Abstract
Drug-related cues are potent triggers for relapse in people with cocaine dependence. Dopamine (DA) release within a limbic network of striatum, amygdala and hippocampus has been implicated in animal studies, but in humans it has only been possible to measure effects in the striatum. The objective here was to measure drug cue-induced DA release in the amygdala and hippocampus using high-resolution PET with [18F]fallypride. Twelve cocaine-dependent volunteers (mean age: 39.6±8.0 years; years of cocaine use: 15.9±7.4) underwent two [18F]fallypride high-resolution research tomography–PET scans, one with exposure to neutral cues and one with cocaine cues. [18F]Fallypride non-displaceable-binding potential (BPND) values were derived for five regions of interest (ROI; amygdala, hippocampus, ventral limbic striatum, associative striatum, and sensorimotor striatum). Subjective responses to the cues were measured with visual analog scales and grouped using principal component analysis. Drug cue exposure significantly decreased BPND values in all five ROI in subjects who had a high-, but not low-, craving response (limbic striatum: p=0.019, associative striatum: p=0.008, sensorimotor striatum: p=0.004, amygdala: p=0.040, and right hippocampus: p=0.025). Individual differences in the cue-induced craving response predicted the magnitude of [18F]fallypride responses within the striatum (ventral limbic: r=0.581, p=0.048; associative: r=0.589, p=0.044; sensorimotor: r=0.675, p=0.016). To our knowledge this study provides the first evidence of drug cue-induced DA release in the amygdala and hippocampus in humans. The preferential induction of DA release among high-craving responders suggests that these aspects of the limbic reward network might contribute to drug-seeking behavior.
Keywords: addiction, craving, reward, striatum, limbic, conditioning
INTRODUCTION
The amygdala and hippocampus potently influence learning and memory (Robbins et al, 2008), responses to motivationally important cues (Tracy et al, 2001; Tye and Janak, 2007), and the development and expression of habit-like behaviors (Lingawi and Balleine, 2012). Less attention has been given to how they affect responses to drug-related cues, but lesioning or inactivating these regions diminishes cue-precipitated drug-seeking behaviors (Meil and See, 1997; Kantak et al, 2002; Rogers and See, 2007), whereas electrical stimulation increases them (Vorel et al, 2001; Hayes et al, 2003). In humans, functional neuroimaging studies have identified both amygdala and hippocampal activations to drug-related cues (Grant et al, 1996; Childress et al, 1999; Wexler et al, 2001), but the neurotransmitters mediating these effects remain unknown.
One plausible candidate transmitter is dopamine (DA). Mesolimbic DA transmission is thought to influence the ability of drug cues to capture and sustain interest, and foster the development and expression of habit-like, stimulus-response behaviors (Berridge, 2007). In laboratory animals, these effects have been studied primarily within the striatum. However, exposure to cocaine cues can also induce DA release within the amygdala (Weiss et al, 2000), an effect known to influence cue-induced cocaine-seeking behavior (See et al, 2001; Ledford et al, 2003; Berglind et al, 2006). The role of hippocampal DA transmission on responses to drug cues remains unknown, but emerging evidence supports an influence in the formation and activation of emotionally potent memories (Shohamy and Adcock, 2010). Together, these observations highlight the importance of DA transmission within multiple regions in the acquisition, selection and maintenance of reward-seeking behaviors.
In humans, drug cue-induced DA responses have been reported in the striatum (Volkow et al, 2006; Wong et al, 2006; Boileau et al, 2007), but not elsewhere in the brain reflecting limitations of the PET tracer, [11C]raclopride. A more recently developed tracer, though, [18F]fallypride, has higher affinity than [11C]raclopride for D2/D3 receptors enabling the measurement of DA release in regions where the concentration of DA receptors is substantially lower than striatum (Mukherjee et al, 2002; Slifstein et al, 2010). In the present study, we used this tracer with high-resolution PET to assess the ability of drug cues to induce DA release in the amygdala, hippocampus, and striatum of volunteers meeting diagnostic criteria for cocaine dependence.
MATERIALS AND METHODS
Participants
Non-treatment-seeking cocaine users who met DSM-IV criteria (American Psychiatric Association, 2000) for current cocaine dependence were recruited from the community through local advertisements. Volunteers who tentatively met the entry criteria following a brief telephone screen were invited to a more in-depth face-to-face evaluation using the Structured Clinical Interview for DSM-IV (First, 1997). Participants were free of current axis I psychiatric disorders other than substance use, had never experienced head trauma with loss of consciousness, and were physically healthy as determined by a medical exam, electrocardiogram, and standard laboratory tests. Women were excluded if they had a seropositive pregnancy test. All participants had a current or past history of other illicit substance use, but reported cocaine as their drug of choice (Supplementary Table 1). No participants were currently seeking treatment for their substance use problems or planning to quit within the month following the study. The study was carried out in accordance with the Declaration of Helsinki and approved by the Research Ethics Board of the Montreal Neurological Institute. All participants provided written, informed consent.
