Abstract
Purpose
To establish feasibility, maximum tolerated dose, and potential efficacy of ablative dose total marrow irradiation (TMI) delivered by helical tomotherapy, in patients with multiple myeloma (MM).
Experimental Design
Patients with responding or stable MM received tandem autologous transplants (TASCT), first with melphalan 200 mg/m2, and ≥ 60 days later, with TMI. TMI doses were to be escalated from 1000 cGy, by increments of 200 cGy. All patients received thalidomide and dexamethasone maintenance.
Results
Twenty two of 25 enrolled patients (79%) received TASCT: TMI was administered a median of 63.5 days (44–119) after melphalan. Dose limiting toxicities at level 5 (1800 cGy) included reversible grade 3 pneumonitis, congestive heart failure, and enteritis (1), and grade 3 hypotension (1). The estimated median radiation dose to normal organs was 11–81% of the prescribed marrow dose. Late toxicities included reversible enteritis (1), and lower extremity deep venous thrombosis during maintenance therapy (2). The complete and very good partial response rates were 55% and 27%, following TASCT and maintenance therapy. At a median of 35 months of follow-up (21-50+ months) progression-free and overall survival for all patients are 49 % (95% CI 0.27-0.71) and 82% (0.67-1.00).
Conclusion
Ablative dose TMI as part of TASCT is feasible, and the complete response rate is encouraging. Careful monitoring of late toxicities is needed. Further assessment of this modality is justified at the 1600 cGy maximum tolerated dose level in MM patients who are candidates for ASCT.
Keywords: multiple myeloma, total marrow irradiation, tandem autologous transplantation
INTRODUCTION
Complete (CR) and very good partial response (VGPR) are considered surrogate markers of survival for patients with multiple myeloma (MM) (1-3). Single and tandem autologous stem cell transplantation (TASCT) have improved CR and VGPR rates, leading to improved progression-free (PFS) and overall survival (OS) (4-6). Although controversy still exists regarding the merits and best timing of TASCT (7) (8), data suggest that ASCT consolidation provides the greatest benefit when applied relatively early—within a year from diagnosis— and, prior to development of refractory disease.(9) (10) Data have also been emerging in support of maintenance therapy following ASCT (11-13). The incorporation of thalidomide, bortezomib, and lenalinomide as components of MM regimens, has lead to improved CR and very VGPR rates.(14-18) However, at least in patients under age 65, (8) the best long-term results for PFS and OS have been observed following treatment with ASCT and/or TASCT consolidation with maintenance (10, 13).
Fractionated total body irradiation (FTBI) continues to be an important part of conditioning regimens for patients undergoing SCT for a wide variety of hematologic malignancies (19). The optimal ablative dose for FTBI has not been clearly defined (19). Attempts to include FTBI as an adjunct to high-dose melphalan-based conditioning in patients with (20) MM were unsuccessful due to unacceptable toxicities (primarily mucositis). (21) Low-dose FTBI has been applied as part, or as the sole modality, in the context of non-myeloablative allogeneic SCT, but the role of such therapy in patients with MM is still to be defined. (22-24)
Recently, we reported on the concept of using helical tomotherapy (Tomotherapy Hi-Art System, Madison, WI) to deliver a more targeted, conformal form of FTBI. Dosimetry studies demonstrated reduced doses to adjacent critical normal organs which predicted for reduced toxicities.(25-27) We therefore set out to evaluate the feasibility and efficacy of total marrow irradiation (TMI) as part of TASCT in patients with MM, after they recovered from melphalan-based ASCT. Patients subsequently received maintenance therapy with thalidomide (28) and dexamethasone. Here, we describe the observed acute and early chronic toxicities, dose-limiting toxicities (DLTs) and maximum tolerated dose (MTD), CR and VGPR rates, and early results of PFS and OS in patients treated during the dose-finding phase of this trial.
MATERIALS AND METHODS
Patient Characteristics
Patients with Salmon-Durie stage I-III MM (29), within 18 months from diagnosis, in response, or with stable disease, and ≤ 70-years of age were eligible for this trial. Patient characteristics are provided in Table 1. Eligibility criteria included a Karnofsky status ≥ 70%, creatinine clearance > 50 ml/min, cardiac left ventricular ejection fraction ≥ 50%, SGOT and SGPT < 2.5 times the upper limits of institutional normal, adequate pulmonary function as demonstrated by a forced expiratory volume of > 60% and DLCO > 59% of predicted lower normal limit, and ≤ grade 1 peripheral neuropathy. Patients were pre-assessed for their ability to lie supine for approximately one hour, the time needed for one session of TMI. All patients voluntarily signed an informed consent form approved by the Institutional Review Board of the City of Hope Cancer Center.
Table 1.
