Abstract
Production of new neurons from stem cells is important for cognitive function, and the reduction of neurogenesis in the aging brain may contribute to the accumulation of age-related cognitive deficits. Restriction of calorie intake and prolonged treatment with rapamycin have been shown to extend the lifespan of animals and delay the onset of age-related decline in tissue and organ function. Using a reporter line in which neural stem and progenitor cells are marked by the expression of GFP, we examined the effect of prolonged exposure to calorie restriction (CR) or rapamycin on hippocampal neural stem and progenitor cell proliferation in aging mice. We show that CR increases the number of dividing cells in the dentate gyrus (DG) of female mice. The majority of these cells corresponded to Nestin-GFP-expressing neural stem or progenitor cells; however, this increased proliferative activity of stem and progenitor cells did not result in a significant increase in the number of doublecortin-positive newborn neurons. Our results suggest that restricted calorie intake may increase the number of divisions that neural stem and progenitor cells undergo in the aging brain of females.
Keywords: adult neurogenesis, stem cells, aging, calorie restriction, rapamycin
Introduction
Adult hippocampal neurogenesis is linked to behavior (e.g., facilitating pattern separation in similar contexts), response to therapy (e.g., mediating response to antidepressants), and neural tissue repair (Zhao et al., 2008; Deng et al., 2010; Aimone et al., 2011; Ming & Song, 2011; Sahay et al., 2011; Samuels & Hen, 2011). Production of new neurons diminishes dramatically with age and this decline in neurogenesis may underlie age-related cognitive impairment (Kuhn et al., 1996; Cameron & McKay, 1999; Leuner et al., 2007). The decrease in production of new neurons is to a large degree driven by the decrease in the number of neural stem cells (Encinas et al., 2011b; Encinas & Sierra, 2012), which may be further exacerbated by their decreased propensity to divide (Hattiangady & Shetty, 2008). This decrease may potentially be attenuated by symmetric divisions of stem cells (Bonaguidi et al., 2011) or through proliferative activity of specific subpopulations of stem cells (Lugert et al., 2010). Radial glia-like hippocampal neural stem cells are largely quiescent in the adulthood. Our results suggest that upon activation they undergo a rapid series of divisions followed by astrocytic differentiation and, while physically still present in the DG, leave the pool of functional stem cells (Encinas et al., 2011b). Given the link between hippocampal neurogenesis and cognitive function, it is plausible that slowing down the loss in neural stem cells or increasing their productivity in getting rise to new neurons may help to ameliorate the age-related cognitive deficits.
Restriction of calorie intake can increase the lifespan of a diverse range of species, including mammals (Anderson & Weindruch, 2010; Houtkooper et al., 2010; Anderson & Weindruch, 2012; Mercken et al., 2012). Lifespan can also be significantly extended by prolonged treatment with the mTOR pathway inhibitor rapamycin, even when treatment is started late in life (Harrison et al., 2009; Houtkooper et al., 2010; Miller et al., 2011). Both CR and rapamycin, usually applied for a limited period of time, have been shown to affect adult neurogenesis (Bondolfi et al., 2004; Stangl & Thuret, 2009; Paliouras et al., 2012). However, it is not clear whether prolonged treatment with CR or rapamycin has similar benefits and whether such benefits are observed in old animals. It is also not clear which specific subclasses of neural stem and progenitor cells and which steps of the neuronal differentiation cascade are targeted by CR or rapamycin. Here we used a reporter transgenic mouse line in which different subclasses of progenitor cells can be distinguished based on their expression of the GFP transgene, morphology, and mitotic behavior (Mignone et al., 2004; Enikolopov & Overstreet-Wadiche, 2008) to investigate the effects of CR and rapamycin on hippocampal stem and progenitor cells in aging animals.
Materials and Methods
Animals
Nestin-GFP reporter mouse line is described in (Mignone et al., 2004). Nestin-GFP homozygous transgenic mice, previously backcrossed to C57BL/6 animals for over 10 generations, were bred and initially housed in the animal facility of Cold Spring Harbor Laboratory in a standard light- and temperature-controlled environment (12-hour light/dark cycle; light on at 7:00 AM; 21oC) with access to food and water ad libitum. Mice were then shipped to the Barshop Institute for Longevity and Aging Studies at UT Health Science Center and were maintained there during the entire treatment period. Prior to six months of age, all mice were maintained with access to standard Purina Mills Test diet ad libitum. Starting at 6 months, mice were switched onto the experimental diet treatments. The mice were randomly divided into three groups: control (n=28, 14 females and 14 males), CR (n= 26; 12 females and 14 males), and rapamycin (n=28; 14 females and 14 males). Control group received standard diet ad libitum. CR was established by feeding mice 80% of ad libitum consumption for one week and then lowering it to 60% for the remainder of the study (Fok, 2012). Mice in the rapamycin group were fed ad libitum the Purina Mills chow containing 14 ppm of encapsulated rapamycin in the diet as described (Harrison et al., 2009; Miller et al., 2011; Fok, 2012). Empty capsules were added to the ad libitum control diet. After 12 months of treatment, half of the mice were randomly selected to be sacrificed for analysis (control n=12: 5 females and 7 males; CR n=12: 5 females and 7 males; rapamycin n=12, 6 females and 6 males). Diet treatment on the remaining mice was continued for an additional 10 months, at which point all remaining mice were sacrificed for analysis.
