Abstract
Study Design
Investigation of injectable nucleus pulposus (NP) implant.
Objective
To assess the ability of a recently developed injectable hydrogel implant to restore non-degenerative disc mechanics through support of NP functional mechanics.
Summary of Background Data
While surgical intervention for low back pain is effective for some patients, treated discs undergo altered biomechanics and adjacent levels are at increased risk for accelerated degeneration. One potential treatment as an alternative to surgery for degenerated disc includes the percutaneous delivery of agents to support NP functional mechanics. The implants are delivered in a minimally invasive fashion, potentially on an outpatient basis, and do not preclude later surgical options. One of the challenges in designing such implants include the need to match key NP mechanical behavior and mimic the role of native non-degenerate NP in spinal motion.
Methods
The oxidized hyaluronic acid gelatin implant material was prepared. In vitro mechanical testing was performed in mature ovine bone-disc-bone units in three stages: intact, discectomy, and implantation vs. sham. Tested samples were cut axially for qualitative structural observations.
Results
Discectomy increased axial range of motion (ROM) significantly compared to intact. Hydrogel implantation reduced ROM 17% (p < 0.05) compared to discectomy and returned ROM to intact levels (ROM intact 0.71 mm, discectomy 0.87 mm, post-implantation 0.72 mm). While ROM for the hydrogel implant group was statistically unchanged compared to the intact disc, ROM for sham discs, which received a discectomy and no implant, was significant increase compared to intact. The compression and tension stiffness were decreased with discectomy and remained unchanged for both implant and sham groups, as expected because the annulus fibrosus was not repaired. Gross morphology images confirmed no ejection of NP implant.
Conclusion
An injectable implant that mimics non-degenerate NP has the potential to return motion segment ROM to normal subsequent to injury.
Keywords: Lumbar Spine, Hydrogel, Compression, Nucleus Pulposus, Implant, Mechanics, Sheep
Introduction
The nucleus pulposus (NP) of the intervertebral disc is a hydrated material with a high proteoglycan content that generates a large osmotic pressure. The NP osmotic pressure functions to provide load support in compression and places the surrounding annulus fibrosus (AF) into circumferential tension.1 In the setting of disc degeneration, the NP proteoglycan content decreases, reducing pressure and altering the overall disc load support, resulting in increased range of motion (ROM) in axial loading.2,3,4 Similar to degeneration, in laboratory studies, when NP is removed to simulate degeneration or discectomy surgery, the ROM increases.5,6,7 Restoration of ROM, perhaps via reestablishment of NP swelling pressure, is a key parameter to treatment strategies for disc disease.4,5
There is a clinical need for an injectable NP implant to restore disc mechanical function. While injectable NP implants are under investigation as motion-preserving alternatives to spine fusion, an FDA approved injectable NP implant has yet to be offered. The advantages of injectable implants include minimally invasive delivery, potentially on an outpatient basis, and injectable implants do not preclude later surgical options. Replacement of native NP with an implant, in the setting of healthy AF, may simultaneously reduce pain, slow progression of degeneration and restore non-degenerate spinal mobility.8,9,10,11,12 Significant challenges to implant design include the need to match key NP mechanical behavior ex vivo and mimic the role of native non-degenerate NP in spinal motion.8,11,13,14 Long term development and testing of nucleoplasty devices should include five assessments: 1) mechanical testing, 2) cell-materials interaction assimilation leading to material integration, 3) kinematic testing including ROM and resistance to expulsion, 4) biocompatibility and biodurability, and 5) safety testing.15 Having previously completed mechanical testing16,17 and cell-materials interaction assimilation leading to material integration18, this study will focus on step 3, ROM testing. In this study we specifically investigate the ability of a recently developed NP implant, consisting of oxidized hyaluronic acid (oHA) and gelatin material,16 to restore disc mechanical function characteristics that are most related to the NP.
The ROM is the mechanical behavior most related to the NP function in axial range of motion.3,4,5,17 In order to demonstrate restoration to normal ROM, a model system with a healthy disc is needed, precluding the use of human cadaver samples that are typically older and quite degenerated. The sheep disc is a suitable model of the human disc with respect to geometry and axial mechanics20,21,22,23 and has previously been utilized for in vitro22,23,24,25,26 and in vivo24,27,28,29,30,31,32 studies of discectomy. One advantage of the sheep is that is a suitable model for future in vivo evaluation of the NP implant if in vitro results warrant additional studies.
The objectives of this study were to 1) evaluate the ovine disc as an in vitro model of discectomy for NP implant testing, and 2) to determine if an injectable hydrogel could functionally replace the NP by re-establishing native mechanics, primarily ROM. In the current study, ROM was specifically evaluated as a first step in order to characterize the material in restoring the disc mechanics. It is hypothesized that discectomy will result in increased ROM and hypermobility and that injection of an oHA-gelatin hydrogel NP implant will return ROM to normal levels.