Patient Characteristics
| Patient Population (N:22, 10F/12M) | Median (range) | ||
|---|---|---|---|
| Age, in years | 53 (35–66) | ||
|
| |||
| Time from diagnosis to ASCT, in months | 8 (4–14) | ||
|
| |||
| Time between cycles 1 and 2 of TASCT, in months | 63.5 (44-119) | ||
|
| |||
| Number of patients (: %) | |||
|
| |||
| Stage I II III | 2 (10) | 5 (22) | 15 (68) |
|
| |||
| M protein type: IgG IgA | 15 (68) | 7 (32) | |
|
| |||
| Del 13 by FISH | 9 (41) | ||
| Hyperploidy 5, 9, 15, 14, and /or 17 | 7 (32) | ||
| Hyperploidy 5, 9, or 15 and del 13 | 3 (14) | ||
| Normal | 7 (32) | ||
|
| |||
| Beta2 microglobulin at presentation > 3.5 | 2 (12) | ||
|
| |||
| Received thalidomide/dexamethasone | 8 (36) | ||
|
| |||
| Received bortezomib/dexamethasone | 9 (41) | ||
|
| |||
| Received lenolidomide/dexamethasone | 1 (5) | ||
|
| |||
| Received an anthracycline-containing regimen | 7 (32) | ||
|
| |||
| CR | VGPR | PR/SD | |
| Best response prior to TASCT | 2 (9) | 10 (45.5) | 10 (45.5) |
| Response after TASCT | 5 (23) | 11 (50) | 6 (27) |
| Best response after TASCT and maintenance | 12 (55) | 6 (27) | 4 (18) |
TASCT:tandem autologous stem cell transplant; CR: complete response; VGPR: very good partial response; PR: partial response; SD: stable disease
Treatment Schema
Patients underwent mobilization of peripheral blood progenitor cells (PBPCs) with a combination of cyclophosphamide (1.5 g/m2) and filgrastim (10 μg/kg/day), with the goal to procure ≥ 4 × 106 CD34+ cells/kg. During the first ASCT (cycle 1) patients received melphalan (100 mg/m2/day) for two consecutive days (totaling 200 mg/m2), followed by reinfusion of 50% of collected PBPCs.
Between a minimum of 6 and a maximum of 18 weeks after cycle 1, patients were readmitted for their second cycle of ASCT (cycle 2), consisting of TMI followed by PBPC reinfusion. TMI dose levels were to be escalated in increments of 200 cGy, starting from 1000 cGy to 2000 cGy/per cohorts. At the initial dose level of 1000 cGy, TMI was delivered at 200 cGy once daily over 5 days. Starting with dose level 1200 cGy, second planned fractions were delivered a minimum of 6 hours later. Table 2 describes the planned dose escalation scheme for TMI.
Table 2. Treatment Schema.
Mobilization and PBPC Procurement
Cyclophosphamide 1.5 gm/m2 and G-CSF 10 microgram/kg/day to procure ≥ 4 × 106 CD34+ cells/kg
Cycle 1
Melphalan 100 mg/m2/day × 2 days, then PBPC reinfusion and G-CSF 5 microgram/day starting day 5
Cycle 2
TMI followed by PBCS and G-CSF microgram/kg/day starting on day 0
| Day -5 | Day -4 | Day -3 | Day -2 | Day -1 | TMI dose level # of patients | ||
|---|---|---|---|---|---|---|---|
| cGy time | cGy | treated | |||||
|
| |||||||
| 200 am | 200 | 200 | 200 | 200 | 1000 | 1 | 3 |
|
| |||||||
| 200 am | 200 | 200 | 200 | 200 | 1200 | 2 | 4* |
| 200 pm | |||||||
|
| |||||||
| 200 am | 200 | 200 | 200 | 200 | 1400 | 3 | 3 |
| 200 pm | 200 | ||||||
|
| |||||||
| 200 am | 200 | 200 | 200 | 200 | 1600 | 4 | 6 |
| 200 pm | 200 | 200 | |||||
|
| |||||||
| 200 am | 200 | 200 | 200 | 200 | 1800 | 5 | 6 |
| 200 pm | 200 | 200 | |||||
|
| |||||||
| 200 am | 200 | 200 | 200 | 1600 cGy MTD Phase II dose | |||
| 200 pm | 200 | 200 | 200 | ||||
Due to an unplanned downtime, one patient received only 1200 cGy.
Maintenance: Dexamethasone (40 mg/d × 4 days every 28 days) and thalidomide (50-200 mg/d)
Radiotherapy Technique
Details of the technique have been previously published.(25-27) Briefly, all patients were initially scanned on a large bore (85 cm) CT simulator (Philips Medical System, Eindhoven, The Netherlands) and 4 mm slices were obtained during shallow breathing, inspiration and expiration to account for changes in position of ribs, lung, kidneys, liver, and spleen. A full body vac-lok bag (CIVCO Medical Systems, Kalona, IA) and thermoplastic mask over the head and neck were used as immobilization devices. Target (skeletal bone) and avoidance structures were contoured on an Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA). The mandible and maxillary bones were excluded as target structures in an effort to minimize oral cavity dose and mucositis. Using the Hi-Art Tomotherapy treatment planning system (Tomotherapy, Inc. Madison, WI), plans were designed such that a minimum of 85% of the target structures received the prescribed dose. An example of the radiation dose distribution is shown in Figure 1 for a patient receiving 1600 cGy.