All mice were monitored daily for the general health and lifespan assessment during the diet period. Their body weight and age-related illness were recorded at least once per month. The symptoms of age-related illness which were monitored included tumors, hair loss, aberrant postures (hunchbacked), cataracts, and other eyes problems such as bulging, discoloration, and conjunctivitis. The mice that were judged to be moribund were euthanized humanely.
Mouse maintenance was following the guidelines for the use and treatment of laboratory animals from the National Institutes of Health and all procedures were approved by Animal Care and Use Committees of Cold Spring Harbor Laboratory and University of Texas Health Science Center.
Double S phase pulse labeling of dividing cells and immunohistochemistry
To label proliferating cells, all mice received single injections of 5-chloro-2-deoxyuridine (CldU) (128 mg/kg, i.p; Sigma) and 5-iodo-2-deoxyuridine (IdU) (173 mg/kg, i.p.; Sigma) 24 hr and 2 hr prior to euthanasia, respectively. After an overdose of anesthetic the mice were transcardially perfused with 30 ml of phosphate buffered saline (PBS) and 30 ml of 4% paraformaldehyde (PFA) in PBS, pH 7.4. The extracted brains were further post-fixed in 4% PFA in PBS overnight at 4°C and stored in PBS with 0.1% sodium azide at 4°C until sectioning. The brains were sagittally sectioned at 50 μm thickness by using a vibratome (Vibratome). The brain sections were sequentially collected and subsets of sections at 100 μm intervals were taken for immunohistochemistry and analysis. Brains of 4 mice (of the total 36) were removed from analysis because of damage or malformations. Approximately 40 brain sections per mouse were processed in total. After rinses with PBS, the sections were denatured in 2N HCl at 37°C for 1 hr for detection of CldU- and/or IdU-incorporating stem and progenitor cells. The denatured sections were neutralized with 0.1 M borate, pH 8.0 twice for 20 min. The sections were rinsed with washing solution (PBS with 0.2% Triton X-100) and incubated for blocking and permeabilization in PBS with 2% Triton X-100 and 5% goat serum (GS) at room temperature (RT) for 2 hr. Particularly for the double labeling of CldU and IdU, the brain sections were pre-incubated with rat anti-CldU (Accurate Chemicals, OBT-0030; 1:500 dilution) for 12 hr at RT and overnight at 4°C prior to exposure to other antibodies, in order to lessen cross-reactivity of CldU antigens with the anti-IdU antibody. After rinses with washing solution, the sections were incubated at 4°C overnight in antibody solution (PBS with 0.2% Triton X-100 and 3% GS) containing primary antibodies: chicken anti-GFP (Aves Labs, GFP-1020; 1:400 dilution), rat anti-CldU (Accurate Chemicals, OBT-0030; 1:500 dilution), mouse anti-IdU (BD Bioscience, 347580; 1:300 dilution), rabbit anti-GFAP (Dako, Z-0034; 1:500 dilution), and guinea pig anti-doublecortin (DCX) (Millipore, AB2253, 1:500 dilution). The primary antibody reaction was extended for 2 hr at RT and the sections were rinsed with washing solution. The sections were then incubated for 2 hr at RT with Alexa Fluor (AF) fluorescent dye-conjugated goat secondary antibodies AF-405, AF-488, AF-568, or AF-633 (Invitrogen; 1:400 dilution). After rinses with washing solution, the sections mounted on gelatin-coated slide glasses were cover-slipped over fluorescence mounting media (Dako) for confocal microscopy.