Materials and Methods
Study design
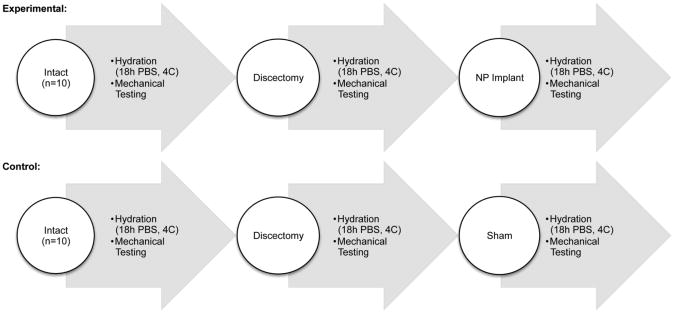
The experimental group consisted of three conditions: intact, discectomy, NP implant (n=10, Figure 1). Axial mechanical properties were measured in each of these conditions for all discs. The control group consisted of the same three conditions and underwent the same protocol in its entirety, but without the addition of an NP implant for the third condition (n=10, Figure 1). Gross morphological evaluation was performed at the end of the test for both the experimental group (NP treated discs) and control group (discectomy but no NP implant), and separate intact discs were also included for comparison of morphological structure.
Figure 1.
Experimental Design. Mechanical tests were performed in three conditions for each disc: intact, post-discectomy, and following implant treatment (n=10). Another set of samples underwent the same procedure, but without implant after discectomy (n=10).
Specimen preparation
Lumbar spines were harvested from twenty skeletally mature sheep previously obtained for a non-spine animal study. All musculature and soft tissue were dissected and facets and transverse processes removed to eliminate any facet and tranverse process contributions on disc mechanics. Bone-disc-bone units, were prepared by making parallel cuts through the vertebral bodies above and below the disc at lumbar spine level L4-L5. Samples were potted in polymethyl-methacrylate bone cement. Kirschner wires were placed through the bone cement and vertebral body to increase pull out strength. The potted samples were wrapped in saline soaked gauze throughout preparation to prevent dehydration. Prior to each mechanical testing step, the sample was thawed and hydrated for 18 hours in a 4°C refrigerated PBS bath to establish a uniform initial hydration condition.33
Mechanical testing
Mechanical tests were performed in PBS bath on an Instron 8874 servohydrolic test frame (Instron, Canton, MA). The displacement resolution of the Instron 8874 is 0.004 mm. Mechanical testing protocol consisted of 20 sinusoidal cycles from -300 N compression to +300 N tension at 1 Hz, as previously described.20 The maximum cyclic compressive load of -300 N was selected to represent approximately 1.5 times human body weight scaled for differences in cross sectional area of the human and ovine intervertebral discs.20,21,22,23 Therefore, this load represents a moderately high stress within the physiological range of the human. This test protocol was repeated three times per sample (intact, discectomy, NP implant/sham).
The 20th test cycle was selected for data analysis, with the first 19 cycles of loading serving as preconditioning to establish a repeatable hydration level and force-displacement hysteresis response.5,34 Axial range of motion (ROM) was directly measured as the total peak-to-peak displacement. The compression stiffness was calculated from a linear regression of load-displacement between -200 and -300 N and the tension stiffness similarly calculated between +200 and +300 N.
Discectomy
A standard discectomy approach was performed. The sample was thawed then the right posterior AF was incised with an 11-blade (2.5 mm incision). Micro-rongeur was inserted into the nuclear cavity and loose NP material was removed. Microcurette was used to release additional nuclear material and micropituitary rongeur was used to resect remaining loose NP. The amount of nuclear material removed (0.024 ± 0.003 g, approximately 35% of the total NP mass) was determined by immediately weighing the sample. The sample was then rehydrated, mechanical testing performed as above, and refrozen.
Implant/sham
Following discectomy and mechanical testing, the sample was thawed and the experimental group was injected with a hydrogel implant previously designed and tested for this purpose,17 while the control group underwent the same procedures without an NP implant. The implant consisted of an oxidized hyaluronic acid (oHA) and gelatin material. One gram of hyaluronan (Mw 1.5×106 Engelhard, Inc.) was dissolved in 80 ml of water in a shaded flask and sodium periodate, dissolved in 20 ml water (pH 5.4), was added dropwise. After incubating at ambient temperature, 10 ml of ethylene glycol was added to terminate the reaction followed by stirring for one hour. The product was dialyzed for 3 days and lyophilized to obtain oxidized hyaluronic acid oHA (yield: 50-67%). Gelatin (Bloom 300, Type A, Mw 100,000) solution of 20% (w/v) concentration (in pH 9.4, 0.1 M borax) was prepared. The oHA and gelatin solutions were mixed in the weight ratio of 7:3 stirred for 1 min at 37°C.