Figure 1.
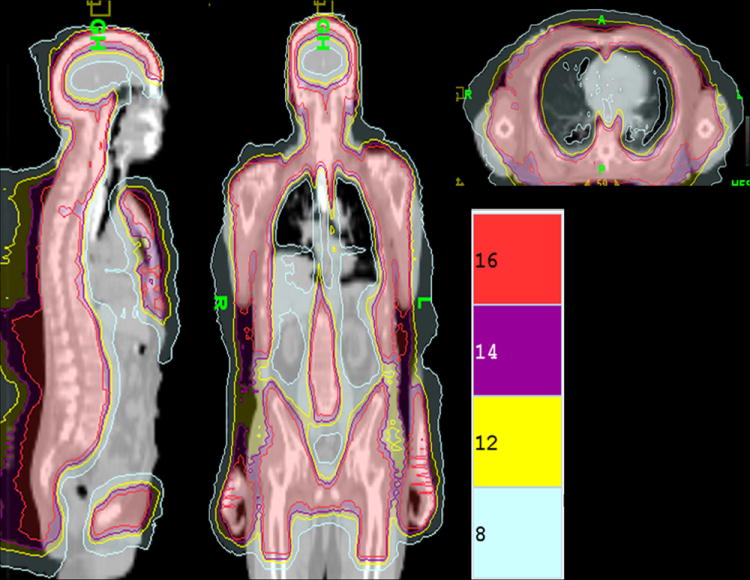
Treatment Plan Dose Distribution Color Wash From a Patient treated With 1600 cGy of TMI
Maintenance Therapy
Dexamethasone (40 mg/d × 4 days every 28 days) and thalidomide (50–200 mg/d) were initiated ≥ 30 days after TMI, and were administered for 6 months following achievement of complete remission, or, for at least 12 months for patients with persistent evidence of residual disease (Table 2). Patients also received zoledronic acid (4 mg, intravenously) every 3 months.
Supportive Care
During cycles 1 and 2, mandatory prophylactic antibiotics included levaquin, acyclovir, and fluconazole, and broad spectrum intravenous antibiotics were prescribed according to the treating physician’s choice for patients who developed neutropenic fever. Total parenteral nutrition, intravenous fluid support, red blood cell and platelet transfusions, and antiemetics were prescribed as per physician’s choice during cycle 1. However, in order to optimize control of nausea/emesis, the administration of antiemetics was standardized with the cohorts of patients treated at TMI doses 1,600 and 1,800 cGy, and consisted of aprepitant, ondasentron, and dexamethasone. No patient received palifermin, or erythropoietin. Filgrastim 5 μg/kg was started 5 days after PBPC reinfusion during the first cycle, and on the day of PBPC reinfusion, during cycle 2, and was administered through recovery of the absolute granulocyte count recovery to ≥1000 / μl, for 3 consecutive days.
Follow-up
Mandatory evaluations included physical assessment, routine hemogram and comprehensive chemistry panel, and serum protein electrophoresis every 3 months, and bone x-rays and bone marrow biopsies at 30 days, 6, and 12 months post TMI, and yearly thereafter.
Statistical Design
Patients were accrued in cohorts of 3–6 following a conventional phase I trial design. In case of observing DLT among the first 3 patients, a maximum of 6 patients were to accrue at that dose level. TMI dose escalation was not allowed until all patients treated at the previous dose level recovered hematologic function. DLT was defined as grade 3 or 4 non-hematologic toxicity (except for fatigue, electrolyte abnormalities, and febrile neutropenia) or grade 4 leukopenia or thrombocytopenia of greater than 28 days duration. Time to platelet independence was reported as the duration between ASCT and the day after the last platelet infusion. (30) Toxicities were assessed according to the NCI Common Toxicity Criteria (CTC) version 3. If ≥ 2 of 6 patients developed grade 3–4 toxicities, the MTD was to be established at the preceding (DLT-1) dose level.
Response criteria:CR was defined as complete absence of serum and urinary M-protein and no more than 5% plasma cells on bone marrow; VGPR as ≥ 90% reduction in bone marrow plasma cells and blood M-protein levels; partial response as ≥ 50% reduction in blood and bone marrow findings; and stable disease as < 25% reduction in blood and bone marrow findings for a minimum of 3 months.(31, 32) Progression was defined as > 25% increase in M-protein, > 25% increase of bone marrow plasma cells, or new bone lesions. During this phase I trial, a combined CR and VGPR rate of > 50% was required, to warrant further testing of the regimen. Additional outcomes examined included PFS and OS, which were calculated from day 1 of administering high-dose melphalan during cycle 1 of TAST. PFS was defined as time to any type of recurrence or death from any cause. Standard Kaplan-Meier and Cox regression methods were applied for survival analysis using the SAS/STAT and S-Plus software).
