How obesity promotes its associated comorbidities of insulin resistance, diabetes mellitus, and cardiovascular disease continues to attract and confound legions of basic and clinical researchers. Certain fundamentals of the problem seem to be established, particularly a central role of adipose tissue as a regulator of whole-body insulin sensitivity and metabolism.1,2 This role likely reflects the unique capacity of adipose tissue to store and sequester triglycerides and other fatty molecules away from tissues like skeletal muscle and liver, which can be disrupted by these molecules.3 Dysfunction in lipid handling by adipocytes in conditions such as obesity thus has deleterious consequences in these peripheral organs. However, adipose tissue is composed of several cell types, including stromal fibroblasts, capillary endothelial cells, and immune cells, in addition to adipocytes. Indeed, half of the cells that make up adipose tissue of obese mice were identified as macrophages,4 associated with low-grade systemic inflammation. It follows that understanding adipose tissue physiology in obesity requires considering the entire composition and architecture of the tissue, including the extracellular matrix, microcirculation, and cells of hematopoietic origin that influence the pathophysiology of the disease.
A key role of insulin in mammals is to stimulate the synthesis and storage of triglycerides. Thus, the impairment of insulin signaling (insulin resistance) in obesity is a major contributor to adipose tissue dysfunction. One current hypothesis explaining insulin resistance in adipocytes derives from findings showing a reduction in blood flow and oxygen tension in adipose tissue in obese humans and rodents.5–9 In cultured mouse adipocytes, hypoxia appears to promote the release of fatty acids9 and the expression of proinflammatory genes,10 suggesting that in adipose tissue hypoxia could help further attract immune cells.11 This would in turn increase local concentrations of cytokines such as tumor necrosis factor-α, a known inhibitor of fat storage capacity in adipocytes. This intriguing model, in which hypoxia induces adipocyte insulin resistance, is dealt a troubling blow by the study of Goossens et al12 in this issue of Circulation. Surprisingly, they report that adipose tissue of obese human subjects displays enhanced rather than decreased oxygen tension, despite the confirmation of low blood flow, providing data that directly conflict with conclusions from previous studies.8,13,14
What might account for the discrepant results in which Goossens et al12 observe hyperoxia in adipose tissue of obese humans and previous studies indicate hypoxia? One possible reason is the relatively small size of the study populations in all reports so far and the relatively large variance observed in these reports. The significant differences in age, ethnicity, sex, and possibly antihypertensive medication taken by the subjects might also underlie differences in results among laboratories. Insulin sensitivity, inferred indirectly from fasting insulin levels, may also be a source of variability. In the Goossens et al study, obese subjects may be less insulin resistant relative to control subjects than in previous studies.
Perhaps more salient are the differences in methods used to measure oxygen tension in adipose tissue of obese subjects. In studies by Kabon et al14 and Pasarica et al,8,13 oxygen is measured with a small Clarke electrode placed within the adipose tissue so that values are recorded at several intervals over a 30-minute period. In the present work by Goossens et al,12 oxygen is measured quasicontinuously in the perfusate of a microdialysis probe. The latter method samples a greater area within the tissue. However, the effects of the perfused fluid, which is supplemented with 50 mmol/L ethanol, could complicate the measurements. In addition, it remains possible that oxygen tension values may display large variations among heterogeneous regions of adipose tissue. Goossens et al correctly point out that such hypothetically localized hypoxic areas within adipose tissue cannot be ruled out by their studies. At this stage, we are left with another puzzling discrepancy between findings in this field that will have to be clarified by multiple laboratory groups in future studies. Furthermore, even if the hyperoxia that Goossens et al report is confirmed in abdominal subcutaneous adipose tissue, it is possible that differences among various adipose depots will be encountered, as suggested by studies in rats.5
Interestingly, despite the inconsistent results related to oxygen tension, the various studies agree in their findings of lower vascular endothelial growth factor (VEGF) levels in adipose tissue from obese individuals compared with control subjects. The production of VEGF, a major stimulator of angiogenesis, is generally increased in response to hypoxia. Increased angiogenesis in response to VEGF in turn results in improved oxygen supply to hypoxic tissue, thus returning to homeostasis. Thus, if the level of VEGF is taken to be an indicator of oxygen sufficiency, the decreased VEGF found by both Pasarica et al8 and Goossens et al12 is consistent with an absence of hypoxia in obese adipose tissue. The absence of hypoxia and its associated proangiogenic milieu are also consistent with the decreased capillary density observed in both studies, as well as the diminished angiogenic potential of adipose tissue from morbidly obese individuals recently reported by our group.15
An immediate paradox is raised by the above considerations: How can a deficit in blood flow into the tissue be associated with a corresponding increase in oxygen within the tissue? Goossens et al address this problem through measurements of oxygen consumption in adipose tissue, revealing a large decrease in the obese subjects. Consistent with other studies,16–18 they also find that expression of mitochondrial markers is lower in adipose tissue from obese individuals. They hypothesize that a decrease in mitochondrial density may underlie the low oxygen consumption, leading to increased oxygen pressure and a consequently impaired angiogenic response. The much larger adipocyte size in obesity itself decreases the density of mitochondria within the cells, but other factors that suppress mitochondrial biogenesis may also be involved. Thus, as illustrated in the Figure, a deficiency in adipocyte mitochondria or in the architecture of the adipose tissue, leading to impaired oxygen delivery to mitochondria, could be the primary cause of the observed paucity of capillaries in adipose tissue of obese subjects.