Confocal microscopy and quantification
Quantitative analysis of cell populations was performed by means of design-based stereology as described (Encinas & Enikolopov, 2008; Park & Enikolopov, 2010; Encinas et al., 2011a; Encinas et al., 2011b). For quantification of proliferating stem and progenitor cells, we performed confocal microscopy by using a spinning disk confocal microscope (PerkinElmer UltraVIEW Vox, PerkinElmer). The images were taken under x4 pixel binning, with 12 bits image depth, and the 40x objective air lens. Optical serial Z stack were sliced at 1.8 μm intervals, directed from the bottom to the top. To diminish the potent crosstalk which frequently occurs from multiple fluorophore labeling, the entire optical Z stack was imaged for each channel sequentially. The laser power was set to 30% for 3-20 ms of exposure time. Stitched composite images were created with 10% overlap. The same scan conditions were applied to the entire set of sections. The confocal images were further analyzed for quantification by using Volocity 6.0.1 (PerkinElmer). Quantification of immunolabeled cells was achieved by several image view options, including extended focus, Z planes, and 3D opacity-elicited rotation. Representative images were collected using a laser scanning microscope LSM 710 (Carl Zeiss). Images were imported into Adobe Photoshop 13.0 (Adobe Systems) and were minimally processed to adjust the brightness, contrast, and background.
Statistical analysis
Data are presented as the mean and S.E.M. We used the Bartlett's test and the Brown-Forsythe test to evaluate the normality of the data distribution in order to determine whether a parametric (ANOVA) or nonparametric (Kruskall-Wallis test) test, followed by post-hoc comparisons using t-tests or Mann-Whitney tests, was appropriate. Statistical analysis was performed using Prism6 software (GraphPad Software, Inc.).
Results
We exposed Nestin-GFP mice to standard diet ad libitum (control group), calorie restricted diet (CR group), or standard diet supplemented with microencapsulated rapamycin (rapamycin group). The treatments started at 6 months and were carried out for 12 or 22 months. All mice in three experimental groups survived by 18 months of age (12 months of treatment). We did not see any reliable difference in the lifespan of those mice that died between 18 and 28 months (i.e., between 12 and 22 months treatment time points). This is compatible with other lifespan studies where 30-40% mice survived at that age (Harrison et al., 2009; Miller et al., 2011) (i.e., the timespan and sample size of our experiment would prevent observing reliable differences in longevity between the treatment groups). However, we noticed that CR-exposed females had lower incidence of age-related signs and illnesses: when most of the animals (31 mice) showed loss of hair, cataracts, tumors, hunchback, dermatitis, or protruding xyphoid, only one of the CR-exposed females showed such signs.
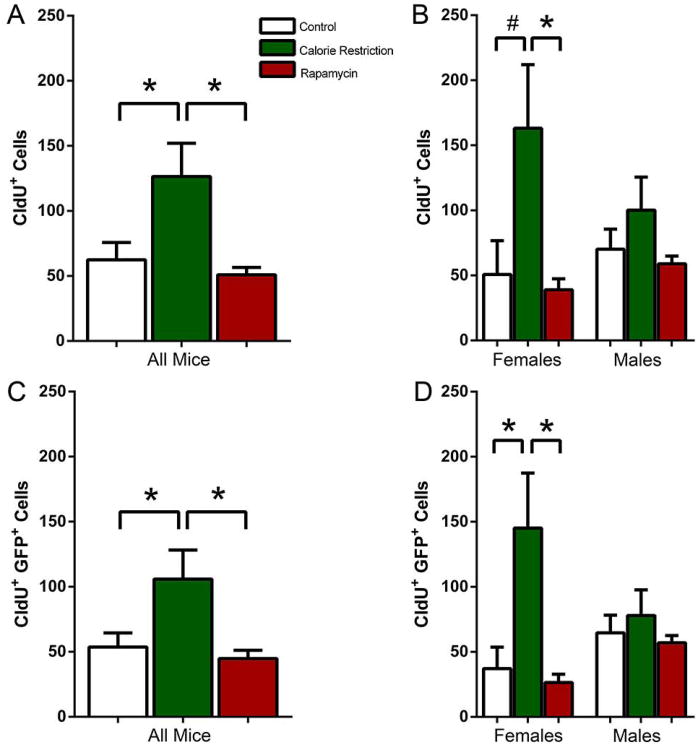
We next examined whether long term (12 months) exposure of adult mice to CR or rapamycin can change the level of hippocampal neurogenesis as judged by the incorporation of nucleotide analogues CldU and IdU. We injected CldU and 24 hr later determined the total number of CldU-positive cells. The number of CldU+ cells in the subgranular zone (SGZ) of the DG was increased in CR animals compared to both control and Rapamycin (KW2,29=6.498, P=0.039; CR 2.0 fold increase over Control MW2-P=0.042; CR 2.5 fold increase over Rapamycin MW2-P=0.025). There was no difference between the control and the rapamycin-fed mice (Fig. 1A). However, when we analyzed the results for males and females separately, we found that CR increased the number of CldU-positive cells in females, but not in males, in relation to the control and rapamycin groups (KW2,10=6.267, P=0.032; CR 3.2 fold increase over Control, MW1-P=0.056; CR 4.2 fold increase over rapamycin, MW2-P=0.016). Rapamycin did not affect the CldU-positive cell count in females or males (Fig. 1B). Note that in this and following series of experiments the low number of labeled cells reflects the advanced age of the analyzed animals, with the number of dividing cells in old rodent brain over hundred times lower than in the young (e.g., 1 month old) animals (Kuhn et al., 1996; Cameron & McKay, 1999; Olariu et al., 2007; Encinas et al., 2011b). Such experiments were also carried out after 22 months of treatment (i.e., at 28 months of age), but there were too few dividing cells to get reliable estimates (not shown).