RESULTS
Patient Population
Twenty-five patients were enrolled between February 2005 and October 2007, 22 of whom received TASCT. Of the three patients who did not proceed to TMI, one refused, one suffered a septic episode during cycle 1, and her left ventricular ejection fraction remained lower then what was allowed (< 50%) to proceed with TMI, and a third patient was found to be ineligible due to a pre-existing, possibly malignant thyroid nodule.
Patient characteristics for the 22 patients who had received TMI are given in Table 1. The median age was 53 (range, 35–66). The majority of patients were treated for stage III disease. Thirty-five percent of patients received thalidomide and dexamethasone induction alone, 17% received thalidomide and dexamethasone plus either bortezomib or an anthracycline, 30% received an anthracycline-based regimen only, 13% received bortezomib and dexamethasone, and 4% received lenalidomide and dexamethasone as part of the induction regimen. No patients had received prior radiotherapy. Two patients were in CR (9%) and ten were in VGPR (45.5%) prior to initiating cycle 1 of TASCT. Patients were enrolled on this trial an average of 8 months (range, 4–14) from diagnosis.
Toxicities
Table 3 illustrates grade 3 and 4 non-hematologic toxicities among the 22 patients who received TASCT. Reversible grade 3 non-hematologic toxicities by TMI dose levels included febrile neutropenia (levels 1, 2, 4, 5: 1 patient each), fatigue (level 1: 1 patient, levels 4 and 5: 2 patients each). Other non-DLT toxicities included metabolic/electrolyte abnormalities (level 1: 1 patient, level 2: 1 patient, levels 4 and 5: 4 patients each). We observed one case of early engraftment syndrome in a patient treated at the lowest 1000 cGy dose level. The observed higher grade toxicities of nausea/emesis in the patients treated at 1600 and 1800 cGy were felt to be due to non-compliance and insufficient intensity of prescribed antiemetics: once the antiemetic regimen was adjusted and enforced (see supportive care section), no further incidence of > grade 2 nausea or emesis was observed. The incidence and types of non-dose limiting grade 3-4 toxicities were similar to those observed following cycle 1 (melphalan). DLT was observed in 2 patients treated at level 5 (1800 cGy), and consisted of reversible grade 3 radiation pneumonitis, congestive heart failure, and enteritis requiring parenteral feeding (1 patient), and reversible grade 3 hypotension and enteritis requiring pressor support (1 patient), thereby defining the maximum tolerated dose (MTD) at 1600 cGy (200 cGy twice daily × 4 days).
Table 3.
Grade 3 Non-hematologic Toxicities by Cycle and Dose Levels&
| Cycle 1 Melphalan 200 mg/m2 |
Cycle 2 TMI 1000 cGy |
Cycle 2 TMI 1200 cGy |
Cycle 2 TMI 1400 cGy |
Cycle 2 TMI 1600 cGy |
Cycle 2 TMI 1800 cGy |
Cycle 2 TMI all dose levels |
|
|---|---|---|---|---|---|---|---|
| # of patients treated | 22* | 3 | 4 | 3 | 6 | 6 | 22 |
| Toxicities | N (%) | ||||||
| Febrile neutropenia | 6 (27) | 1 | 1 | 0 | 1 | 1 | 4 (18) |
| Fatigue/anorexia | 3 (14) | 1 | 0 | 0 | 2 | 2 | 5 (22) |
| Nausea/emesis | 2 (9) | 0 | 0 | 0 | 2† | 2 | 3 (14) |
| Mucositis | 1 (4.5) | 0 | 0 | 0 | 0 | 0 | 0 (0) |
| Enteritis/colitis | 3 (14) | 0 | 0 | 0 | 0 | 2* | 2 (9) |
| Engraftment syndrome | 0 (0) | 1 | 0 | 0 | 0 | 0 | 1 (5) |
| Pneumonitis | 0 (0) | 0 | 0 | 0 | 0 | 1* | 1 (5) |
| Congestive heart failure/hypotension | 0 (0) | 0 | 0 | 0 | 0 | 1ˆ | 1 (5) |
| Metabolic/electrolyte abnormalities | 5 (23) | 1 | 1 | 0 | 4 | 4 | 10 (45) |
TMI, total marrow irradiation
non-compliance with antiemetics, not DLT;
one case of infectious colitis [non-DLT], and one case of DLT: reversible enteritis, pneumonitis, congestive heart failure in the same patient;
DLT: hypotension, requiring pressure support;
only patients who received both cycles are included
The median time between cycles 1 and 2 was 63.5 days (range, 44-119). The median number of days to reach a granulocyte count of > 1000/μl was 14 after cycles1 and 2 (range; 12-17 versus 13-18). The period to reach platelet independence was 13 days (range; 0-15 versus (0-17) following cycles 1 and 2. There were no delayed/secondary graft failures.
Late (30 days after ASCT) grade 3-4 toxicities included reversible enteritis in a patient previously experiencing similar toxicities during cycle 2 (one of the cases defining DLT), and 2 cases of deep venous thrombosis. There were no secondary malignancies.