Procedure
Each subject had one MRI and two PET sessions carried out on separate days. Subjects were asked to abstain from psychotropic drugs for at least 24 h before the PET sessions, and on the morning of each test day, urine drug screens were administered (Triage Drugs of Abuse Panel, Biosite Diagnostics, sensitive to amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, opiates, and phencyclidine) and results were recorded. Female participants were given a urine pregnancy test before each PET session; none tested positive (Assure FastRead hCG Cassette, Conception Technologies, San Diego, California, USA). As gonadal hormone levels fluctuate during the menstrual cycle and these changes are thought to influence reward-related neurotransmission (Becker, 2009), female participants were tested during their follicular phase when estradiol and progesterone levels are lower and more stable. Menstrual phase was verified by self-report and all were tested in the first 7 days of their cycle.
On the neutral cue session (Figure 1), participants developed, 2 h before scanning began, an autobiographical script with the investigator in which they recalled a relaxing, uneventful day that they could clearly remember, and narrate in detail. The development and rehearsal of this script lasted for 30 min. They were then presented with paperclips, pencils, and erasers, asked to doodle or write a few sentences and erase them, and manipulate the paperclips. This object manipulation lasted about 15 min. Subjects were then shown a 10-min video clip of people in everyday situations. Additional non-drug-themed neutral videos were watched while lying on the PET bed.
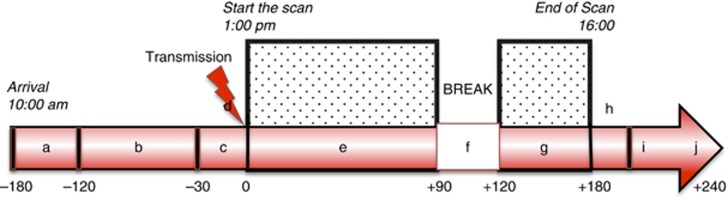
Figure 1.
Test day procedures and timing. Time points are defined according to start of emission scan (time point 0). a: Arrival at the PET unit, baseline measurements, and urine drug test. b: Develop autobiographical script, manipulate paraphernalia, watch video highlights (context different on neutral and cue day as described in Supplementary Section in full detail). c: Collect subjective measures, lay down in camera, insert intravenous catheter for tracer injection. d: Six-min transmission scan. e: Emission scan, watching videos through video glasses. f: 30-min break. g: Reinstall in the scanner, continue neutral, or cue videos. h: Six-min transmission scan. i: End of the scan, removal from the scanner, self report of subjective measures. j: Debriefing.
Procedures were similar on the cocaine cue test session (Figure 1). Two hours before scanning began participants developed an autobiographical script with the investigator, in which they described in detail a subjectively positive drug experience. Intranasal cocaine powder users were presented with a mirror, a razor blade, a straw, and a bag of white powder (lactose). Crack cocaine users were provided with a crack pipe, a spoon and a rock-shaped crystal (salt). Subjects were told that the substance was genuinely cocaine or crack. Subjects were asked to use the razor to divide the powder into lines several times and to hold the straw, or touch and smell the crystal and put it in the pipe or spoon. This object manipulation lasted for 15 min. For the following 15 min, subjects watched a cocaine-themed video. Additional cocaine-themed videos were watched while lying on the PET bed. The videos showed images of people buying, using, and becoming intoxicated by cocaine (powder or crack depending on the subject's preferred form of the drug), as well as images of the drug itself and drug paraphernalia.
Neuroimaging
Each participant underwent two PET scans on a Siemens high-resolution research tomograph and one T1-weighted MRI session for PET/MR co-registration. PET sessions consisted of a bolus injection of 3.30±0.24 mCi [18F]fallypride and two dynamic image acquisition scans (90-min and 60-min) separated by a 30-min break. A 6-min 137Cs transmission scan for attenuation correction was performed at the beginning and end of every scan session.
[18F]Fallypride non-displaceable binding potential values (BPND=FND * (Bavail/KD; please see Supplementary Methods) were calculated (Cunningham et al, 1991; Innis et al, 2007) using the Simplified Reference Tissue Model (Lammertsma and Hume, 1996) with the basis functions method (Gunn et al, 1997). The gray matter of the cerebellum was used as the reference region, as it is devoid of D2/D3 receptors. Regions of interest (ROIs) were defined on each individual's MRI in stereotaxic space, and BPND values were derived for inter-group comparisons using Turku PET centre tools (http://www.turkupetcentre.net/). Regional BPND values were weighted with the volume size when combining both hemispheres (see Supplementary Section for additional details).