Figure.
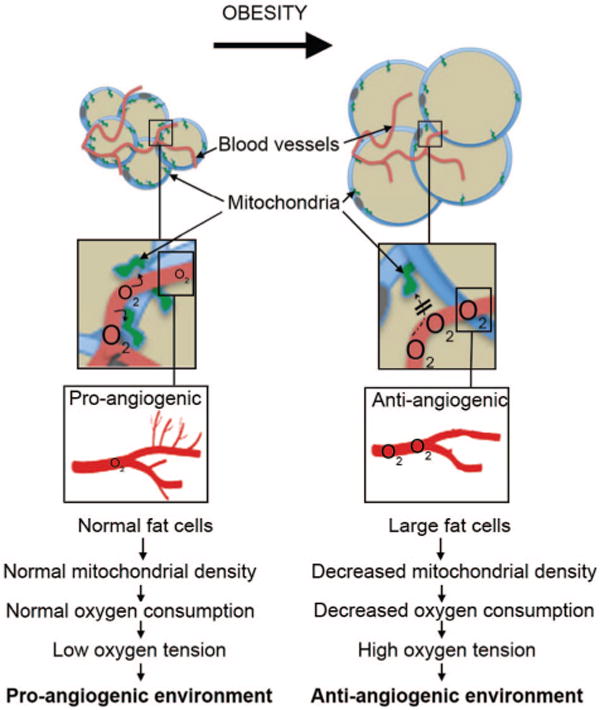
A deficiency in adipocyte mitochondria could be the primary cause of the observed paucity of capillaries in adipose tissue of obese subjects.
A key question raised by these findings is how elevated oxygen tension, decreased oxygen consumption, and impaired angiogenic homeostatic mechanisms relate to insulin resistance, the cardinal feature of obesity. Previous models, in which inflammation driven by hypoxia putatively causes insulin resistance, are countered by the hyperoxia that Goossens et al report. Furthermore, despite the claim by Goossens et al that adipose tissue of the obese subjects in their study displays increased expression of macrophage markers, none other than monocyte chemoattractant protein-1 rises to the level of statistical significance between the groups. Despite clear insulin resistance in the obese group, there were no significant differences between lean and obese subjects in the levels of the cytokines interleukin-6, interleukin-8, or tumor necrosis factor-α, consistent with a growing sense that the major role of macrophages in expanding adipose may not be in an inflammatory context but rather to mitigate lipolysis and remove adipocyte debris.19,20 In keeping with this concept, evidence of adipose inflammation is not compelling in this study. From this standpoint, free fatty acids coming from adipocytes are reported to be the important attractant for macrophages. A causative role for increased lipolysis in the recruitment of macrophages into adipose tissue is indeed supported by the data of Yin et al,9 showing high lipolytic activity in adipose tissue of ob/ob mice. Although inflammatory cytokines are known to impair adipocyte insulin signaling mechanisms and the inflammatory pathway as a cause of insulin resistance remains a major paradigm in the field, this explanation will likely be scrutinized more critically over the next few years.
