Abstract
This fMRI study shows that, compared to healthy subjects, children and adults with bipolar disorder (BD) exhibit impaired memory for emotional faces and abnormal fusiform activation during encoding. Fusiform activation abnormalities in BD were correlated with mania severity and may therefore represent a trait and state BD biomarker.
Keywords: fMRI
1.Introduction
Both adults and children with Bipolar Disorder (BD) display deficits in face memory (McClure et al., 2005; Glahn et al., 2010) and affect recognition (Getz et al., 2003; Rich et al., 2008b). It is important to delineate the neural correlates of these deficits. While data demonstrate neural dysfunction in pediatric BD during emotional-face encoding (Dickstein et al., 2007), it is unknown whether such dysfunction manifests in adult BD.
Using a whole-brain analysis, we examined brain regions engaged by successful and unsuccessful emotional-face encoding in children and adults with BD vs. healthy subjects. We hypothesized that both BD children and adults would demonstrate behavioral deficits during emotional-face encoding (i.e., low d’ scores; Dickstein et al., 2007). We also hypothesized that BD subjects would have neural abnormalities during successful encoding i.e., increased activation in emotional regions, including striatum (Dickstein et al., 2007) and amygdala (Roberson-Nay et al., 2006), and decreased activation in face encoding areas, including frontal regions and hippocampus (Leube et al., 2001).
2.Methods
120 subjects participated: 22 adult BD (mean age 41.3±9.9y), 35 adult healthy volunteers (HV; 36.2±10.4y), 29 child BD (14.5±2.7y), and 34 child HV (14.8±2.3y). Subjects provided informed consent/assent; for recruitment/assessment see (Dickstein et al., 2007; Kim et al., 2012). Based on DSM-IV criteria, BD subjects were “narrow phenotype” (Leibenluft et al., 2003). HV inclusion criteria were: negative history for Axis I disorders in the proband and mood disorders in first-degree relatives; normal physical and neurologic examinations; and no current medication use. For demographic and clinical information, see Supplementary Table 1. Data from 13 child HV and 15 child BD appeared previously (Dickstein et al., 2007).
We employed a subsequent-memory paradigm (Dickstein et al., 2007). fMRI data were acquired while subjects viewed gray-scale faces (happy, angry, fearful, neutral) in an event-related design (encoding phase). In separate blocks, they rated hostility, subjective fear, or nose width of the faces, or viewed them passively. Memory for these faces was tested in a surprise recognition test outside the scanner. The memory test consisted of viewing neutral faces, including previously-viewed actors who had displayed emotions in-scanner, and novel actors. Subjects indicated whether they had seen each face before.
Imaging data were “binned” according to whether the subject correctly or incorrectly recognized previously viewed faces, thereby mapping brain regions engaged during successful encoding. Correct identifications were “hits”; faces that were labeled incorrectly as not seen during the scan were “misses.” A d’ score was calculated to measure performance during the post-scan memory task: d'=Zhits-Zfalse alarms (false alarm was a face not presented during the scan that was labeled incorrectly as previously-seen). Higher d’ indicates better performance and memory discrimination.
For GE 3T scanning parameters, see (Dickstein et al., 2007). Image processing used SPM8 software. Pre-processing included slice-timing and motion correction, normalization to MNI space, voxel resampling to 2×2×2mm, smoothing (8mm Gaussian kernel), and intensity normalization. Subject-level statistical models and contrasts were constructed. A whole-brain repeated-measures ANOVA was then calculated using diagnosis (HV,BD) and age (adult,child) as between-subjects factors and condition (faces remembered accurately: Hit-Fix, faces not remembered: Miss-Fix) as a within-subject factor. Hits and misses were collapsed across all face emotion types to maximize statistical power and increase total trials per condition to approximately 45-50 Hit and Miss trials each (versus ~15 trials each for Hit and Miss per emotion). Clusters surpassing a p<0.001, uncorrected voxel-level threshold with ≥20 voxels were considered significant, and beta values from such clusters were extracted from each subject for post-hoc analyses in SPSS.
3.Results
Groups did not differ on IQ or sex (p's>0.11). There was a trend age difference between HV and BD adults (p=0.07).
3.1.Behavior
GLM analysis comparing the effects of diagnosis, age group, and their interaction on d’ revealed only a main effect of diagnosis (HV>BD, p<0.01).
