Abstract
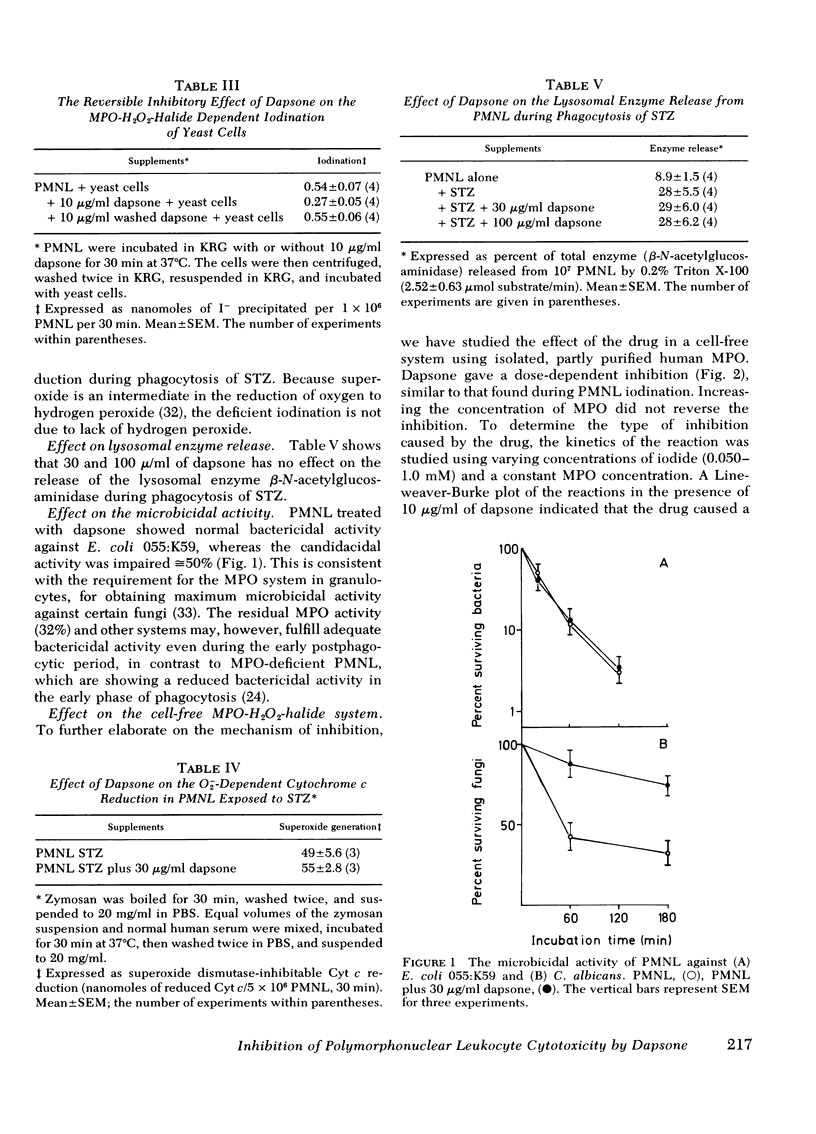
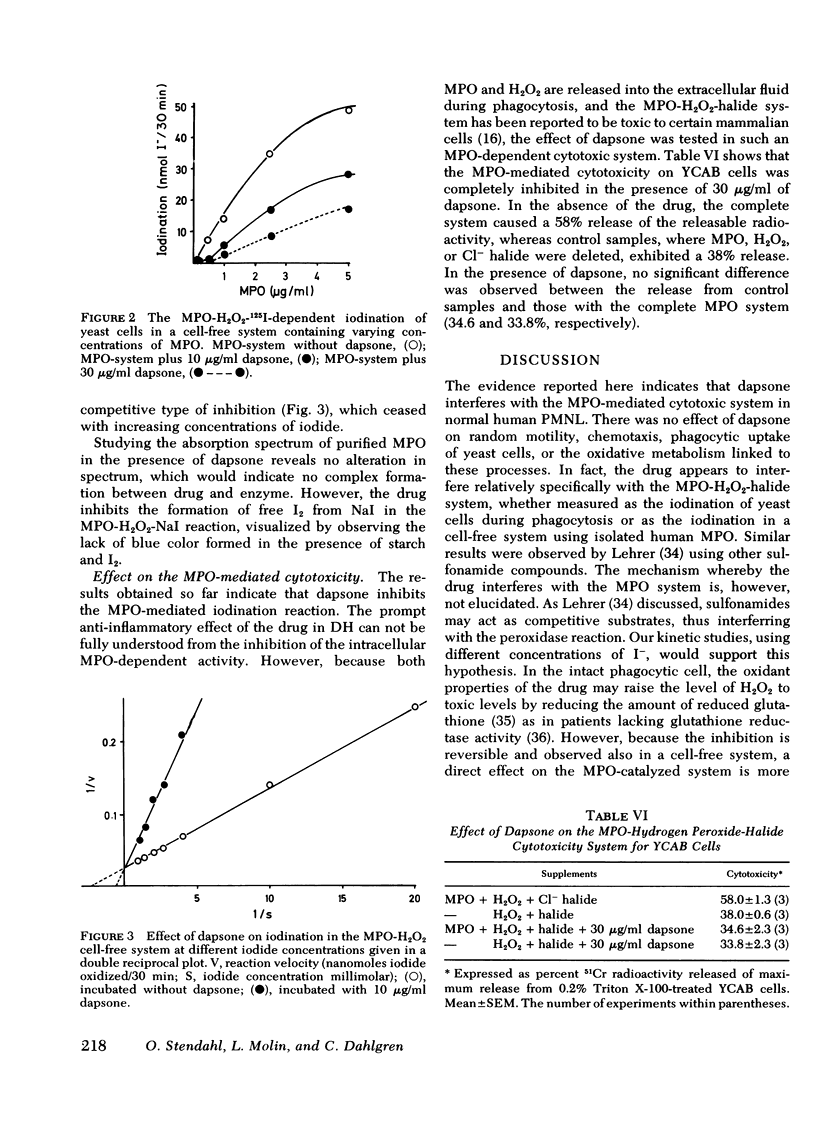
The effect of the sulfone compound 4,4′-diaminodiphenyl sulfone (dapsone) on normal human polymorphonuclear leukocytes (PMNL) has been investigated in vitro. The drug has a dramatically beneficial effect in dermatitis herpetiformis in which the PMNL and immune complexes has been stressed to be of importance for the development of the skin lesions. Pruritus disappears and the inflammatory eruptions clear within a few days of starting therapy. The effect of dapsone has been evaluated on the different stages of phagocytosis. Using dapsone concentrations (1-30 μg/ml) comparable with those found after therapeutic doses, we have found that the drug interferes primarily with the myeloperoxidase (MPO)-H2O2-halide-mediated cytotoxic system in the PMNL. No effect was observed on random locomotion, chemotaxis, phagocytic ingestion, oxidative metabolism, or the release of lysosomal enzymes. Kinetic studies in a cell-free system with purified MPO revealed a competitive type of inhibition using varying concentrations of NaI. Furthermore, the inhibition resulted in reduced candidicidal activity during phagocytosis of Candida albicans, and reduced cytotoxicity to adjacent mammalian cells measured as the 51Cr release from virus-induced lymphoma cells. Because the MPO-H2O2-halide system not only fulfills the antimicrobial activity but is suggested to be a modulator of the inflammatory reaction as well, the action of dapsone in dermatitis herpetiformis may in part be explained by its effect on this system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barranco V. P. Inhibition of lysosomal enzymes by dapsone. Arch Dermatol. 1974 Oct;110(4):563–566. [PubMed] [Google Scholar]
- CHANG Y. T., WOLCOTT R. R., DOULL J. A. Sulfone therapy of leprosy. Med Clin North Am. 1954 Mar;11:599–610. doi: 10.1016/s0025-7125(16)34902-1. [DOI] [PubMed] [Google Scholar]
- CORNBLEET T. Sulfozone (diasone) sodium for dermatitis herpetiformis. AMA Arch Derm Syphilol. 1951 Dec;64(6):684–687. doi: 10.1001/archderm.1951.01570120019003. [DOI] [PubMed] [Google Scholar]
