 ms long, and delivered in three 5-minute blocks of seven sprays each, with pseudorandomized intertrial intervals. Subjective ratings were acquired between blocks (ie, after the 7th, 14th, and 21st flavor sprays). Subjects were reminded to remain awake, alert, and still during scanning.
Image Acquisition and Processing
MRI was acquired for anatomic coregistration of PET images (see Supplemental Materials and Methods). RAC synthesis was as described previously (Fei et al, 2004). RAC PET scans were acquired on a Siemens EXACT HR+ (3-D mode; septa retracted). Before each PET scan, a 10-min transmission scan using three internal rod sources (68Ge) was acquired for attenuation correction. PET was initiated with the IV infusion of 551±4 MBq RAC (mass dose 0.110±0.006 nmol/kg, mean±SEM) over 1.5 min, with dynamic acquisition over 45 min (Yoder et al, 2009).
PET images were motion-corrected, registered to MRI volumes, and spatially normalized (see Supplemental Materials and Methods for details). D2/D3 receptor availability was indexed by binding potential (BPND), operationally defined as the bound RAC concentration relative to non-displaceable RAC concentration (Innis et al, 2007). BPND was estimated using the multilinear reference tissue model (MRTM2: Ichise et al, 2003) for all voxels within striatum, with the cerebellar time-activity curve as the input function. BPND images were then smoothed with a 4-mm full-width at half-maximum isotropic Gaussian kernel. Voxel-wise changes in BPND, expressed as a percentage of control condition, were calculated according to: ΔBPND=(BPND[Gatorade]−BPND[beer])/BPND[Gatorade], where only voxels with BPND values >0.75 in both beer and Gatorade scans were considered (eg, Joutsa et al, 2012).
Statistics
Subject characteristics
FH differences in demographics were assessed with one-way ANOVA; post-hoc t-tests identified pair-wise group differences (Supplementary Table S1).
Subjective ratings
Ratings (pleasantness, intensity, desire, and wanting) after flavor delivery were averaged in each condition for each subject, reflecting perception during scanning. As water baseline ratings did not differ between scan conditions (Ps>0.5), these scores were averaged across scans. Subjective ratings were analyzed using a 3(FH Group) × 3(Flavor) ANOVA, with flavor as a repeated measure, and post-hoc paired t-tests used to examine differences by condition.
Imaging data
Voxel-wise analyses were performed on parametric ΔBPND images. As our hypotheses concerned the VST, all voxel-wise results reported here were evaluated in left and right VST search volumes (defined anatomically as in Mawlawi et al, 2001), with peak voxel significance corrected for family-wise error (FWE) PFWE<0.05 within this search volume. Only clusters larger than 10 voxels were considered (threshold P<0.01). One-sample t-tests (ΔBPND differs from 0) were performed in the full sample (n=49) to test if DA was higher or lower during beer relative to Gatorade flavor conditions. Voxel-wise regression assessed correlations between ΔBPND and: drinks/week, AUDIT, mean desire and wanting, and inter-flavor differences in pleasantness and intensity. Ratings of beer flavor assessed desire and wanting, while (Gatorade—beer) differences were calculated for pleasantness and intensity. Independent sample t-tests assessed differences between stimulus order groups (ie, beer first), as well as FH groups. All voxel-wise image statistics were calculated with SPM8; subjective ratings were analyzed with SPSS for Windows (ver. 19.0.0).
RESULTS
Subject Characteristics
Subjects were stratified by FH for post-hoc analyses, with groups of FH positive (FHP, n=12; at least one first-degree relative with probable alcoholism), FH ambiguous (FHA, n=18; only second degree relatives with probable alcoholism), and FH negative (FHN, n=19; no first- or second-degree relatives with probable alcoholism), Supplementary Table S1. Number of AUD relatives was greater in FHP than in FHA t(28)=2.6, P=0.014. FH groups did not differ by drinks per week (one-way ANOVA, P>0.2; mean±SD 20.8±12, 16.9±9, and 13.4±12 for FHP, FHA, and FHN respectively) or by comparing FHP to the combined (FHA+FHN) groups (t-test, P>0.1). There was a trend for group differences in AUDIT (P=0.055), but age, education, drinks per drinking day, and heavy drinking days per week did not differ (Ps>0.09).