ROI Analysis
We focused on a restricted number of a priori defined ROIs based on the areas implicated in cue responsivity and the ability of [18F]fallypride to detect effects there. The striatal sub-regions were based on the functional organization of limbic, associative and sensorimotor sub-compartments as proposed by Laruelle, Haber and colleagues (Haber and McFarland, 1999; Mawlawi et al, 2001; Martinez et al, 2003): ventral striatum (limbic striatum), pre-commissural dorsal caudate (posterior caudate/associative striatum), pre-commissural dorsal putamen (posterior putamen/associative striatum), post-commissural caudate (anterior caudate/associative striatum), and post-commissural putamen (anterior putamen/sensorimotor putamen). The two extra-striatal regions were hippocampus and amygdala. Regions were segmented using F.I.R.S.T. (FMRIB's Integrated Registration and Segmentation Tool) (http://www.fmrib.ox.ac.uk/fsl/first/index.html; Patenaude et al, 2011), and then checked and modified manually if necessary.
Behavioral Measures
Drug craving and subjective mood states were assessed using 17 Likert-like visual analog scale (VAS) items (happy, rush, high, euphoria, excited, anxious, energetic, mind-racing, alert, bored, interested, urge for cocaine, desire cocaine, crave cocaine, want cigarette, want alcohol, and want other drug). The VAS questionnaire was administered at baseline, 30 min before the start of the scan and then every 30 min after the start of the scan. The Cocaine Selective Severity Assessment Scale was administered as a measure of early cocaine abstinence symptoms at the baseline of each scan day (Kampman et al, 1998). The total score was used as a measure of subjective withdrawal state.
Statistical Analysis
All data were analyzed using IBM SPSS Version 20 for Macintosh. Data were analyzed using the General Linear Model GLM procedure for repeated measures to model three within-subject factors of hemisphere (left and right), region (limbic striatum, associative striatum, sensorimotor striatum, amygdala, and hippocampus), and session (neutral, cocaine cue), and one between-subjects factor of group (high craving, low craving). Mauchly's test of sphericity suggested that the GLM, including both striatal and extrastriatal regions, violated the assumption of homogeneity of variance. We corrected for this by using lower-bound estimates to assess significance in the ANOVA; this is the most conservative correction available. Reanalyzing the data as separate ANOVAs for striatal and extrastriatal regions avoided the homogeneity issue but increased the risk of type I errors due to failure to correct for multiple testing. As the results were consistent with both analyses, we included all ROIs in one ANOVA and chose the more conservative option (lower-bound estimates of sphericity). Planned pairwise comparisons were performed to delineate the source of significant differences on ANOVA.
To estimate cue-induced change in subjective states, an average change from baseline score was calculated for each individual in each test session (delta score) and compared with Student's paired t-test. Because of substantial colinearity of the VAS items, distinct factors were generated. In brief, differences in VAS delta scores between the two sessions were calculated. These double delta scores were then grouped using principal component analysis. Factors with eigenvalues above one were extracted and varimax rotated when more than one factor was detected.
Individual differences in the magnitude of regional BP changes (%ΔBPND=(BPND_Neutral—BPND_cue)/BPND_Neutral * 100) were correlated with subjective states using Pearson product moment correlations. In all analyses, statistical significance was set as p⩽0.05. Data normality for BP change scores were assessed with the Shapiro–Wilk test and met the assumption of normality.
RESULTS
Characteristics of Participants
Twelve volunteers completed the study (Table 1). Participants reported smoking crack cocaine (N=9) or taking it intra-nasally (N=3) at least once a week for an average of 16 years (range: 3–25 years, average 7.5±4.5 grams of cocaine per week). All participants had a current or past history of other illicit substance use (Supplementary Table 1) but reported cocaine as their drug of choice. No participants were currently seeking treatment for their substance use problems.
Table 1. Characteristics of Research Participants (N=12).
| Characteristics | Value (mean±SD) |
|---|---|
| Age (years) | 39.5±8.0 (range: 31–48) |
| Sex (number) | Male (10/12) |
| Ethnicity | 3 African Americans, 1 Aboriginal, 8 Europeans |
| Age of first use (years)a | 23.7±6.5 |
| Duration of use (years) | 15.9±7.4 (range: 3–25) |
| Lifetime use (days) | 2100.3±1548.5 |
| Cocaine use days/week, past 5 yearsb | 4.3±2.1 |
| Amount/week (g) | 7.5±4.5 |
| Primary route of administration | 9 Smoked cocaine, 3 intranasal powder |
| Cigarette smokers | 9 Current smokers |
Drug use information refers to cocaine use and was collected through a self-report retrospective interview.
For subjects with less than 5 years history of use, this number equals lifetime use.