- Cikes M., Friberg S., Jr, Klein G. Progressive loss of H-2 antigens with concomitant increase of cell-surface antigen(s) determined by Moloney leukemia virus in cultured murine lymphomas. J Natl Cancer Inst. 1973 Feb;50(2):347–362. doi: 10.1093/jnci/50.2.347. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J., Einstein A. B., Fefer A. Peroxidase-H2O2-halide system: Cytotoxic effect on mammalian tumor cells. Blood. 1975 Feb;45(2):161–170. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975 Jun 1;141(6):1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Peroxidase-mediated mammalian cell cytotoxicity. J Exp Med. 1973 Jul 1;138(1):318–323. doi: 10.1084/jem.138.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry L., Seah P. P., Riches D. J., Hoffbrand A. V. Clearance of skin lesions in dermatitis herpetiformis after gluten withdrawal. Lancet. 1973 Feb 10;1(7798):288–291. doi: 10.1016/s0140-6736(73)91539-0. [DOI] [PubMed] [Google Scholar]
- Keller H. U., Sorkin E. Studies on chemotaxis. VI. Specific chemotaxis in rabbit polymorphonuclear leucocytes and mononuclear cells. Int Arch Allergy Appl Immunol. 1967;31(6):575–586. [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Inhibition by sulfonamides of the candidacidal activity of human neutrophils. J Clin Invest. 1971 Dec;50(12):2498–2505. doi: 10.1172/JCI106750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren S., Enerbäck L., Freiberg N. Oral acantholytic itching disease responding to dapsone. Dermatitis herpetiformis, pemphigus, or a new disease? Oral Surg Oral Med Oral Pathol. 1976 Nov;42(5):597–605. doi: 10.1016/0030-4220(76)90211-5. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan A. L., Champion R. H. Generalized pustular psoriasis treated with dapsone. Br J Dermatol. 1973 Feb;88(2):183–185. doi: 10.1111/j.1365-2133.1973.tb07523.x. [DOI] [PubMed] [Google Scholar]
- Mier P. D., van den Hurk J. J. Inhibition of lysosomal enzymes by dapsone. Br J Dermatol. 1975 Oct;93(4):471–472. doi: 10.1111/j.1365-2133.1975.tb06523.x. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Molin L., Rajka G. Phagocytic activity of neutrophil leucocytes in pustulosis palmo-plantaris, chronic discoid lupus erythematosus and erysipelas. Acta Derm Venereol. 1971;51(2):138–140. [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Olsson I., Olofsson T., Odeberg H. Myeloperoxidase-mediated iodination in granulocytes. Scand J Haematol. 1972;9(5):483–491. doi: 10.1111/j.1600-0609.1972.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasbridge M. R., Scott G. L. The haemolytic action of dapsone: the effect on red-cell glycolysis. Br J Haematol. 1973 Feb;24(2):169–181. doi: 10.1111/j.1365-2141.1973.tb05737.x. [DOI] [PubMed] [Google Scholar]