Stimulus Delivery
Total fluid volume was 14.2±0.4 and 15.4±0.4 ml for beer and Gatorade, respectively. The former resulted in a negligible alcohol dose of 0.0061±0.0003 g/kg body weight (for comparison, one standard 12 oz. beer of 4.2% alcohol v/v would result in a dose of 0.168 g/kg in a 70-kg person).
Subjective Ratings
Stimulus qualities
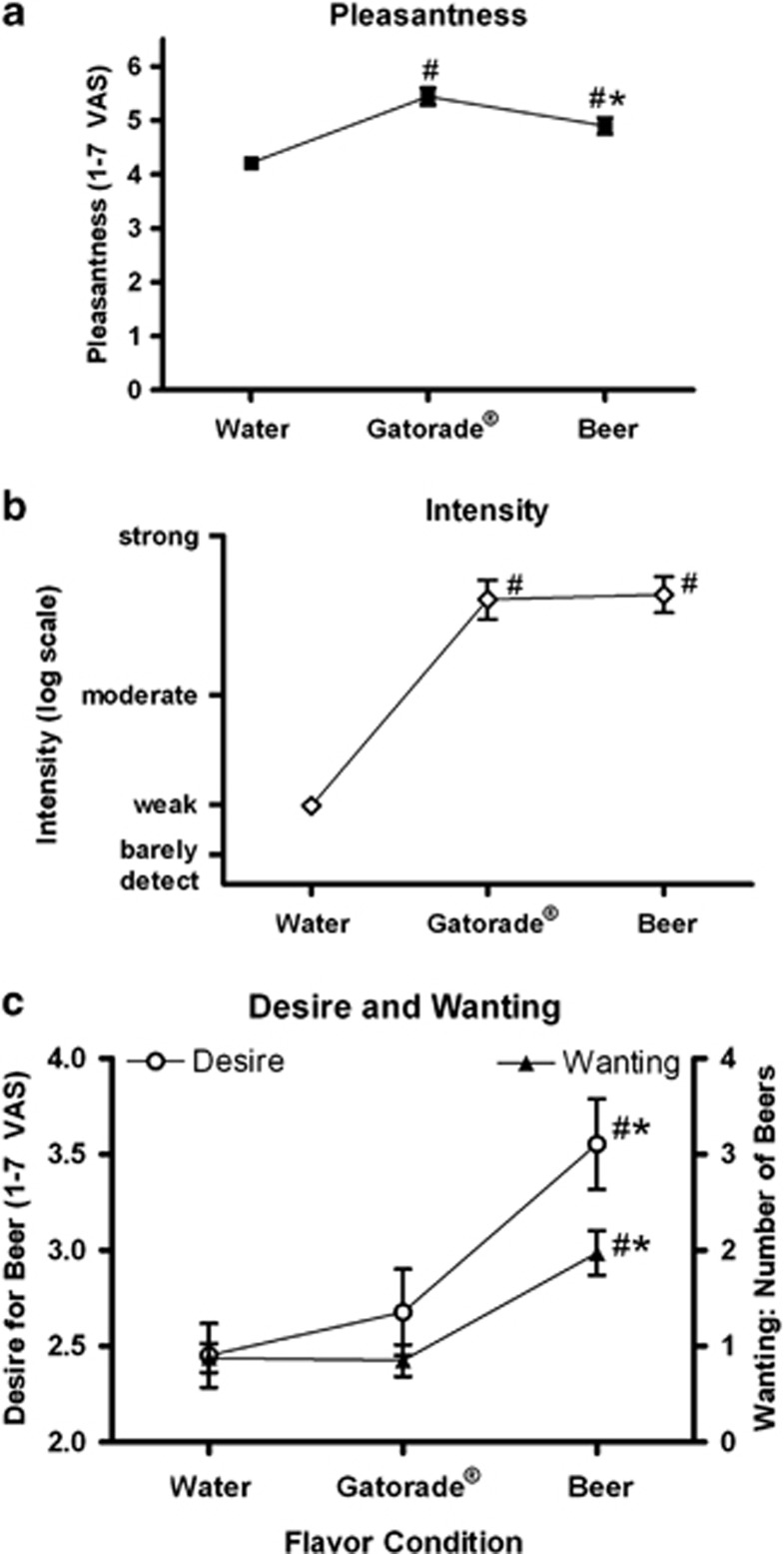
Pleasantness ratings differed by tastant, F(2,96)=24.3, P<0.001, with both beer and Gatorade rated as more pleasant than water, ts(48)>4.0, Ps<0.001, and Gatorade rated as more pleasant than beer, t(48)=2.7, P=0.009 (Figure 2a). Intensity ratings differed by tastant, F(2,96)=89.2, P<0.001, with both beer and Gatorade rated as stronger than water, ts(48)>10.5, Ps<0.001. As intended, however, there was no difference in perceived intensity between beer and Gatorade, (P>0.7; Figure 2b).