If not macrophage-induced inflammation, what causes insulin resistance in adipose tissue? Hyperoxia itself may have deleterious effects on adipose cells through unknown mechanisms. This area of adipocyte biology is poorly understood, but it is possible that the hyperoxia described by Goossens et al disrupts 1 or more factors necessary for the adipose tissue to appropriately expand in response to chronic overnutrition. Growth of all tissues requires concomitant expansion of their capillary network, as is evident during development and in tumor growth. Adipose tissue is relatively unique in its ability to expand by many-fold in adults in a nononcogenic manner, both by hypertrophy of existing adipocytes and by hyperplasia. The angiogenic potential of adipose tissue increases with the initiation of adipose tissue growth,21,22 but seems to be impaired in obesity.15 Normally, localized regions of hypoxia coordinate tissue growth as the proliferating cells become distanced from the existing vasculature. Hypoxia-regulated factors include not only VEGF, but also many other proangiogenic and antiangiogenic factors necessary for the formation of a functional microvasculature. Adipocytes are powerful sources of many proangiogenic and antiangiogenic factors that can work in concert to generate functional capillary network expansion. Examples of such factors are monobutyrin23 and angiopoietin-like protein 4,24 as well as pigment epithelium-derived factor,25,26 which directly impairs insulin action in adipocytes at the level of Akt phosphorylation. Perhaps the expression of such factors is dysregulated by the hyperoxia described in the present study, leading to attenuation of insulin signaling pathways.
Another factor contributing to the insulin resistance of adipose tissue in obesity could be diminished blood flow through the tissue, as Goossens et al and others have reported. It has been claimed that insulin action on glucose transport into skeletal muscle is restricted by blood flow and that the stimulatory effect of hormone on this parameter contributes to the increased glucose uptake in this tissue.27 Could this also be the case in adipose tissue? Certainly, adipocytes isolated in vitro respond directly to insulin because incubation with insulin results in large increases in glucose transport, which is blunted when adipocytes are obtained from obese animals or humans. Furthermore, GLUT4 expression is decreased in adipocytes in obesity, which contributes to the blunted response. However, it is possible that the decreased blood flow in obesity could become rate limiting for optimal glucose clearance in vivo. Remarkably, Goossens et al and another study28 show that obesity also almost completely abolishes the robust stimulatory effect of glucose ingestion on adipose blood flow seen in lean subjects. Thus, in obesity, the lower basal blood flow through adipose tissue, combined with defective hormonal stimulation of flow, could result in an apparent adipocyte insulin resistance when in fact it is the supply of glucose to the adipocytes that is defective. This idea requires further critical evaluation.
In the end, it is not the specific site of inhibition of insulin signaling that is most critical to our interest in the role of adipose tissue on whole-body metabolism and the cause of type 2 diabetes mellitus. Rather, it is this question: How do adipose tissue parameters of blood flow and oxygen tension measured by Goossens et al and others affect lipid storage and sequestration by adipocytes? Insulin signaling certainly plays an important role in lipogenesis and triglyceride storage in adipocytes; the induction of very severe insulin resistance by selective depletion of insulin receptors in adipose tissue from early in mouse development decreases fat mass by about half.29 But, there still is significant triglyceride stored in adipose tissue in these animals, suggesting that other mechanisms regulating lipid metabolism may also be at play. Such other mechanisms are probably important, because in obesity the extent of blunting of insulin action on glucose transport is usually less severe than observed in the receptor knockout mice. The challenge now, in addition to critically testing that general hyperoxia characterizes adipose tissue depots in obese humans, is to define the key ways in which lipid handling by adipose tissue is compromised in obesity.
Acknowledgments
We thank members of our laboratories for many stimulating discussions on this topic.
Sources of Funding
Funding for work by our laboratories cited in this article was provided by grants from the National Institutes of Health (DK080366 to Dr Corvera and DK30898 and DK030638 to Dr Czech).
Footnotes
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
Disclosures
None.