Figure 1.
(A, B) CldU-labeled cells in all mice (A) and separately in females and males (B) after 12 months exposure to CR or rapamycin. (C, D) CldU-labeled Nestin-GFP-positive cells in all mice (C) and separately in females and males (D) after 12 months exposure to CR or rapamycin. ANOVA and Kruskal Wallis tests indicate significance of the changes in all groups except males. # corresponds to p<0.1 for difference between the control and the CR groups in B. * corresponds to p≤0.05.
Incorporation of nucleotide analogues, such as BrdU, CldU, or IdU, marks all cells that were in S phase of the cell cycle at the time of the nucleotide injection. This includes dividing stem cells, transit amplifying progenitor cells, postmitotic neuroblasts, cells undergoing early stages of apoptosis, oligodendrocyte progenitor cells, microglia, pericytes, and endothelial cells of the blood vessels (however, the vast majority of labeled cells correspond to neural progenitors). Nestin-GFP reporter mouse line, in which expression of GFP is controlled by the regulatory elements of the nestin gene, can be used to distinguish neural stem and progenitor cells from other cell types based on their expression of GFP (Mignone et al., 2004; Enikolopov & Overstreet-Wadiche, 2008). Therefore, we next analyzed the number of GFP-expressing cells which were labeled with CldU (i.e., dividing neural progenitors) in the SGZ. The number of CldU+GFP+ cells was increased CR-exposed mice compared to both the control and the rapamycin-treated groups(KW2,29=6.702, P=0.035; CR 2.0 fold over Control MW1-P=0.029; CR 2.4 fold over rapamycin MW2-P=0.019) (Fig. 1C). Characteristically, the number of CldU+GFP+ cells in all groups was somewhat lower than the total number of CldU+ cells, reflecting the fact that some of the CldU-positive cells do not correspond to neural progenitors. Similar to the total CldU+ cells, the distribution of counts was different when the results in females and males were considered separately. The number of dividing neural progenitors in CR-exposed females was increased 3.9 fold compared to females maintained on the normal diet and 5.6 fold compared to the rapamycin-treated females (KW2,10=7.810, P=0.009; CR 3.9 fold over Control MW2-P=0.032; CR 5.6 fold over rapamycin MW2-P=0.016); there was no difference between the control and the rapamycin-treated females(Fig. 1D). In contrast, there was no statistically significant difference between the counts from all three groups when only the male mice were considered (Fig. 1D). Thus, CR, - but not rapamycin, - increases the number of dividing neural progenitors in female, - but not male, -mice after 12 months of treatment.
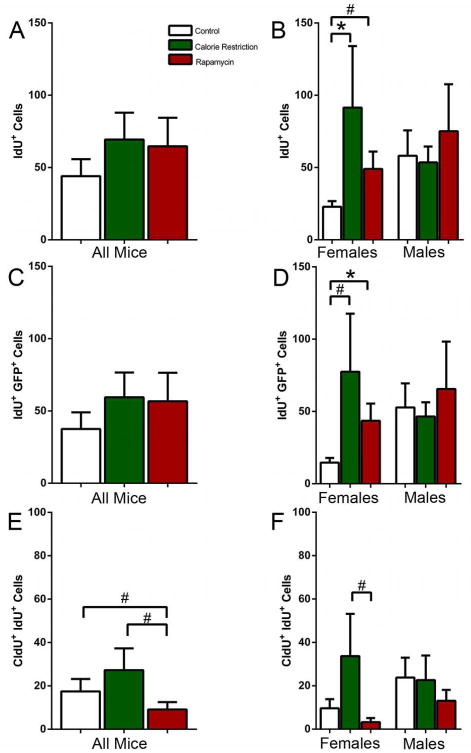
Cell division can be analyzed with higher temporal resolution when a nucleotide label of one type is followed by a label of another type (double S-phase labeling) (Hayes & Nowakowski, 2002; Encinas et al., 2011b). We combined labeling with CldU with subsequent labeling with IdU (22 hr after CldU, 2 hr before the analysis). While the numbers of IdU cells were less than those for CldU (because CldU-labeled cells had a chance to undergo mitoses and thus increase their number), the general trend for IdU labeled cells matched that of CldU cells, with an increase in the number of labeled cells in female, but not male, mice exposed to CR (Female CR 4.0 fold increase over control, MW2-P=0.032)(Fig. 2A, B). Likewise, the trend towards an increase in response to CR in female mice was observed when only the GFP-expressing IdU-labeled progenitors were analyzed (Female CR 5.3 fold increase over control, MW1-P=0.087); again, there was no change in male mice in response to CR or rapamycin(Fig 2C,D).