- Riches D. J., Martin B. G., Seah P. P., Fry L. The ultrastructural changes in the skin in dermatitis herpetiformis after withdrawal of dapsone. Br J Dermatol. 1976 Jan;94(1):31–37. doi: 10.1111/j.1365-2133.1976.tb04338.x. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SNEDDON I. B., WILKINSON D. S. Subcorneal pustular dermatosis. Br J Dermatol. 1956 Dec;68(12):385–394. doi: 10.1111/j.1365-2133.1956.tb12774.x. [DOI] [PubMed] [Google Scholar]
- Seah P. P., Fry L. Immunoglobulins in the skin in dermatitis herpetiformis and their relevance in diagnosis. Br J Dermatol. 1975 Feb;92(2):157–166. doi: 10.1111/j.1365-2133.1975.tb03052.x. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Lindgren S. Function of granulocytes with deficient myeloperoxidase-mediated iodination in a patient with generalized pustular psoriasis. Scand J Haematol. 1976 Feb;16(2):144–153. doi: 10.1111/j.1600-0609.1976.tb01130.x. [DOI] [PubMed] [Google Scholar]
- Thompson D. M., Souhami R. Suppression of the arthus reaction in the guinea-pig by dapsone. Proc R Soc Med. 1975 May;68(5):273–273. doi: 10.1177/003591577506800501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINKELMANN R. K., DITTO W. B. CUTANEOUS AND VISCERAL SYNDROMES OF NECROTIZING OR "ALLERGIC" ANGIITIS: A STUDY OF 38 CASES. Medicine (Baltimore) 1964 Jan;43:59–89. doi: 10.1097/00005792-196401000-00003. [DOI] [PubMed] [Google Scholar]
- WINKELMANN R. K., ROTH H. L. Dermatitis herpetiformis with acantholysis or pemphigus with response to sulfonamides: report of two cases. Arch Dermatol. 1960 Sep;82:385–390. doi: 10.1001/archderm.1960.01580030079010. [DOI] [PubMed] [Google Scholar]
- Ward M., McManus J. P. Letter: Dapsone in Crohn's disease. Lancet. 1975 May 31;1(7918):1236–1237. doi: 10.1016/s0140-6736(75)92216-3. [DOI] [PubMed] [Google Scholar]
- Williams K., Capstick R. B., Lewis D. A., Best R. Anti-inflammatory actions of dapsone and its related biochemistry. J Pharm Pharmacol. 1976 Jul;28(7):555–558. doi: 10.1111/j.2042-7158.1976.tb02794.x. [DOI] [PubMed] [Google Scholar]