Figure 2.
(a) All subjects (n=49) rated the pleasantness (filled squares) of beer and Gatorade, as well as (b) intensity (open diamonds). (c) Subjects also rated the desire for beer (open circles, left y-axis), and the number of beers wanted (filled triangles, right y-axis). In all graphs, mean±SEM are presented; #=differed from water (P<0.05), and *=differed from Gatorade (P<0.05). The Baseline condition was evaluated after two water sprays and before each flavor scan; these are averaged between flavor conditions.
Desire to drink
Desire differed by tastant F(2,96)=24.1, P<0.001. Relative to the water baseline, beer flavor increased desire to drink beer, t(48)=6.2, P<0.001, while Gatorade did not (P>0.09). Beer flavor increased desire for beer more than Gatorade, t(48)=4.6, P<0.001. Similarly, the number of beers wanted differed by tastant, F(2,96)=28.0, P<0.001, as beer flavor increased number of beers wanted relative to water, t(48)=5.9, P<0.001, while Gatorade flavor did not change beer wanting (P>0.7). Beer flavor increased beer wanting more than Gatorade flavor, t(48)=5.3, P<0.001 (Figure 2c). Subjective ratings did not interact with FH (Ps>0.5).
PET Imaging
Flavor effects on BP
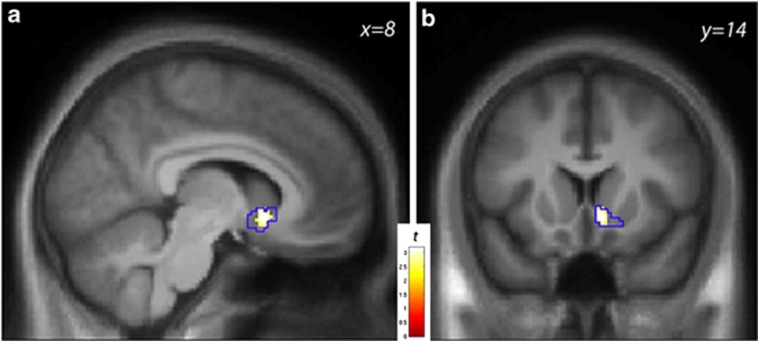
RAC BPND was significantly reduced in the right VST by beer flavor relative to Gatorade (one-sample t-test, ΔBPND>0, n=49), indicating that DA levels were higher during beer tasting (peak voxel coordinate: [8 14−6], Z=3.02, Puncorr=0.001, PFWE=0.034). Average ΔBPND across the R VST anatomical ROI, expressed as percent change, was 3% SEM=1%, and was 5%±2% within the responding region (P<0.01, illustrated in Figure 3). The L VST showed no effect; average ΔBPND was −1%±1%. The opposite contrast (ie, ΔBPND<0) revealed no ventral striatal effects (PsFWE>0.18). There were no significant effects of tastant order.
Figure 3.
(a) Statistical map in the sagittal (a) and coronal (b) views illustrating the striatal response to beer relative to Gatorade taste [ΔBPND>0] of [11C]raclopride in male drinkers (n=49). The color bar indexes the t-statistic at voxels in which DA levels were higher in the beer condition relative to the Gatorade condition. The anatomical right ventral striatum search volume is outlined in blue; voxel-wise display threshold, P<0.01, uncorrected.