Figure 2.
(A, B) IdU-labeled cells in all mice (A) and separately in females and males (B) after 12 months exposure to CR or rapamycin. (C, D) IdU-labeled Nestin-GFP-positive cells in all mice (C) and separately in females and males (D) after 12 months exposure to CR or rapamycin. (E, F) Double labeled CldU+IdU+ cells in all mice (E) and in females and males (F) after 12 months exposure to CR or rapamycin. # corresponds to p<0.1, * corresponds to p≤0.05.
Among CldU- and IdU-labeled cells a fraction is labeled with both thymidine analogues. These cells correspond to progenitor cells that have undergone a round of division after the first (CldU) label and have entered the S phase for the second time at the time of the second label (IdU) injection. For the double-labeled CldU+IdU+ cells the trend was again similar, with an increased number of double labeled cells in females exposed to CR (Fig. 2E, F). These results suggest that 20-40% of cells that were in the S phase at the time of CldU injection, undergo another round of DNA synthesis at the time of the IdU injection (note, however, that the total number of labeled progenitor cells in the SGZ is very low at this age and the absolute number of double-labeled cells is lower yet, complicating the precise analysis and comparison of the fraction of the double-labeled cells between experimental groups).
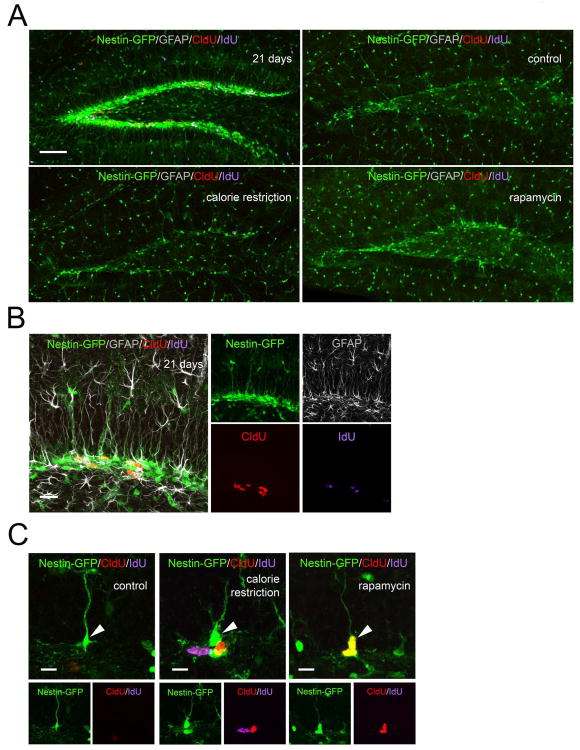
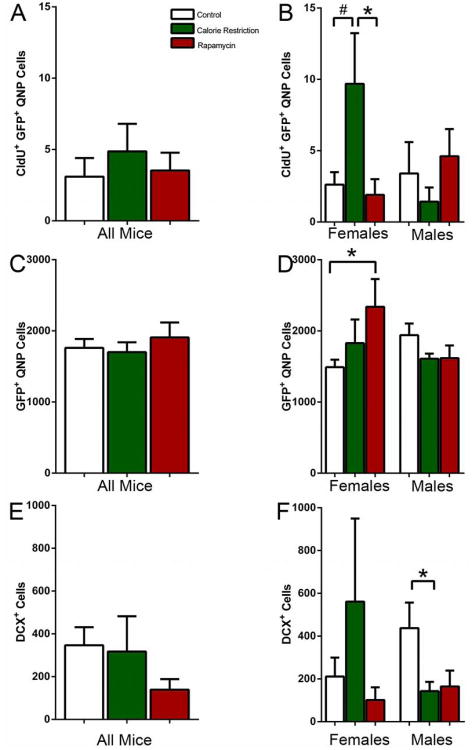
The majority of GFP-labeled cells in the reporter line DG correspond to the amplifying neural progenitors, while a smaller fraction corresponds to radial glia-like stem cells. These cells are largely quiescent under normal conditions, with only a small fraction of these cells labeled by thymidine analogs (Encinas et al., 2006; Encinas et al., 2011b). These quiescent neural progenitors (QNP) can be distinguished from the rest of GFP-expressing cells by their morphology and expression of astrocytic markers, even though their morphology changes in the aging mice: the apical process becomes thicker and more branched with additional ramifications at the top of the process we present the view of the dentate gyrus o and of individual stem cells in the young brain for comparison (Fig. 3A-C). While the overall number of labeled QNPs was, as expected, very low in all groups, their number was higher in CR-exposed females (Females CR 3.7 fold increase over control t-P=0.06; CR 5.1 fold increase over rapamycin t-P=0.05)(Fig. 4A,B). Since such increase may reflect a change in either the total number of QNPs or the proliferating fraction of the QNP population, we determined the total number of QNPs. There was no detectable difference in the number of QNPs between genders or types of treatment except for rapamycin-treated females (however, the fraction of dividing QNPs did not differ from the control group) (Fig. 4C,D). This suggests that prolonged exposure to CR does not change the overall number of hippocampal neural stem cells but the fraction of dividing stem cells increases in females exposed to CR.
Figure 3.