3.2.Neuroimaging
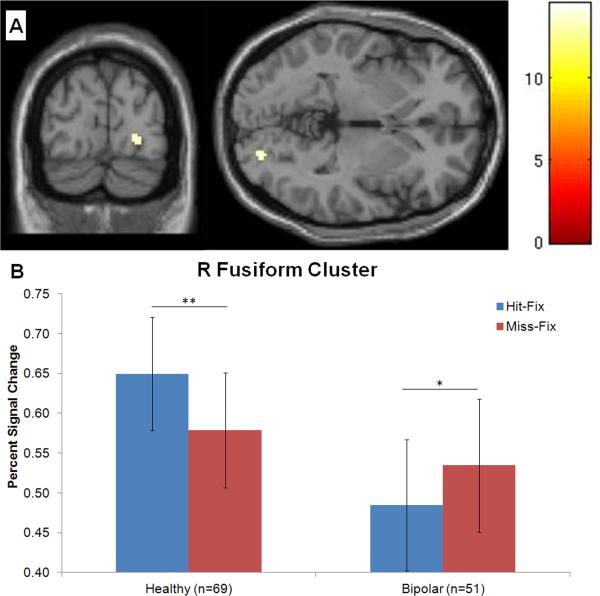
The model revealed a diagnosis-by-condition interaction in right fusiform gyrus (cluster defined by p<0.001 uncorrected, k=25; peak MNI coordinates: 24, -86, -2;Fig.1A), and a diagnosis-by-age-by-condition interaction in left middle frontal gyrus (MFG; p<0.001 uncorrected, k=30; -40, 12, 48).
Fig1.
Condition (successfully-encoded faces, forgotten faces) by diagnosis interaction in right fusiform gyrus (A), due to greater activation for Hit vs. Fixation than Miss vs. Fixation in healthy volunteers (HV), while subjects with bipolar disorder (BD) exhibited the opposite pattern (B). Percent signal averaged across entire cluster (k=25 voxels; cluster peak MNI coordinates: 24, -86, -2). **p<0.01; *p<0.05.
Post-hoc Analyses
Post-hoc analyses to decompose the diagnosis-by-condition interaction in the fusiform cluster indicated greater activation for Hit-Fix than Miss-Fix (p<0.01) among HV subjects, while BD exhibited the opposite pattern (p=0.02) (Fig.1B).
In MFG, HV children showed greater activation to Hit-Fix than Miss-Fix (p=0.01), while BD children showed the opposite pattern (p=0.02). Also, HV adults had a greater response to Miss-Fix than did BD adults (p=0.04). Within the HV group, adults showed greater activation to Miss-Fix than Hit-Fix (p=0.01), a pattern opposite to that seen in HV children; HV adults also showed a greater response than HV children to Miss-Fix (p=0.001) (Supplemental Figure).
Associations with Symptoms and Medications
The difference between Hit-Fix and Miss-Fix fusiform activation correlated negatively with YMRS scores (n=50; r=-0.30; p=0.04). BD subjects currently in hypomanic or mixed state (n=12; no manic subjects in the sample) had lower difference scores than other BD subjects (n=38; p=0.03). However, the diagnosis-by-condition interaction remained significant when only euthymic BD patients (n=29) were included in the model with HV subjects (p=0.04). Depressive symptoms did not correlate with difference activation in either cluster. Fusiform activity did not differ between BD subjects taking vs. not taking lithium (p's>0.21), stimulant (p's>0.21), atypical antipsychotic medication (p's>0.43), or valproate (p's>0.72 ) for Hit-Fix, Miss-Fix, or the difference between these two contrasts.
4.Discussion
In this first study of the neural correlates of successful emotional face encoding in children and adults with BD, patients with BD, regardless of age, exhibited impaired memory and an opposite pattern of fusiform activation than HV. HV showed greater fusiform activation in response to subsequently remembered vs. forgotten faces, while BD showed the opposite pattern. This pattern in BD is both a trait and state marker: while it was present in euthymic patients, mania severity correlated with fusiform activity pattern. Contrary to hypotheses, we did not find between-group differences in the striatum, amygdala, or hippocampus; however, whether this represents type II error or a true null finding cannot be determined.