Desire to drink and recent drinking
In either R or L VST, there were no voxel-wise correlations between ΔBPND and desire for beer, number of beers wanted, drinks per week, or AUDIT (PsFWE>0.14).
Flavor properties
In either R or L VST, there were no significant voxel-wise correlations between ΔBPND and mean difference scores (Gatorade—beer) of pleasantness or intensity.
Family history
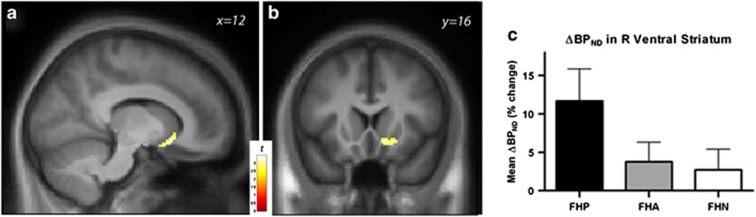
To maximize sensitivity to FH effects, we first assessed the R VST response by testing for differences across FH extremes (ie, (FHP>FHN)). FHP showed increased ΔBPND in the right VST relative to FHN (peak voxel [12 18 −8], Z=2.80, Puncorr=0.003, PFWE=0.061, Figure 4a–c). FHP also had a significantly greater response when tested against all subjects without any first-degree AUD relatives (n=37; peak coordinate [12 16 –10], Z=2.96, Puncorr=0.002, PFWE=0.040). Correspondingly, only the FHP sample showed a ventral striatal DA response when analyzed alone (peak location at [8 14 –10], Z=3.60, Puncorr<0.001, PFWE=0.006), while FHA and FHN showed no effect: mean ΔBPND (functional cluster, P<0.01) as a percentage±SEM=11.7±4.1; 3.8±2.5; and 2.7±2.7, respectively (Figure 4c). There were no correlations between recent drinking and R VST ΔBPND within any of the groups.
Figure 4.
(a) Statistical map in the sagittal and (b) coronal views illustrating the location of increased striatal DA response in FHP (FHP>FHN). Voxel-wise display threshold, p<0.01, uncorrected. (c) For illustration, mean (±SEM) values were extracted using all voxels with ΔBPND>0 in the full n=49 sample (voxel-wise P<0.01, uncorrected) that were within the anatomically defined right ventral striatum. ΔBPND values (mean±SEM) in the right ventral striatum indicate that subjects with first-degree relatives with probable alcoholism (FHP, n=12) exhibited larger relative increase in ΔBPND (eg, higher DA levels during the beer condition) compared with both family history ambiguous (FHA: second degree alcoholic relatives only, n=18) and family history negative (FHN: no alcoholic relatives, n=19). AUD, alcohol use disorder; BP, binding potential; DA, dopamine.
Effects of dependence
Mean R VST ΔBPND values from those subjects who met DSM-IV criteria for dependence (n=4) were not significantly different from those of the rest of the sample, (P>0.6; mean±SEM 3.2%±2.3, and 3.5%±1.5, respectively). The distribution of subjects meeting criteria for DSM-IV dependence across groups was: ns=1 and 3 for FHP and FHA, respectively. An exploratory analysis of flavor effects on BP in 45 nondependent subjects revealed a slightly weakened effect (peak [8 14 –6], Z=2.80, Puncorr=0.003, PFWE=0.059), but the same R VST localization of ΔBPND>0.
Extra-ventral striatal flavor effects
Analysis of flavor effects on RAC BPND (ΔBPND differing from zero) outside the VST did not reveal effects in dorsal caudate or putamen, (PsFWE>0.1, corrected by region).