Subjective States Analysis
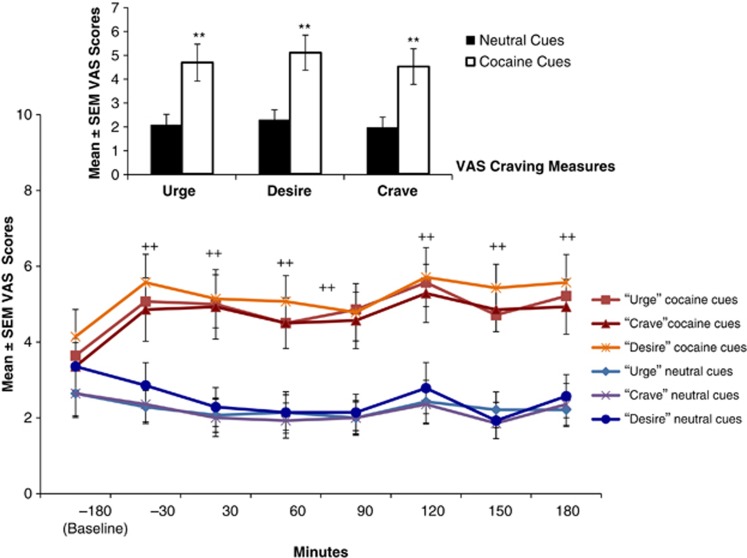
Exposure to the cocaine cues, as compared to the neutral cues, significantly increased drug craving scores (urge, desire, crave cocaine), effects that were maintained throughout the PET scanning session (p-values <0.005; Figure 2). Cocaine cue exposure also increased scores for Rush, Anxious, Excited, Mind-racing, Interested, and Euphoria (all t (11)> 2, p<0.04); however, as many of the VAS measures were highly inter-correlated, reflecting a smaller number of latent constructs, principal component analysis was used to extract factors from the time-averaged double delta VAS scores. Six distinct factors were identified (Supplementary Table 2). The first factor accounted for 31% of the variance and included four items: crave cocaine (0.94), desire cocaine (0.85), urge for cocaine (0.85), and alert (0.75). This factor appeared to represent focused craving for cocaine; it was used in the subsequent correlational analysis and to divide subjects into those who did (n=6) vs did not (n=6) report positive changes in the crave factor score.
Figure 2.
Changes of mean craving scores of self-report visual analog scales on neutral and cocaine cue day. Time points are defined according to start of emission scan (time point 0). Cue exposure starts approximately 1 h after baseline time point. Error bars represent standard errors of the mean (All t-values>3.5, df=11, **p values<0.005).
PET [18F]Fallyride BPND Data: Effect of Cocaine Cues
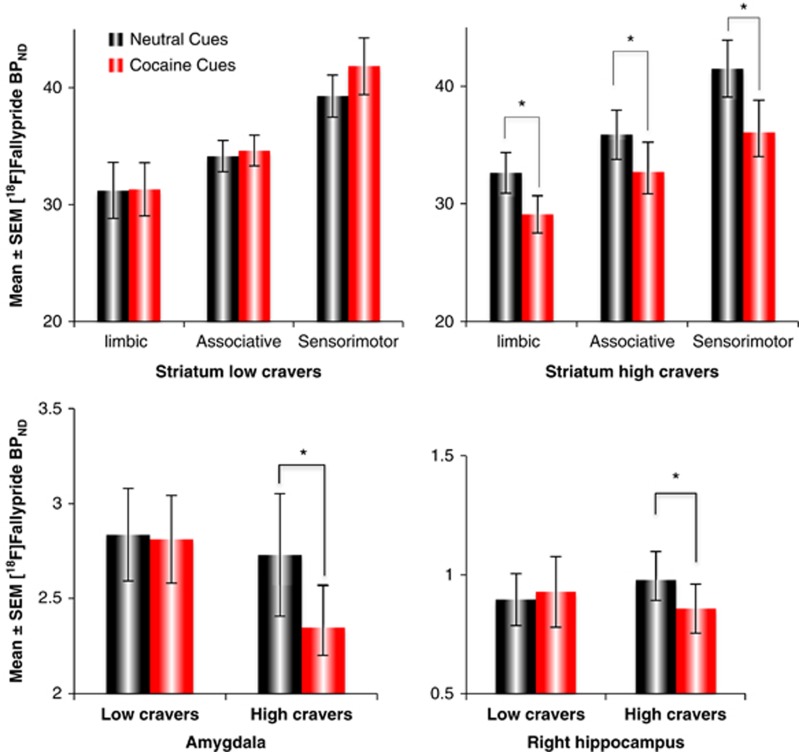
The four-way group × session × ROI × hemisphere ANOVA of BPND values yielded a three-way group × session × ROI interaction (F1,10=9.02, p=0.013). Decomposition of the interaction indicated that this reflected significant cue-induced decreases in [18F]fallypride BPND among the high-craving subjects (Figure 3; Supplementary Figure 1; Supplementary Table 4). Among subjects exhibiting high-craving factor scores, exposure to the cocaine cues, compared with the neutral ones, led to significantly lower BPND values in the limbic p=0.019, associative p=0.008, and sensorimotor p=0.004 striatum, as well as the amygdala (p=0.040). Significant effects were not seen in the whole hippocampus. However, further exploration suggested an effect in the right hippocampus (Least Significant Difference post hoc: p=0.047; t-test: t(5)=3.15, p=0.025). These effects were not seen in subjects with low-craving scores (p⩾0.1) (Figure 3; Supplementary Table 4).