HV showed encoding-related activity in right fusiform to later-remembered faces, replicating previous studies (Lehmann et al., 2004). In contrast, patients showed increased fusiform activity to later-missed faces. Studies of BD children and adults report structural (Frazier et al., 2005; Lyoo et al., 2006) and functional (e.g., Chen et al., 2006; Pavuluri et al., 2009) abnormalities during face-emotion processing in the fusiform. Fusiform activity is influenced by attention and emotion; attentional influences are mediated by fronto-parietal networks, and emotional influences via amygdala (Vuilleumier and Pourtois, 2007). Possibly, inappropriate modulation by these networks results in abnormal fusiform activity and impaired encoding in BD. Evidence of decreased functional connectivity between amygdala and fusiform gyrus in children with BD vs. HV during face stimulus presentation (Rich et al., 2008a) supports this hypothesis.
Unlike a recent study (Kim et al., 2012), we did not find developmental differences, either neural or behavioral, in patients. While Type II error cannot be excluded, this may indicate that face-memory deficits reflect stable psychopathology and thus, may serve as a BD biomarker. The inverse correlation between fusiform response and mania symptoms also suggests that fusiform dysfunction during emotional-face encoding may be a biomarker for BD.
Supplementary Material
Acknowledgements
This research was supported (in part) by the Intramural Research Program of the NIMH. Dr. Olsavsky's research was made possible through the Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer Inc. (via a grant to the Foundation for NIH from Pfizer Inc.). Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine in major depression; he has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors report no financial relationships with commercial interests. We would like to thank the staff of the Emotion and Development Branch at NIMH and the subjects and families for their participation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen CH, Lennox B, Jacob R, Calder A, Lupson V, Bisbrown-Chippendale R, Suckling J, Bullmore E. Explicit and implicit facial affect recognition in manic and depressed States of bipolar disorder: a functional magnetic resonance imaging study. Biological Psychiatry. 2006;59:31–9. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Rich BA, Roberson-Nay R, Berghorst L, Vinton D, Pine DS, Leibenluft E. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disorders. 2007;9:679–92. doi: 10.1111/j.1399-5618.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Breeze JL, Makris N, Giuliano AS, Herbert MR, Seidman L, Biederman J, Hodge SM, Dieterich ME, Gerstein ED, Kennedy DN, Rauch SL, Cohen BM, Caviness VS. Cortical gray matter differences identified by structural magnetic resonance imaging in pediatric bipolar disorder. Bipolar Disorders. 2005;7:555–69. doi: 10.1111/j.1399-5618.2005.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz GE, Shear PK, Strakowski SM. Facial affect recognition deficits in bipolar disorder. Journal of the International Neuropsychological Society. 2003;9:623–32. doi: 10.1017/S1355617703940021. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Almasy L, Barguil M, Hare E, Peralta JM, Kent JW, Dassori A, Contreras J, Pacheco A, Lanzagorta N, Nicolini H, Raventós H, Escamilla MA. Neurocognitive endophenotypes for bipolar disorder identified in multiplex multigenerational families. Archives of General Psychiatry. 2010;67:168–77. doi: 10.1001/archgenpsychiatry.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Thomas LA, Rosen BH, Moscicki AM, Brotman MA, Zarate CA, Blair RJ, Pine DS, Leibenluft E. Differing Amygdala Responses to Facial Expressions in Children and Adults With Bipolar Disorder. The American Journal of Psychiatry. 2012 doi: 10.1176/appi.ajp.2012.11081245. undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann C, Mueller T, Federspiel A, Hubl D, Schroth G, Huber O, Strik W, Dierks T. Dissociation between overt and unconscious face processing in fusiform face area. NeuroImage. 2004;21:75–83. doi: 10.1016/j.neuroimage.2003.08.038. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. The American Journal of Psychiatry. 2003;160:430–7. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- Leube DT, Erb M, Grodd W, Bartels M, Kircher TT. Differential activation in parahippocampal and prefrontal cortex during word and face encoding tasks. Neuroreport. 2001;12:2773–7. doi: 10.1097/00001756-200108280-00035. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee JY, Kim SJ, Kim N, Dunner DL, Renshaw PF. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disorders. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- McClure EB, Treland JE, Snow J, Dickstein DP, Towbin KE, Charney DS, Pine DS, Leibenluft E. Memory and learning in pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:461–9. doi: 10.1097/01.chi.0000156660.30953.91. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:308–19. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Fromm SJ, Berghorst LH, Dickstein DP, Brotman MA, Pine DS, Leibenluft E. Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2008a;49:88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Grimley ME, Schmajuk M, Blair KS, Blair RJ, Leibenluft E. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Development and Psychopathology. 2008b;20:529–46. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, Charney DS, Leibenluft E, Blair J, Ernst M, Pine DS. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An FMRI study. Biological Psychiatry. 2006;60:966–73. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–94. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.