The estimated median radiation dose to normal organs ranged from 11-81% of the prescribed target bone marrow dose (supplement: Table S3). The median doses to lens and oral cavity were < 25% of the prescribed target dose and to lungs, heart, GI tract and thyroid approximately 33-55%. Figure 2 illustrates the median organ doses with TMI. With the escalation of the prescribed TMI dose median doses for all organs increased as expected, but still remained below that would have been expected for standard 1200 cGy FTBI, where all unshielded organs would receive approximately 1200 cGy with the exception of bladder and breast doses in a few patients at the 1800 cGy dose level 27. The observed acute toxicities (Table 3) were consistent with the reduced organ doses predicted from treatment plans. In general, toxicities were not significantly different from what has been observed following high-dose melphalan and ASCT (cycle 1).
Figure 2.
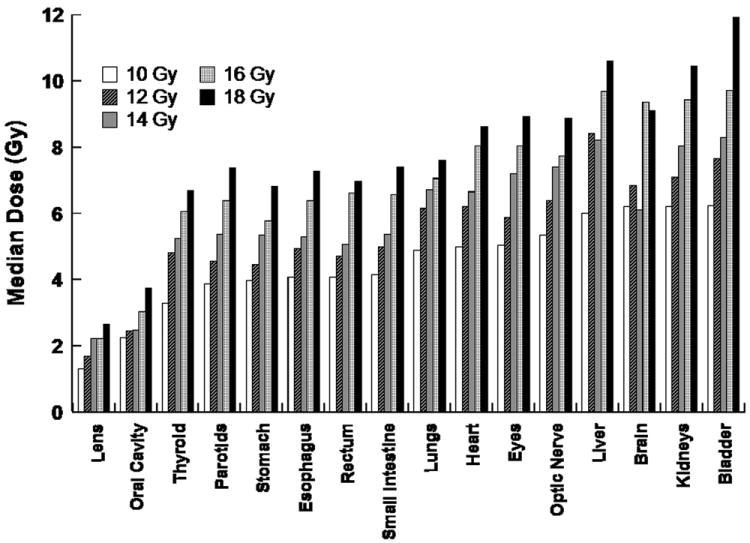
Median Organ Doses with TMI. Median (D50) dose for each organ compartment is shown. Bar graphs represent an average of the dose for the 3-6 patients at each TMI dose level.
Table 1 illustrates changes in response status following TASCT. At the mandated first (30 days after TMI) time point for disease assessment, 23% of patients were in CR and 50% in VGPR. An overall CR rate of 55% and VGPR rate of 27%were observed; the majority of patients achieved CR while on maintenance therapy, at a median time from TASCT of 10 months (range; 1-37 months), At a median of 35 months of follow-up (range:21-50 months) PFS and OS for all 25 patients enrolled are 49 % (95% CI: 0.27-0.71) and 82% (0.67-1.00; Figure 3). PFS and OS are 53% (95% C:I 0.3-0.76) and 84% (0.68-1.00) for the 22 patients who had received their planned TASCT (Figure not shown). Currently, 6 patients are still on maintenance therapy. The very first patient enrolled, remains in CR at 50 months, off maintenance therapy.
Figures 3.
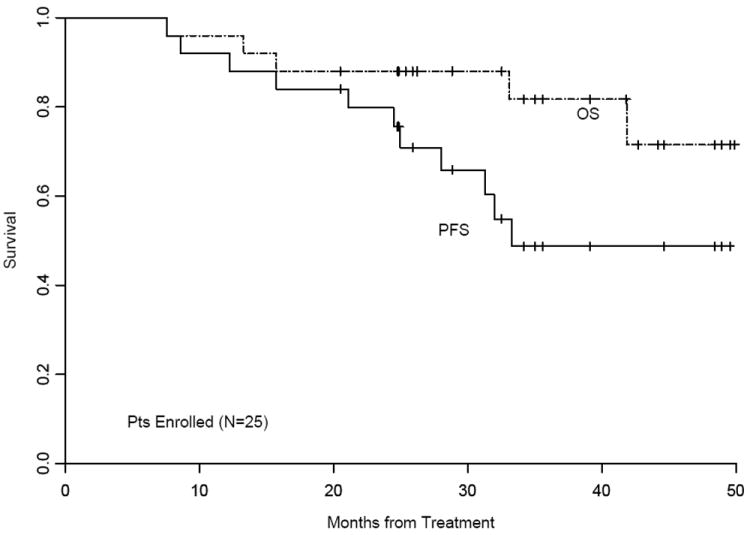
Kaplan-Meier Estimates of Progression-Free and Overall Survival for All 25 Patients Treated by Intent to Treat Analysis
DISCUSSION
Accomplishment of CR and VGPR is predictive of prolonged PFS and OS in patients with MM. Single, and more recently TASCT have resulted in an increased percentage CR and VGPR, and, as a consequence, significant prolongation in PFS has been observed in several well-conducted, prospective, randomized trials (5, 33), with overall survival benefits also seen in at least one trial (5). With the availability of novel therapeutic agents, higher CR and VGPR rates can be achieved both prior to ASCT, and also, when such agents (for example, thalidomide, bortezomib, lenolidomide) are incorporated as part of, or subsequent to ASCT (13, 34). Our current therapeutic dilemmas therefore include the task of identifying those who may benefit from ASCT following novel induction regimens, defining whether tandem ASCT provides benefit over “consolidation” therapy with novel agents, and, if so, in which patient population. Indeed, prospective randomized trials designed by the Bone Marrow Transplant CTN group in the United States, and by the IFM in collaboration with the Dana-Farber group have just began accruals to provide answers to such questions in well-designed, prospective, randomized trials. In the older (≥ 65) patient population data suggest that combinations of melphalan and/or anthracyclines with novel agents (e.g., thalidomide, lenalidomide, bortezomib), may yield high CR and VGPR rates and PFS and OS durations similar to that of single or tandem ASCT (17, 35, 36). However, none of the current regimens (inclusive of novel therapeutics with or with subsequent ASCT) are optimized for sequence, dose, duration, or targeting abilities, and their ability to kill the most resistant myeloma cells, i.e. myeloma stem cells, is limited (37).