(A) Dividing stem and progenitor cells after 12 months of CR or rapamycin treatment. Upper left panel shows the DG of young (21 days) Nestin-GFP mice for comparison. Note the large number of Nestin-GFP cells and of dividing CldU-positive, IdU-positive, and CldU/IdU-double-positive cells in the young, but not the old, brain. (B, C) QNPs change their morphology and division in the DG of old mice (C) compared to young mice (B; shown here for comparison purposes). Note the large number of Nestin-GFP cells and of dividing CldU-positive, IdU-positive, and CldU/IdU-double-positive cells in the young brain. Brain sections were also probed with anti-GFAP antibody to reveal astrocytes and the GFAP-positive radial processes in QNP cells; note the correspondence between the GFP-positive and GFAP-positive radial processes. In (C) are examples of dividing cells in the vicinity of Nestin-GFP QNPs cells; note the changed morphology of QNPs (arrows) in old mice (thicker processes with increased branching and ramifications). Also note double labeled CldU+/IdU+ stem and progenitor cells. Markers (Nestin-GFP, GFAP, CldU, and IdU) are indicated on the individual panels. Scale bar is 100 μm in (A), 20 μm in (B), and 10 μm in (C).
Figure 4.
(A, B) CldU-labeled Nestin-GFP positive stem cells (QNPs) in all mice (A) and separately in females and males (B) after 12 months exposure to CR or rapamycin. (C, D) Total number of Nestin-GFP-positive QNP cells in all mice (C) and separately in females and males (D) after 12 months exposure to CR or rapamycin. (E, F) DCX-labeled neuroblasts and newborn immature neurons in all mice (A) and separately in females and males (B) after 12 months exposure to CR or rapamycin. # corresponds to p=0.06 in B; * corresponds to p≤0.05.
To examine whether the CR-induced increase in division of neural progenitors may be later translated into an increased number of young neurons, we determined the number of doublecortin (DCX)-positive cells in the DG of the experimental groups. While the trend was similar to the results with dividing progenitors, there was no significant difference in the number of DCX-expressing cells (except for a decrease in the CR-exposed males) (Fig. 4E,F).
Discussion
Our results indicate that prolonged exposure to CR increases both the total number of dividing cells and the number of dividing neural stem and progenitor cells in the DG of adult female mice. Our study also support the notion that age-dependent decrease in progenitor cell division is to a large degree due to the loss of stem cells rather than solely a decrease in their ability to divide and produce progeny (Olariu et al., 2007; Encinas et al., 2011b). Interestingly, while the number of dividing stem cells has drastically increased in response to CR, the overall number of stem cells did not change. This may suggest that a larger fraction of the stem cell pool is recruited in division in the CR-treated female brain; however, with a series of divisions acting as a signal for a stem cell to convert into an astrocyte, this mechanism would imply a rapid exhaustion of the total stem cell pool specifically in the CR group (Encinas et al., 2011b; Encinas & Sierra, 2012). Another possibility is that CR induces symmetric divisions of stem cells; however, if this happens during the entire period of CR treatment, one would expect an increase in the total number of stem cells, which was not the case (unless the resulting increase in stem cells is perfectly balanced by an increased loss of these or other stem cells). A model more compatible with our experimental results is that the fraction of the stem cell pool involved in division is the same but each stem cell undergoes more asymmetric divisions; in this case a larger number of stem cells will appear labeled after a single injection of the label but the total number of stem cells would not change. This mechanism could also explain other situations where an increased number of dividing cells is not matched by the changes in the stem cell pool.