Figure 3.
[18F]Fallypride BPND on neutral and cocaine cue days in subjects with high- and low-craving factor scores Top: striatal regions. Bottom left: amygdala. Bottom right: hippocampus. Values represent mean±SEM. *p<0.05.
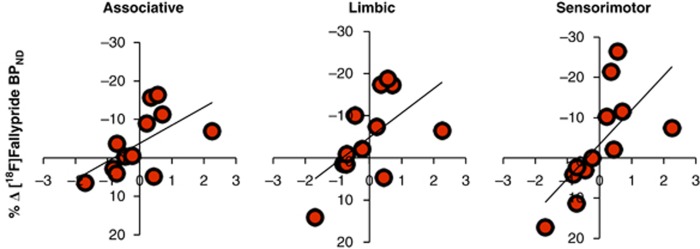
As found in two PET [11C]raclopride studies (Volkow et al, 2006; Wong et al, 2006), individual differences in cocaine cue-induced craving predicted differences in the measure of striatal DA release. The greater the craving response, the greater the DA response. This association was observed in the striatum as a whole (r=0.631, p=0.028) and in all three striatal ROIs: limbic (r=0.581, p=0.048), associative (r=0.589, p=0.044), and sensorimotor (r=0.675, p=0.016; Figure 4). The effects were in the same direction when hemispheres were investigated independently and reached significance in left associative (r=0.604, p=0.038), left sensorimotor (r=0.773, p=0.003), and right limbic striatum (r=0.626, p=0.029). Correlations between craving and changes in [18F]fallypride BPND were not significant in the hippocampus (r=0.213, p=0.51) or amygdala (r=0.258, p=0.42).
Figure 4.
The relationship between changes in cue-induced craving factor score (x axis) and percent changes in [18F]fallypride BPND (y axis) across the test sessions in striatal ROIs (N=12). Associative striatum (r=0.589, p=0.044), ventral limbic striatum (r=0.581, p=0.048), and sensorimotor striatum (r=0.675, p=0.016).
DISCUSSION
To our knowledge, the present study provides the first evidence of drug cue-induced DA release in human amygdala and hippocampus. The amygdala is thought to have an important role in the acquisition and expression of learned associations between emotionally important events. In conjunction with activity in the striatum and hippocampus, these effects influence the ability of motivationally salient stimuli to elicit and sustain focused interest and facilitate the selection of situation appropriate behavioral responses (Robbins and Everitt, 2002; Phillips et al, 2003; Goto and Grace, 2008; Robbins et al, 2008; Shohamy and Adcock, 2010).
In humans, the role of the amygdala in the processing of emotionally relevant stimuli has been studied using various methods, including functional neuroimaging (Chase et al, 2011; Tang et al, 2012), assessments of the effects of naturally occurring selective lesions (Adolphs et al, 1995; Tsuchiya et al, 2009), and following direct electrical stimulation (Rayport et al, 2006). Together, these studies are consistent with a more extensive animal literature indicating that the amygdala can modulate associative learning between discrete cues and rewards, influence the emotional intensity attached to events, and regulate striatal responsiveness and its effects on behavioral approach (Savage and Ramos, 2009; Buffalari and See, 2010). Although few studies have investigated which specific neurotransmitters are implicated, DA is a plausible candidate. For example, in laboratory animals, exposure to cocaine cues increases DA release in the amygdala (Weiss et al, 2000). Moreover, several studies have demonstrated that pharmacological manipulations of DA levels in the amygdala influence the behavioral response to cocaine cues (Alleweireldt et al, 2002; Di Ciano et al, 2003; Berglind et al, 2006) and affect learning and memory of the cue–drug association (Hitchcott and Phillips, 1997). Our own study raises the possibility that cue-induced amygdalar DA release has a similar role in humans.
To the best of our knowledge, this is also the first report of a dopaminergic response to cocaine cues in the hippocampus in both the animal and human literatures. A role of the hippocampus in episodic memory, reward learning, and the generation of contextually appropriate reward seeking has been indicated, though (McDonald and White, 1993; Eichenbaum, 2013; Dickerson and Eichenbaum, 2009). Several studies have demonstrated that DA transmission facilitates hippocampal synaptic long-term potentiation (Jay, 2003; Li et al, 2003) and likely has an important role in the formation and reactivation of reward-related memories (Shohamy and Adcock, 2010; Frey et al, 1990; Otmakhova and Lisman, 1998). In humans, neuroimaging studies have provided evidence of hippocampal activation following exposure to drug cues (Grant et al, 1996; Kilts et al, 2001; Wexler et al, 2001; Chase et al, 2011; Tang et al, 2012). Moreover, activity in dopaminergic midbrain regions evoked by reward anticipation tasks is associated with hippocampal activation and evidence of enhanced hippocampus-dependent long-term memory formation (Wittmann et al, 2005; Adcock et al, 2006). Thus, the hippocampal DA signal may influence neuroplastic changes that facilitate long-term memories of pairings between rewards and context.