TMI is an attractive option for patients with radiosensitive hematologic malignancies, such as MM. Because stem cell/tumorigenic cell properties may include the ability to withstand the effects of radical oxygen species, higher doses of radiation to disease-containing areas is a logical strategy to apply in the treatment sequence of patients with multiple myeloma (37). Several groups have attempted to escalate FTBI doses in combination with chemotherapeutic agents in an effort to improve outcomes via overcoming radiation resistance.The group from Fred Hutchinson Cancer Center reported comparing cyclophosphamide combined with 1200 cGy delivered at 200 cGy/day or 1575 cGy at 225 cGy/day. Lower relapse rates, but unacceptably higher treatment-related mortality rates were noted in patients treated for chronic myelogenous leukemia (24%, at 1200 cGy and 34%, treated at 1575 Gy), resulting in no difference in overall survival between the two arms (38), and confirming the difficulties of combining FTBI either in the allogeneic, or autologous setting, with high dose chemotherapy (21). Similarly, very high -76% -incidence of grade 3 or greater mucositis was observed in a trial which applied radiation therapy, first described by coining the phrase total marrow irradiation, using modified TBI with shielding of the liver and lungs, in combination with busulfan and cyclophosphamide, and resulting in 48% and 41% CR rates, in untreated and more advanced cases of myeloma (39).
Because CR rates are a meaningful surrogate marker for PFS and OS, improving the currently available and standard ASCT and TASCT preparatory regimens in patients with myeloma is worthwhile, whether the improvement is accomplished by adding novel agents (such as bortezomib), or using radiation therapy as the sole modality, but in a technically more precise fashion. Therefore we applied helical tomotherapy for TMI, and indeed, the acute toxicities observed in our TASCT trial following single modality TMI were modest and compared favorably to results from a prior phase I tandem autologous HCT trial of similar design. In that previous study, patients with multiple myeloma or breast cancers or sarcomas with primarily bone metastases were first treated with busulfan, melphalan, and thiotepa, followed by modified FTBI at 1200, 1350 or 1500 cGy. Death from pulmonary toxicity was observed in 3 of 17 patients treated at 1500 cGy, when the “standard” FTBI was applied in the setting of the trial (20).
Bone-seeking radiopharmaceuticals, such as 166Ho-DOTMP (40) have also been undergoing evaluation as part of ASCT conditioning regimens. Due to the unpredictable amounts of these pharmaceuticals that reach the target (bone marrow), and unexpectedly high doses that reach critical organs, such as components of the genitourinarty tract, these modalities are currently do not fit into the standard treatment regimens for myeloma patients.
In this phase I portion of our trial we have demonstrated the safety and clinical utility of TMI using image guided intensity-modulated radiotherapy delivered by helical tomotherapy. Median organ doses were approximately 11–81% of the prescribed bone marrow dose. With dose escalation to 1600 cGy, median organ doses still remained below that for standard FTBI to 1200 cGy, which predicted for reduced acute toxicities. Whether combining melphalan with TMI, or delivering boost doses to areas of greater tumor burden (for example areas of persistent uptake on PET scan following recovery from TASCT) is feasible with simultaneous TMI, would be the subject of alternative study designs.
TMI as the sole ablative modality in a tandem second ASCT following the first cycle of melphalan-ASCT is feasible up to 1600 cGy, and may contribute to the success of the tandem strategy followed by maintenance in this trial in view of the substantial conversion rate (55%) to CR, and VGPR (27%). Reduced radiation doses to major normal organs were predicted, and, indeed, the toxicities observed were moderate. However, further evaluation is needed to better characterize long-term toxicities and assess the impact this new approach will have on disease control. The phase II portion of our trial at TMI of 1600 cGy is ongoing, and pending the outcome, a randomized comparison of single ASCT versus TASCT may be justified.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Great progress has been made in the treatment of multiple myeloma, and, part of the improvement over the past decades in 5-year progression-free survival is due to the administration of autologous stem cell transplantation, following treatment with one or more of the ever improving types of induction therapy. Fractionated total body irradiation had been part of transplantation in combination with high-dose melphalan, but was deemed too toxic. Here, we report on the feasibility of delivering image-guided/sculpted total marrow irradiation as part of consolidation/tandem transplant by applying helical tomotherapy, following first a high-dose melphalan-based autologous transplantation, and allowing for safe, and potentially effective dosing of therapeutic dose total marrow irradiation for myeloma patients. Pending further confirmation, targeting the originating bone marrow and metastatic sites with ablative dose irradiation may allow for improved outcome following autologous transplantation for many thousands of suitable patients, each year.