DISCUSSION
The primary findings of this investigation indicate that the taste of a preferred alcoholic drink (beer), absent a pharmacologically significant dose of alcohol, is capable of inducing relative increases in DA transmission in the brain's VST. Moreover, this effect was most driven by subjects with a first-degree relative with alcoholism (the mean ΔBPND for FHP was approximately fourfold that of the FHN), without clear effects related to recent drinking. Thus, while prior studies of oral alcohol suggest that striatal DA may be released as a function of alcohol's pharmacologic effects (Boileau et al, 2003; Urban et al, 2010; Ramchandani et al, 2011), we believe that alcohol-conditioned taste cues alone may also be sufficient to induce ventral striatal DA release—an interpretation consistent with findings in animals (Doyon et al, 2003; Doyon et al, 2005). Although ethanol can directly excite ventral tegmental DA neurons in vitro (Brodie et al, 1999), alcohol-conditioned cues can alone provoke VST DA responses in behaving animals that are reflective of motivated behavior and alcohol-seeking (Gonzales et al, 2004). We, therefore, suggest at least the possibility that, in those studies where alcohol is ingested (Boileau et al, 2003; Urban et al, 2010), alcohol's intraoral sensory properties may contribute to DA responses, which are in turn thought to promote and maintain drug-seeking behavior and consumption (for review see Berridge, 2007).
A FH of alcoholism doubles the risk of alcohol dependence (eg, Nurnberger et al, 2004). In that context, FHP subjects had the most robust VST DA release, which is consistent with other human imaging studies in which familial alcoholism modulates brain activation to alcohol cues. For instance, we (Kareken et al, 2010) and others (Tapert et al, 2003) have demonstrated that alcohol-associated cues differentially activate the medial prefrontal cortex (which sends axonal projections to the VST; for review see Haber and Knutson, 2010) as a function of FH. In contrast, an fMRI study of children of alcoholics did not detect a difference in VST activation for monetary rewards (Bjork et al, 2008), while another study of FHP subjects found reduced VST activation to monetary rewards compared with FHN subjects (Andrews et al, 2011). However, monetary and alcohol cues may not elicit the same brain activation patterns (Wrase et al, 2007). Whereas fMRI does not identify specific neurotransmitters, our current findings are specific to VST DA, with the data suggesting that familial alcoholism is associated with increased dopaminergic signaling to alcohol-related cues (although see Munro et al, 2006 for null results with amphetamine). These data are also consistent with dopaminergic (and other neurochemical) differences between selectively bred alcohol preferring and non-preferring animal lines (for review, see Murphy et al, 2002).
While our work focused on the VST, there is a body of literature linking dorsal striatal DA release with cocaine-conditioned stimuli (Volkow et al, 2006; Wong et al, 2006; Volkow et al, 2008). Exploratory analysis of the dorsal caudate or putamen did not show DA release in our sample; however, these prior reports differ from the current study in important ways. First, the subjects were current heavy users of cocaine, whereas the present study's subjects had no history of stimulant dependence or current use. Second, the modality of the cue presentation differed considerably, as the cocaine cue studies used visual cues of drugs that are ingested by smoking, snorting, or injection; thus, the sensory cue modality (vision) was not directly tied to the method of drug administration. Flavor cues are, by contrast, intrinsic to alcohol's oral consumption. A conditioned stimulus (orally perceived beer flavor) that activates the same sensory system as the mode of administration of the unconditioned stimulus (intoxication) is more potent in eliciting conditioned responses than less relevant cues (eg, Garcia and Koelling, 1966). Thus, a sensory-related CS may be expected to activate systems closely associated with the delivery modality of the primary reinforcer. It is also possible that, as our subject population was relatively young, nontreatment-seeking, and largely nondependent, their alcohol use may not have reached a chronic level sufficient to engage habitual motor systems thought to be encoded in dorsal striatum (Ito et al, 2002; Porrino et al, 2004).
There is debate as to whether more or less DA release corresponds with addiction risk. In support of the latter position, Volkow et al (2007) and Martinez et al (2005) reported that stimulant-induced DA transmission was blunted in detoxified alcoholics relative to controls. In contrast, our study suggests that (familial) risk for developing alcoholism may be associated with increased DA response to the conditioned cues of alcohol. However, several important differences between these studies and the current data should be noted: first, the prior studies involved subjects who were alcohol dependent, with considerable drinking histories; our study consisted of younger drinkers with shorter and less severe drinking histories. Therefore, it is unclear if lower stimulant-provoked DA release reflects a predisposition for development of alcoholism, or is a consequence of chronic drinking. Additionally, psychostimulants may be less naturalistic probes of dopaminergic function in alcoholism risk than alcohol-conditioned cues.