In the present study, cue-induced DA release was also observed in the striatum. Evidence of cocaine cue-induced striatal DA responses has been seen previously in PET studies with [11C]raclopride (Volkow et al, 2006; Wong et al, 2006). As observed here, individual differences in the magnitude of the striatal DA effect co-varied with self-reported craving. Based on studies conducted in laboratory animals, it has been proposed that cue-induced DA release within the ventral striatum facilitates flexible, goal-directed approach toward reward-related stimuli (Weiss et al, 2000; Nicola et al, 2005; Berridge, 2007). DA release in more dorsal regions of the striatum, in comparison, may more closely reflect the acquisition and promotion of habit-like, stimulus-response behaviors (McDonald and White, 1993; Ito et al, 2002; Vanderschuren et al, 2005). Accumulating evidence, though, suggests that the primate striatum is not parcellated into sharply delineated subregions; rather there is a gradation of limbic cortical input, innervating ventromedial aspects most densely, dorsolateral aspects least so. Whereas the ventral striatum receives dense input from the amygdala, hippocampus and limbic cortex, more dorsal aspects receive more input from associative and sensorimotor cortex (Haber and Knutson, 2010).
The midbrain DA system includes projections from the substantia nigra to dorsal striatum and more limbic-directed projections from the ventral tegmental area to the nucleus accumbens, basolateral, and central nuclei of the medial amygdala, and hippocampus; DAergic innervation of the latter structure is more dense in primates than in rodents (Haber and Knutson, 2010). As noted above, reciprocal innervation is evident also, and stimulating the afferent fibers from the amygdala and hippocampus increases accumbal DA release (Floresco et al, 1998; Floresco et al, 2001). Our finding of DA responses to cocaine cues in all three regions—amygdala, hippocampus, and striatum—supports the view of limbic and striatal structures as components of an integrated system, contributing to the incentive salience of motivationally relevant cues (Robbins and Everitt, 2002; Phillips et al, 2003; Goto and Grace, 2008; Shohamy and Adcock, 2010).
The observation that cue-induced DA responses occurred only in the high-craving subgroup may reflect a number of factors. First, the videos contained narrative detail designed for the local milieu, and the autobiographical script would be expected to enhance these effects, but some participants might be non-responsive to the mostly impersonal cues (O'Brien et al, 1979; Staiger and White, 1991; Conklin et al, 2010). Alternatively, our low-craving participants may have had less intent to use drugs that day; active inhibition of craving can affect cue-induced appetitive states and cortico-limbic activity (Wertz and Sayette, 2001; McBride et al, 2006; Volkow et al, 2010; Prisciandaro et al, 2012). Finally, recent animal studies suggest that DA responses to reward-related cues occur only in those subjects that imbue the cues with incentive salience; individual differences in these tendencies appear to be an inherited trait (Robinson and Flagel, 2009; Flagel et al, 2010). The higher craving individuals in our study might be particularly prone to attribute incentive salience to drug cues. Intriguingly, though, as both sub-groups had extensive cocaine use histories, the observations might identify two separate neurobiological pathways to addiction.