Acknowledgments
The authors acknowledge the transplant coordinators and nurses, especially Donna Hawkins R.N, and Mirjana Kovacic R.N., for their dedication, and the protocol team lead by Annette Brown, for their support.
This work was supported by PO1 CA 30206-21, and P30 CA 33572.
Footnotes
Contribution: G.S., J.W., T. S., S.F., concieved and designed the study; G.S., R.S., S.F., R.S., D.S., L.P., P.P., N.K., F.S., A.K., C.K. provided patients; A.L, T.S., J.W.; provided technical support; S.F., G.S., J.W., T.S., provided administrative, and logistical support; P.F., G.S., J.W, S.F., analyzed and interpreted data; G.S, J.W., S.F., wrote the article; R.S., P.P., F.S., L.P., N.K., D.S., P.F., A. L., T.S., A.K., C.K., F.S., contributed to the article.
Conflict-of interest disclosure: Jeffrey Wong M.D. is the recipient of a research grant from Tomotherapy Hi-Art System, Madison, WI. The other authors declare no competing conflicts of interests
Partially resented at the 50th Annual Meeting of the American Society of Hematology (ASH), San Francisco, December 6-9, 2008.
References
- 1.Lahuerta JJ, Mateos MV, Martinez-Lopez J, et al. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26:5775–82. doi: 10.1200/JCO.2008.17.9721. [DOI] [PubMed] [Google Scholar]
- 2.Harousseau JL, Avet-Loiseau H, Attal M, et al. Achievement of at least very good partial response is a simple and robust prognostic factor in patients with multiple myeloma treated with high-dose therapy: long-term analysis of the IFM 99-02 and 99-04 Trials. J Clin Oncol. 2009;27:5720–6. doi: 10.1200/JCO.2008.21.1060. [DOI] [PubMed] [Google Scholar]
- 3.Chanan-Khan AA, Giralt S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol. 28:2612–24. doi: 10.1200/JCO.2009.25.4250. [DOI] [PubMed] [Google Scholar]
- 4.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 5.Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 6.Barlogie B, Attal M, Crowley J, et al. Long-term follow-up of autotransplantation trials for multiple myeloma: update of protocols conducted by the intergroupe francophone du myelome, southwest oncology group, and university of arkansas for medical sciences. J Clin Oncol. 28:1209–14. doi: 10.1200/JCO.2009.25.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–6. [PubMed] [Google Scholar]
- 8.Anderson KC, Alsina M, Bensinger W, et al. NCCN clinical practice guidelines in oncology: multiple myeloma. J Natl Compr Canc Netw. 2009;7:908–42. doi: 10.6004/jnccn.2009.0061. [DOI] [PubMed] [Google Scholar]
- 9.Pineda-Roman M, Barlogie B, Anaissie E, et al. High-dose melphalan-based autotransplants for multiple myeloma: the Arkansas experience since 1989 in 3077 patients. Cancer. 2008;112:1754–64. doi: 10.1002/cncr.23327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barlogie B, Attal M, Crowley J, et al. Long-Term Follow-Up of Autotransplantation Trials for Multiple Myeloma: Update of Protocols Conducted by the Intergroupe Francophone du Myelome, Southwest Oncology Group, and University of Arkansas for Medical Sciences. J Clin Oncol. doi: 10.1200/JCO.2009.25.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–94. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 12.Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009;27:1788–93. doi: 10.1200/JCO.2008.18.8573. [DOI] [PubMed] [Google Scholar]
- 13.Ghobrial IM, Stewart AK. ASH evidence-based guidelines: what is the role of maintenance therapy in the treatment of multiple myeloma? Hematology Am Soc Hematol Educ Program. 2009:587–9. doi: 10.1182/asheducation-2009.1.587. [DOI] [PubMed] [Google Scholar]
- 14.Richardson PG, Weller E, Jagannath S, et al. Multicenter, phase I, dose-escalation trial of lenalidomide plus bortezomib for relapsed and relapsed/refractory multiple myeloma. J Clin Oncol. 2009;27:5713–9. doi: 10.1200/JCO.2009.22.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gay F, Hayman SR, Lacy MQ, et al. Lenalidomide plus dexamethasone versus thalidomide plus dexamethasone in newly diagnosed multiple myeloma: a comparative analysis of 411 patients. Blood. 115:1343–50. doi: 10.1182/blood-2009-08-239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–18. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 18.Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 28:2259–66. doi: 10.1200/JCO.2009.26.0638. [DOI] [PubMed] [Google Scholar]
- 19.Adkins DR, DiPersio JF. Total body irradiation before an allogeneic stem cell transplantation: is there a magic dose? Curr Opin Hematol. 2008;15:555–60. doi: 10.1097/MOH.0b013e32831188f5. [DOI] [PubMed] [Google Scholar]