The alcohol flavor effect on relative DA levels was observed only in the right VST. This right lateralized effect is consistent with some reports, such as cocaine-induced glucose metabolism (London et al, 1990), and cocaine cue-induced DA release in high cravers (Wong et al, 2006). Other studies have not detected lateralized effects with cues plus intoxication (Boileau et al, 2003; Volkow et al, 2008), or did not analyze hemispheres separately (Urban et al, 2010). However, our laboratory has previously found lateralized effects to both uncued/unanticipated alcohol-induced DA release in the left VST, while visual and olfactory alcohol cues that errantly predicted alcohol delivery reduced DA release in the right VST (Yoder et al, 2009). In rats, Besson and Louilot (1995) found a lateralized DA response in the VST, such that conditioned olfactory stimuli to appetitive stimuli induced DA release in right VST, while aversive stimuli induced DA release in left VST. One recent study employing random monetary rewards detected DA release in the right but not left VST in males (Martin-Soelch et al, 2011), while an fMRI study found activation to visual alcohol cues in right VST, which was attenuated by a partial DA agonist (Myrick et al, 2010). Together, these data support the idea that DA coding for appetitive conditioned stimuli may be biased toward the right VST.
Compared with previous studies of intoxication from orally ingested alcohol, the flavor cues in this study did not provoke ventral striatal DA as strongly (R VST ΔBPND=5% compared with VST ΔBPND of 17% in Boileau et al, 2003, and 12% (male social drinkers) in Urban et al, 2010). The FHP subjects in the current study showed a more similar response of near 12%, but only on the right side. These comparisons suggest that alcohol intoxication could alone exert an independent effect on ΔBPND. Collectively, these findings suggest that both alcohol-paired cues, as well as intoxication, mediate VST DA release.
There are limitations to consider when interpreting these results. The current study is limited by the lack of a true resting RAC scan, which would enable a more precise interpretation of the nature of the cue-induced change in BPND relative to a neutral baseline. Thus, we cannot be absolutely certain that that the beer condition ‘increased' DA. However, the absence of an appetitive control would have precluded accounting for the nonspecific effects of flavor, general appetitive stimulation, and the effects of oral-sensory stimulation (see Yoder et al, 2011 for a review of these considerations in PET). While we did not observe statistically significant differences in drinks per week between FH groups, our ability to detect differences may have been limited by group size. Thus, we cannot definitively rule out a FH by drinking interaction, which remains for future research.
In summary, the results demonstrate for the first time the important role of an alcoholic drink's flavor, absent alcohol's pharmacological effects, in human ventral striatal DA release, as well as how DA transmission may relate to familial alcoholism. In addition to implicating conditioned stimuli in addiction-relevant striatal DA function, the findings also indicate the need to consider the effects of alcohol's conditioned cues when measuring alcohol's effects on ventral striatal DA release.
Acknowledgments
We gratefully acknowledge Kevin Perry, Wendy Territo, Michele Beal, Courtney Robbins (Dept. of Radiology and Imaging Sciences), Dr William Eiler, Traci Mitchell, Cari Lehigh (Dept. of Neurology), and Melissa Walker (Indiana University School of Medicine) for technical assistance, and Dwight Hector for design and construction of the gustometer. This study was supported by R01 AA017661 (DAK) for design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript; the Indiana Alcohol Research Center P60 AA007611 for additional support to DAK; TR000006 (Indiana Clinical and Translational Sciences Institute, Clinical Research Center at Indiana University School of Medicine) for conduct of the study, and collection and management of the data; T32 AA007462 (BGO) for data collection, analysis, interpretation of the data; and preparation, review, and approval of the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, et al. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biol Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Besson C, Louilot A. Asymmetrical involvement of mesolimbic dopaminergic neurons in affective perception. Neuroscience. 1995;68:963–968. doi: 10.1016/0306-4522(95)00255-h. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Hommer DW. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103:1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Fowler JS, Wolf AP. Striatal binding of the PET ligand 11C-raclopride is altered by drugs that modify synaptic dopamine levels. Synapse. 1993;13:350–356. doi: 10.1002/syn.890130407. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem. 2005;93:1469–1481. doi: 10.1111/j.1471-4159.2005.03137.x. [DOI] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Evans AH, Pavese N, Lawrence AD, Tai YF, Appel S, Doder M, et al. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol. 2006;59:852–858. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- Fei X, Mock BH, DeGrado TR, Wang JQ, Glick-Wilson BE, Sullivan ML, et al. An improved synthesis of PET dopamine D2 receptors radioligand [11C]raclopride. Synthetic Communications. 2004. p. 34.