Our findings should be interpreted in light of the following considerations. First, consistent with two PET [11C]raclopride studies in cocaine dependent participants (Volkow et al, 2006; Wong et al, 2006), we observed evidence of cue-induced DA responses in the dorsal striatum. In comparison, in healthy volunteers administered only three doses of d-amphetamine, exposure to drug-paired cues led to DA release in the ventral striatum (Boileau et al, 2007). In the present study, cue-induced DA responses were seen in both the dorsal and ventral striatum. This more widespread effect could reflect the presence of relatively more diverse cues (eg, videos, autobiographical memories, and paraphernalia), the fact that our participants were not inpatients but free to depart after the test sessions and potentially use cocaine, or the use of a different tracer plus higher-resolution camera. These features noted, the statistically most robust effect was seen in sensorimotor striatum, which overlaps with the area preferentially activated in the [11C]raclopride studies (Volkow et al, 2006; Wong et al, 2006). Moreover, the present results suggest that exposure to a mix of personalized and novel cues evocative of highly learned reward-related memories and behaviors can lead to activations of both ventral and dorsal aspects of the striatum. Second, in our study, individual differences in craving did not correlate with the magnitude of DA response in the amygdala and hippocampus. One possibility is that, compared with the striatum, DA responses in these regions are somewhat less closely related to the initiation of approach behaviors, and more closely related to stimulus intensity, context, and associative learning (Everitt and Robbins 2005). Third, our PET scans were 3 h in duration (time post-tracer injection). There is broad consensus that 60–90 min is sufficient to detect effects outside of the basal ganglia (ie, amygdala and hippocampus); within the striatum, longer scans are required due to the greater time needed for fallypride to reach steady-state levels. Simulation experiments suggest that a striatal signal begins to emerge after 2 h (Ceccarini et al, 2012); empirical data indicate that tracer equilibrium is clearly achieved by 3 h (Vernaleken et al, 2011). The present study plus work conducted elsewhere further confirm that scans of 180–210 min are sufficient to measure striatal DA release (Buckholtz et al, 2010a; Buckholtz et al, 2010b; Treadway et al, 2012). Fourth, the associations between cue-induced DA release and self-reported drug craving are correlations and do not indicate causality. However, other evidence indicates that DA contributes to susceptibility to craving states; eg, diminishing cocaine cue-induced increases in DA transmission leads to decreases in craving (Berger et al, 1996; Leyton et al, 2005). Fifth, the order of scans was fixed (neutral day first, followed by cocaine day) to avoid pairing the PET environment with drug cues before the neutral test session. This benefit of the design was considered reasonable as [18F]fallypride binding exhibits good test–retest reliability (Mukherjee et al, 2002); indeed, if the effect seen here was due to the order of scan, it would not have been observed only in those subjects reporting high levels of craving. Finally, our study had a small number of female participants. Future studies will be needed to address possible effects of gender.
Acknowledgments
We thank R Fukasawa, G Sauchuck, and S Mattei for technical assistance at the PET unit; D Jolly and M Kovacevic for preparation of radiotracers. Funding for the study came from an operating grant to ML from the Canadian Institutes of Health Research (CIHR, MOP-36429).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleweireldt A, Weber S, Kirschner K, Bullock B, Neisewander J. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacol. 2002;159:284–293. doi: 10.1007/s002130100904. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Desk reference to the diagnostic criteria from DSM-IV-TR. Amer Psychiatric Pub Inc; 2000. [Google Scholar]
- Becker JB. Sexual differentiation of motivation: A novel mechanism. Horm Behav. 2009;55:646–654. doi: 10.1016/j.yhbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SP, Reid MS, Delucchi K, Hall S, Hall S, Berger SP, et al. Haloperidol antagonism of cue-elicited cocaine craving. Lancet. 1996;347:504–508. doi: 10.1016/s0140-6736(96)91139-3. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE. Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience. 2006;137:99–706. doi: 10.1016/j.neuroscience.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Berridge K. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, et al. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci. 2007;27:3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 2010a;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, et al. Dopaminergic network differences in human impulsivity. Science. 2010b;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari D, See R. Amygdala mechanisms of Pavlovian psychostimulant conditioning and relapse. Behav Neurosci Drug Addict. 2010;3:73–99. doi: 10.1007/7854_2009_18. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Vrieze E, Koole M, Muylle T, Bormans G, Claes S, et al. Optimized in vivo detection of dopamine release using 18F-Fallypride PET. J Nuclear Med. 2012;53:1565–1572. doi: 10.2967/jnumed.111.099416. [DOI] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA, Robin N, McClernon FJ, Salkeld RP. Bringing the real world into the laboratory: personal smoking and nonsmoking environments. Drug Alcohol Depend. 2010;111:58–63. doi: 10.1016/j.drugalcdep.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham VJ, Pike VW, Bailey D, Freemantle CA, Page BC, Jones AK, et al. A method of studying pharmacokinetics in man at picomolar drug concentrations. Br J Clin Pharmacol. 1991;32:167–172. doi: 10.1111/j.1365-2125.1991.tb03877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D 3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2009;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Memory on time. Trends Cogn Sci. 2013;17:81–88. doi: 10.1016/j.tics.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- First MB. Amer Psychiatric Pub Inc: Washington, DC; 1997. User's guide for the structured clinical interview for DSM-IV axis I disorders SCID-I: clinician version. [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2010;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci. 2001;21:2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Yang CR, Phillips AG, Blaha CD. Association basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur J Neurosci. 1998;10:1241–1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990;522:69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008;31:552–558. doi: 10.1016/j.tins.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. NeuroImage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, McFarland NR. The concept of the ventral striatum in nonhuman primates. Ann N Y Acad Sci. 1999;877:33–48. doi: 10.1111/j.1749-6632.1999.tb09259.x. [DOI] [PubMed] [Google Scholar]