- 20.Zaucha RE, Buckner DC, Barnett T, et al. Modified total body irradiation as a planned second high-dose therapy with stem cell infusion for patients with bone-based malignancies. Int J Radiat Oncol Biol Phys. 2006;64:227–34. doi: 10.1016/j.ijrobp.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–5. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 22.Lahuerta JJ, Martinez-Lopez J, Grande C, et al. Conditioning regimens in autologous stem cell transplantation for multiple myeloma: a comparative study of efficacy and toxicity from the Spanish Registry for Transplantation in Multiple Myeloma. Br J Haematol. 2000;109:138–47. doi: 10.1046/j.1365-2141.2000.01979.x. [DOI] [PubMed] [Google Scholar]
- 23.Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–20. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 24.Garban F, Attal M, Michallet M, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107:3474–80. doi: 10.1182/blood-2005-09-3869. [DOI] [PubMed] [Google Scholar]
- 25.Wong JY, Liu A, Schultheiss T, et al. Targeted total marrow irradiation using three-dimensional image-guided tomographic intensity-modulated radiation therapy: an alternative to standard total body irradiation. Biol Blood Marrow Transplant. 2006;12:306–15. doi: 10.1016/j.bbmt.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Wong JY, Rosenthal J, Liu A, Schultheiss T, Forman S, Somlo G. Image-guided total-marrow irradiation using helical tomotherapy in patients with multiple myeloma and acute leukemia undergoing hematopoietic cell transplantation. Int J Radiat Oncol Biol Phys. 2009;73:273–9. doi: 10.1016/j.ijrobp.2008.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultheiss TE, Wong J, Liu A, Olivera G, Somlo G. Image-guided total marrow and total lymphatic irradiation using helical tomotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1259–67. doi: 10.1016/j.ijrobp.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 28.Sahebi F, Spielberger R, Kogut NM, et al. Maintenance thalidomide following single cycle autologous peripheral blood stem cell transplant in patients with multiple myeloma. Bone Marrow Transplant. 2006;37:825–9. doi: 10.1038/sj.bmt.1705339. [DOI] [PubMed] [Google Scholar]
- 29.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–54. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 30.Somlo G, Sniecinski I, ter Veer A, et al. Recombinant human thrombopoietin in combination with granulocyte colony-stimulating factor enhances mobilization of peripheral blood progenitor cells, increases peripheral blood platelet concentration, and accelerates hematopoietic recovery following high-dose chemotherapy. Blood. 1999;93:2798–806. [PubMed] [Google Scholar]
- 31.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 32.Lahuerta JJ, Martinez-Lopez J, Serna JD, et al. Remission status defined by immunofixation vs. electrophoresis after autologous transplantation has a major impact on the outcome of multiple myeloma patients. Br J Haematol. 2000;109:438–46. doi: 10.1046/j.1365-2141.2000.02012.x. [DOI] [PubMed] [Google Scholar]
- 33.Cavo M, Tosi P, Zamagni E, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434–41. doi: 10.1200/JCO.2006.10.2509. [DOI] [PubMed] [Google Scholar]
- 34.Cavo M, Di Raimondo F, Zamagni E, et al. Short-term thalidomide incorporated into double autologous stem-cell transplantation improves outcomes in comparison with double autotransplantation for multiple myeloma. J Clin Oncol. 2009;27:5001–7. doi: 10.1200/JCO.2009.22.7389. [DOI] [PubMed] [Google Scholar]
- 35.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–17. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 36.Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112:3107–14. doi: 10.1182/blood-2008-04-149427. [DOI] [PubMed] [Google Scholar]
- 37.Ghosh N, Matsui W. Cancer stem cells in multiple myeloma. Cancer Lett. 2009;277:1–7. doi: 10.1016/j.canlet.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clift RA, Buckner CD, Appelbaum FR, Sullivan KM, Storb R, Thomas ED. Long-term follow-Up of a randomized trial of two irradiation regimens for patients receiving allogeneic marrow transplants during first remission of acute myeloid leukemia. Blood. 1998;92:1455–6. [PubMed] [Google Scholar]
- 39.Einsele H, Bamberg M, Budach W, et al. A new conditioning regimen involving total marrow irradiation, busulfan and cyclophosphamide followed by autologous PBSCT in patients with advanced multiple myeloma. Bone Marrow Transplant. 2003;32:593–9. doi: 10.1038/sj.bmt.1704192. [DOI] [PubMed] [Google Scholar]
- 40.Giralt S, Bensinger W, Goodman M, et al. 166Ho-DOTMP plus melphalan followed by peripheral blood stem cell transplantation in patients with multiple myeloma: results of two phase 1/2 trials. Blood. 2003;102:2684–91. doi: 10.1182/blood-2002-10-3250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