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, et al. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Koelling RA. Relation of cue to consequence in avoidance learning. Psychonomic Science. 1966;4:123–124. [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the 'Labeled Magnitude Scale' for measuring sensations of taste and smell. Chem Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutsa J, Johansson J, Niemela S, Ollikainen A, Hirvonen MM, Piepponen P, et al. Mesolimbic dopamine release is linked to symptom severity in pathological gambling. Neuroimage. 2012;60:1992–1999. doi: 10.1016/j.neuroimage.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, et al. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage. 2010;50:267–276. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Morse P. Reactivity to alcohol-related stimuli in the laboratory and in the field: predictors of craving in treated alcoholics. Addiction. 2000;95:889–900. doi: 10.1046/j.1360-0443.2000.9568896.x. [DOI] [PubMed] [Google Scholar]
- London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, et al. Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry. 1990;47:567–574. doi: 10.1001/archpsyc.1990.01810180067010. [DOI] [PubMed] [Google Scholar]
- Marciani L, Pfeiffer JC, Hort J, Head K, Bush D, Taylor AJ, et al. Improved methods for fMRI studies of combined taste and aroma stimuli. J Neurosci Methods. 2006;158:186–194. doi: 10.1016/j.jneumeth.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Szczepanik J, Nugent A, Barhaghi K, Rallis D, Herscovitch P, et al. Lateralization and gender differences in the dopaminergic response to unpredictable reward in the human ventral striatum. Eur J Neurosci. 2011;33:1706–1715. doi: 10.1111/j.1460-9568.2011.07642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Oswald LM, Wong DF, Zhou Y, Brasic J, et al. Striatal dopamine release and family history of alcoholism. Alcohol Clin Exp Res. 2006;30:1143–1151. doi: 10.1111/j.1530-0277.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Myrick H, Li X, Randall PK, Henderson S, Voronin K, Anton RF. The effect of aripiprazole on cue-induced brain activation and drinking parameters in alcoholics. J Clin Psychopharmacol. 2010;30:365–372. doi: 10.1097/JCP.0b013e3181e75cff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Wiegand R, Bucholz K, O'Connor S, Meyer ET, Reich T, et al. A family study of alcohol dependence: coaggregation of multiple disorders in relatives of alcohol-dependent probands. Arch Gen Psychiatry. 2004;61:1246–1256. doi: 10.1001/archpsyc.61.12.1246. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16:809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2012;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Singleton EG, Tiffany ST, Henningfield JE. Alcohol Craving Questionnaire (ACQ-NOW): Background, Scoring, and Administration. Intramural Research Program, National Institute on Drug Abuse: Baltimore, MD, USA; 2000. [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, et al. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, et al. Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [(1)(1)C]raclopride. Biol Psychiatry. 2010;68:689–696. doi: 10.1016/j.biopsych.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Wang C, Morris ED. Change in binding potential as a quantitative index of neurotransmitter release is highly sensitive to relative timing and kinetics of the tracer and the endogenous ligand. J Nucl Med. 2004;45:903–911. [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Morris ED. Assessing dopaminergic neurotransmission with PET: basic theory and applications in alcohol research. Curr Med Imag Rev. 2011;7:118–124. [Google Scholar]
- Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O'Connor SJ, et al. When what you see isn't what you get: alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcohol Clin Exp Res. 2009;33:139–149. doi: 10.1111/j.1530-0277.2008.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.