References
- 1.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 4.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West DB, Prinz WA, Francendese AA, Greenwood MR. Adipocyte blood flow is decreased in obese Zucker rats. Am J Physiol. 1987;253:R228–R233. doi: 10.1152/ajpregu.1987.253.2.R228. [DOI] [PubMed] [Google Scholar]
- 6.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 7.Andersson J, Sjostrom LG, Karlsson M, Wiklund U, Hultin M, Karpe F, Olsson T. Dysregulation of subcutaneous adipose tissue blood flow in overweight postmenopausal women. Menopause. 2010;17:365–371. doi: 10.1097/gme.0b013e3181c12b26. [DOI] [PubMed] [Google Scholar]
- 8.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin J, Gao Z, He Q, Zhou D, Guo Z, Ye J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab. 2009;296:E333–E342. doi: 10.1152/ajpendo.90760.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Rourke RW, White AE, Metcalf MD, Olivas AS, Mitra P, Larison WG, Cheang EC, Varlamov O, Corless CL, Roberts CT, Jr, Marks DL. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia. 2011;54:1480–1490. doi: 10.1007/s00125-011-2103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez de Heredia F, Wood IS, Trayhurn P. Hypoxia stimulates lactate release and modulates monocarboxylate transporter (MCT1, MCT2, and MCT4) expression in human adipocytes. Pflugers Arch. 2010;459:509–518. doi: 10.1007/s00424-009-0750-3. [DOI] [PubMed] [Google Scholar]
- 12.Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JWE, Čajlaković M, Ribitsch V, Clément K, Blaak EE. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 13.Pasarica M, Rood J, Ravussin E, Schwarz JM, Smith SR, Redman LM. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J Clin Endocrinol Metab. 2010;95:4052–4055. doi: 10.1210/jc.2009-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabon B, Nagele A, Reddy D, Eagon C, Fleshman JW, Sessler DI, Kurz A. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100:274–280. doi: 10.1097/00000542-200402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech MP, Thompson M, Perugini RA, Corvera S. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mustelin L, Pietilainen KH, Rissanen A, Sovijarvi AR, Piirila P, Naukkarinen J, Peltonen L, Kaprio J, Yki-Jarvinen H. Acquired obesity and poor physical fitness impair expression of genes of mitochondrial oxidative phosphorylation in monozygotic twins discordant for obesity. Am J Physiol Endocrinol Metab. 2008;295:E148–E154. doi: 10.1152/ajpendo.00580.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rong JX, Qiu Y, Hansen MK, Zhu L, Zhang V, Xie M, Okamoto Y, Mattie MD, Higashiyama H, Asano S, Strum JC, Ryan TE. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes. 2007;56:1751–1760. doi: 10.2337/db06-1135. [DOI] [PubMed] [Google Scholar]
- 18.Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prieur X, Mok CY, Velagapudi VR, Nunez V, Fuentes L, Montaner D, Ishikawa K, Camacho A, Barbarroja N, O’Rahilly S, Sethi JK, Dopazo J, Oresic M, Ricote M, Vidal-Puig A. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60:797–809. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 22.Christiaens V, Lijnen HR. Angiogenesis and development of adipose tissue. Mol Cell Endocrinol. 2010;318(1–2):2–9. doi: 10.1016/j.mce.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Wilkison WO, Choy L, Spiegelman BM. Biosynthetic regulation of monobutyrin, an adipocyte-secreted lipid with angiogenic activity. J Biol Chem. 1991;266:16886–16891. [PubMed] [Google Scholar]
- 24.Gealekman O, Burkart A, Chouinard M, Nicoloro SM, Straubhaar J, Corvera S. Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab. 2008;295:E1056–E1064. doi: 10.1152/ajpendo.90345.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crowe S, Wu LE, Economou C, Turpin SM, Matzaris M, Hoehn KL, Hevener AL, James DE, Duh EJ, Watt MJ. Pigment epithelium-derived factor contributes to insulin resistance in obesity. Cell Metab. 2009;10:40–47. doi: 10.1016/j.cmet.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Famulla S, Lamers D, Hartwig S, Passlack W, Horrighs A, Cramer A, Lehr S, Sell H, Eckel J. Pigment epithelium-derived factor is one of the most abundant proteins secreted by human adipocytes and induces insulin resistance and inflammatory signaling in muscle and fat cells ]published online ahead of print October 12, 2010] Int J Obes (Lond) doi: 10.1038/ijo.2010.212. [DOI] [PubMed] [Google Scholar]
- 27.St-Pierre P, Genders AJ, Keske MA, Richards SM, Rattigan S. Loss of insulin-mediated microvascular perfusion in skeletal muscle is associated with the development of insulin resistance. Diabetes Obes Metab. 2010;12:798–805. doi: 10.1111/j.1463-1326.2010.01235.x. [DOI] [PubMed] [Google Scholar]
- 28.Blaak EE, van Baak MA, Kemerink GJ, Pakbiers MT, Heidendal GA, Saris WH. Beta-adrenergic stimulation and abdominal subcutaneous fat blood flow in lean, obese, and reduced-obese subjects. Metabolism. 1995;44:183–187. doi: 10.1016/0026-0495(95)90262-7. [DOI] [PubMed] [Google Scholar]
- 29.Bluher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]