The increase in the number of dividing stem and progenitor cells in CR-treated females was not observed in the population of advanced progenitors, neuroblasts, and immature neurons which express DCX (although compatible with the general trend for an increase). This suggests that the CR-induced increase in progenitor cell proliferation is not fully translated into the generation of mature new neurons. However, it should be noted that maturation of young neurons is controlled independently of progenitor cell proliferation (Plumpe et al., 2006). Further studies, perhaps by using a more targeted immunohistochemical approaches, fate-mapping, or behavioral analysis, will help to clarify this issue.
CR and rapamycin have both been shown to increase the lifespan of a variety of species (Harrison et al., 2009; Anderson & Weindruch, 2010; Houtkooper et al., 2010). In addition, rapamycin is an effector of the mTOR signaling pathway, the same pathway through which CR affects tissue maintenance and remodeling (Anderson & Weindruch, 2010; Houtkooper et al., 2010). Thus, it may be surprising that we did not observe obvious effects of rapamycin either on the basal level of progenitor cell division or on the size of the stem cell pool in female or male mice (except for an increase in the total number of QNP cells in rapamycin-treated females; however, this increase is not translated in the increased number of dividing progenitors or DCX-positive progenitors). —Our results may indicate that CR relies on some additional signaling pathway(s) besides the mTOR pathway to support higher levels of hippocampal neurogenesis and that this pathway(s) is not shared with rapamycin.
Importantly, the CR-induced increase in the number of dividing cells, including dividing neural stem cells, was observed only in female mice. While there is no evident gender difference in the basal level of hippocampal neurogenesis in young adult mice (Lagace et al., 2007), the situation may be less clear in old animals, where changes in hormonal levels may affect neurogenesis in males and females to a different degree. Our results on the gender difference in response to CR may reflect the fact that the gonadal hormones have profound effect of neurogenesis (Tanapat et al., 1999; Galea, 2008) and that the reproductive function and sex hormones levels decrease earlier in females than in males. Since CR reduces the manifestation of physiological age-related changes (Harrison et al., 2009; Anderson & Weindruch, 2010; 2012), it is possible that a slower impairment of the female hormone status acts to support a higher level of hippocampal neurogenesis even in older females. Interestingly, while the number of analyzed animals was not sufficient for statistically significant estimates, females exposed to CR not only had more robust neurogenesis, but showed less signs of age-related pathologies (alopecia, dermatitis, cataracts, or tumors).
The neural stem cell pool may not be the only target of CR: for instance, even a shorter exposure to CR increases the availability and activity of skeletal muscle stem cells in old animals (Cerletti et al., 2012). Notably, the GFP signal in the Nestin-GFP mouse line, while faithfully reporting the stem and progenitor status of cells of the neural lineage, also highlights stem and progenitor cells of a number of other lineages, e.g., in the anterior pituitary, liver, hair follicle, muscle, and bone marrow (Gleiberman et al., 2005; Day et al., 2007; Mignone et al., 2007; Gleiberman et al., 2008; Mendez-Ferrer et al., 2010); this would allow a parallel analysis of the effects of CR and rapamycin on stem and progenitor populations in several tissues of the same animal.
Changes in hippocampal neurogenesis are linked to changes in cognitive function, with increased levels of neurogenesis usually corresponding to improved performance in learning and memory tests and reduced anxiety (Sahay & Hen, 2007; Aimone et al., 2011; Ming & Song, 2011; Sahay et al., 2011). In the future it will be interesting to examine a possible correlation between the number of dividing stem cells and young neurons in individual mice after different periods of CR of and their performance in behavioral tasks, to determine whether life-long or even short-term CR may benefit the cognitive function of the aging animals.
Acknowledgments
This work was supported by grants from the National Institute of Aging (R01AG040209), National Institute of Mental Health (R01MH092928), The Ellison Medical Foundation, New York State Stem Cell Science (NYSTEM), and Russian Ministry of Education and Science to G. Enikolopov and National Institute of Aging grant (5RC2-AG036613) to W. Ward and A. Richardson.