- Hayes RJ, Vorel SR, Spector J, Liu X, Gardner EL. Electrical and chemical stimulation of the basolateral complex of the amygdala reinstates cocaine-seeking behavior in the rat. Psychopharmacology (Berl) 2003;168:75–83. doi: 10.1007/s00213-002-1328-3. [DOI] [PubMed] [Google Scholar]
- Hitchcott PK, Phillips GD. Amygdala and hippocampus control dissociable aspects of drug-associated conditioned rewards. Psychopharmacology. 1997;131:187–195. doi: 10.1007/s002130050283. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Progr Neurobiol. 2003;69:375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D'Angelo L, Epperson LE. Reliability and validity of the cocaine selective severity assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, et al. Neural activity related to drug craving in cocaine addiction. Archives General Psychiatry. 2001;58:334. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Ledford CC, Fuchs RA, See RE. Potentiated reinstatement of cocaine-seeking behavior following D-amphetamine infusion into the basolateral amygdala. Neuropsychopharmacology. 2003;28:1721–1729. doi: 10.1038/sj.npp.1300249. [DOI] [PubMed] [Google Scholar]
- Leyton M, Casey KF, Delaney JS, Kolivakis T, Benkelfat C. Cocaine craving, euphoria, and self-administration: a preliminary study of the effect of catecholamine precursor depletion. Behav Neurosci. 2005;119:1619–1627. doi: 10.1037/0735-7044.119.6.1619. [DOI] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Lingawi NW, Balleine BW. Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits. J Neurosci. 2012;32:1073–1081. doi: 10.1523/JNEUROSCI.4806-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang D-R, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: Amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang D-R, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav Neurosc. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, et al. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Taha SA, Kim SW, Fields HL. Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience. 2005;135:1025–1033. doi: 10.1016/j.neuroscience.2005.06.088. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. D1/D5 dopamine receptors inhibit depotentiation at CA1 synapses via cAMP-dependent mechanism. J Neurosci. 1998;18:1270–1279. doi: 10.1523/JNEUROSCI.18-04-01270.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C, Greenstein R, Ternes J, McLellan A, Grabowski J. Unreinforced self-injections: effects on rituals and outcome in heroin addicts. Probl Drug Depend. 1979;27:275–281. [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Howland JG. Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neurosci Biobehav Rev. 2003;27:543–554. doi: 10.1016/j.neubiorev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S, Brady KT.2012Brain activation to cocaine cues and motivation/treatment status Addict Bioldoi: 10.1111/j.1369-1600.2012.00446.x [DOI] [PMC free article] [PubMed]
- Rayport M, Sani S, Ferguson SM. Olfactory gustatory responses evoked by electrical stimulation of amygdalar region in man are qualitatively modifiable by interview content: case report and review. Int Rev Neurobiol. 2006;76:35–42. doi: 10.1016/S0074-7742(06)76003-2. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 2007;87:688–692. doi: 10.1016/j.nlm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage LM, Ramos RL. Reward expectation alters learning and memory: The impact of the amygdala on appetitive-driven behaviors. Behav Brain Res. 2009;198:1–12. doi: 10.1016/j.bbr.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends Cogn Sci. 2010;14:464–472. doi: 10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Kegeles LS, Xu X, Thompson JL, Urban N, Castrillon J, et al. Striatal and extrastriatal dopamine release measured with PET and [18F] fallypride. Synapse. 2010;64:350–362. doi: 10.1002/syn.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger PK, White JM. Cue reactivity in alcohol abusers: stimulus specificity and extinction of the responses. Addict Behav. 1991;16:211–221. doi: 10.1016/0306-4603(91)90014-9. [DOI] [PubMed] [Google Scholar]
- Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: A meta-analysis of functional MRI studies. Physiol Behav. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Tracy AL, Jarrard LE, Davidson TL. The hippocampus and motivation revisited: appetite and activity. Behav Brain Res. 2001;127:13–23. doi: 10.1016/s0166-4328(01)00364-3. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, et al. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya N, Moradi F, Felsen C, Yamazaki M, Adolphs R. Intact rapid detection of fearful faces in the absence of the amygdala. Nat Neurosci. 2009;12:1224–1225. doi: 10.1038/nn.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Janak PH. Amygdala neurons differentially encode motivation and reinforcement. J Neurosci. 2007;27:3937–3945. doi: 10.1523/JNEUROSCI.5281-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Di Ciano P, Everitt BJ. Involvement of the Dorsal Striatum in Cue-Controlled Cocaine Seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernaleken I, Peters L, Raptis M, Lin R, Buchholz HG, Zhou Y, et al. The applicability of SRTM in [18F]fallypride PET investigations: impact of scan durations. J Cereb Blood Flow Metab. 2011;31:1958–1966. doi: 10.1038/jcbfm.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Telang F, Logan J, Jayne M, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. NeuroImage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci. 2000;97:4321. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity on self-reported urge. Exp Clin Psychopharmacol. 2001;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Düzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.