Abbreviations
- CldU
5-chloro-2-deoxyuridine
- CR
calorie restriction
- DCX
doublecortin
- DG
dentate gyrus
- GS
goat serum
- IdU
5-iodo-2-deoxyuridine
- PFA
paraformaldehyde
- PBS
phosphate buffered saline
- QNP
quiescent neural progenitor
- RT
room temperature
- SGZ
subgranular zone
- MW2
two-tailed Mann-Whitney test
References
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010;21:134–141. doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. The caloric restriction paradigm: implications for healthy human aging. Am J Hum Biol. 2012;24:101–106. doi: 10.1002/ajhb.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25:333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Cerletti M, Jang YC, Finley LW, Haigis MC, Wagers AJ. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell. 2012;10:515–519. doi: 10.1016/j.stem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol. 2007;304:246–259. doi: 10.1016/j.ydbio.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Enikolopov G. Identifying and quantitating neural stem and progenitor cells in the adult brain. Methods Cell Biol. 2008;85:243–272. doi: 10.1016/S0091-679X(08)85011-X. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Hamani C, Lozano AM, Enikolopov G. Neurogenic hippocampal targets of deep brain stimulation. J Comp Neurol. 2011a;519:6–20. doi: 10.1002/cne.22503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011b;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Sierra A. Neural stem cell deforestation as the main force driving the age-related decline in adult hippocampal neurogenesis. Behav Brain Res. 2012;227:433–439. doi: 10.1016/j.bbr.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enikolopov G, Overstreet-Wadiche L. Transgenic reporter lines for studying adult neurogenesis. In: Gage F, K G, Song H, editors. Adult Neurogenesis. CSHL Press; 2008. [Google Scholar]
- Fok W, Zhang Y, Salmon AB, Bhattacharya A, Gunda R, Jones D, Ward WF, Richardson A, Perez V. Short-term treatment with rapamycin and dietary restriction have overlapping and distinctive effects in young mice. J Gerontol A Biol Sci. 2012 doi: 10.1093/gerona/gls127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–341. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Gleiberman AS, Encinas JM, Mignone JL, Michurina T, Rosenfeld MG, Enikolopov G. Expression of nestin-green fluorescent protein transgene marks oval cells in the adult liver. Dev Dyn. 2005;234:413–421. doi: 10.1002/dvdy.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleiberman AS, Michurina T, Encinas JM, Roig JL, Krasnov P, Balordi F, Fishell G, Rosenfeld MG, Enikolopov G. Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci U S A. 2008;105:6332–6337. doi: 10.1073/pnas.0801644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes NL, Nowakowski RS. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res Dev Brain Res. 2002;134:77–85. doi: 10.1016/s0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Williams RW, Auwerx J. Metabolic networks of longevity. Cell. 2010;142:9–14. doi: 10.1016/j.cell.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17:175–180. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc Natl Acad Sci U S A. 2007;104:17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Mignone JL, Roig-Lopez JL, Fedtsova N, Schones DE, Manganas LN, Maletic-Savatic M, Keyes WM, Mills AA, Gleiberman A, Zhang MQ, Enikolopov G. Neural potential of a stem cell population in the hair follicle. Cell Cycle. 2007;6:2161–2170. doi: 10.4161/cc.6.17.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olariu A, Cleaver KM, Cameron HA. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J Comp Neurol. 2007;501:659–667. doi: 10.1002/cne.21268. [DOI] [PubMed] [Google Scholar]
- Paliouras GN, Hamilton LK, Aumont A, Joppe SE, Barnabe-Heider F, Fernandes KJ. Mammalian target of rapamycin signaling is a key regulator of the transit-amplifying progenitor pool in the adult and aging forebrain. J Neurosci. 2012;32:15012–15026. doi: 10.1523/JNEUROSCI.2248-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Enikolopov G. Transient elevation of adult hippocampal neurogenesis after dopamine depletion. Exp Neurol. 2010;222:267–276. doi: 10.1016/j.expneurol.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumpe T, Ehninger D, Steiner B, Klempin F, Jessberger S, Brandt M, Romer B, Rodriguez GR, Kronenberg G, Kempermann G. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006;7:77. doi: 10.1186/1471-2202-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BA, Hen R. Neurogenesis and affective disorders. Eur J Neurosci. 2011;33:1152–1159. doi: 10.1111/j.1460-9568.2011.07614.x. [DOI] [PubMed] [Google Scholar]
- Stangl D, Thuret S. Impact of diet on adult hippocampal neurogenesis. Genes Nutr. 2009;4:271–282. doi: 10.1007/s12263-009-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]