For the experimental group, the implant was injected directly into the NP cavity through the annular opening created during discectomy with a blunt 21-gauge needle. The NP cavity was filled with the liquid formulation of the implant (0.26 ± 0.09 ml) by syringe. For the control group, the 21-gauge needle was inserted but no material was injected. For all discs, the AF was not repaired or filled with material. Samples were maintained at 37°C, body temperature, for one hour to permit gel formation and then frozen until rehydration and mechanical testing, as described above.
Statistical analysis
The data are presented as mean values ± standard deviation. A single factor repeated measures ANOVA (levels: intact, discectomy, implant/sham) with post-hoc Bonferoni test was applied separately to the experimental and control groups. Dependent variables were ROM, compression stiffness, and tension stiffness. To confirm that the two groups were only different with respect to the final treatment condition, a two-way ANOVA with post-hoc Bonferoni test was performed between the experimental and control groups. Significance was set at p < 0.05.
Results
Discectomy
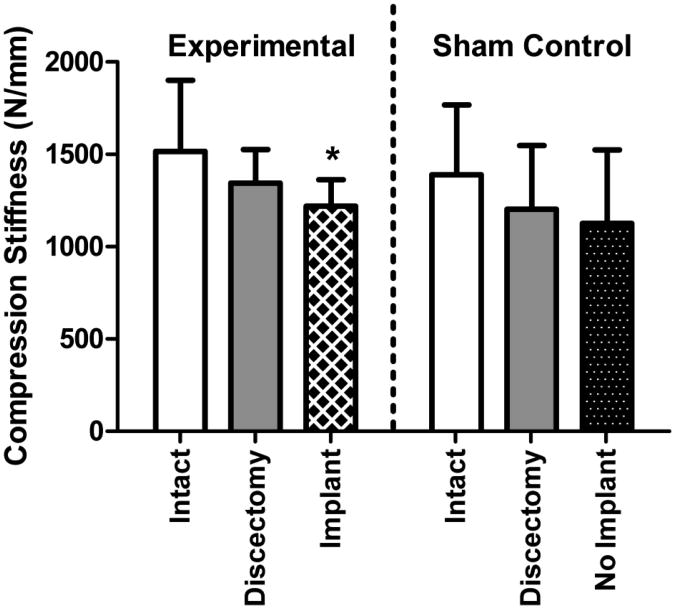
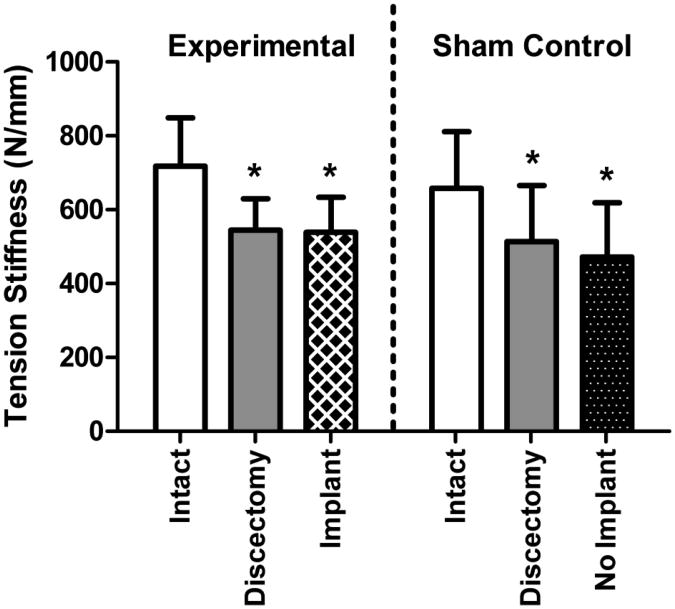
Discectomy significantly altered mechanical function compared to intact samples. The ROM significantly increased for both the experimental and control groups (Figure 2); for the experimental group the ROM increased 18% from 0.71 mm intact to 0.87 mm following discectomy. The ROM of sham and experimental groups were not statistically different (p > 0.05). The compression stiffness was not significantly altered following discectomy (Figure 3) and for the experimental group was 1517 N/mm intact and 1344 N/mm following discectomy. The tension stiffness significantly decreased for both the experimental and control groups (Figure 4); for the experimental group the tension stiffness decreased 24% from 718 N/mm intact to 545 N/mm following discectomy. The intact and discectomy conditions were not significantly different between the experimental and control groups for ROM, compression stiffness, or tension stiffness (p > 0.05).
Figure 2.
Range of Motion (ROM). Discectomy alone significantly increased ROM for both groups. ROM following NP implant treatment significantly decreased, back to its intact state. * p<0.05.
Figure 3.
Compression Stiffness. Compressive stiffness was not significantly altered following discectomy for both groups. Compression stiffness following NP implant treatment was significantly lower than intact control, but not significantly different than the discectomy condition. * p<0.05.
Figure 4.
Tension Stiffness. Tension stiffness was significantly decreased following discectomy for both groups. Tension stiffness following NP implant treatment was significantly lower than intact control, but not significantly different than the discectomy condition. * p<0.05.
Treatment with a nucleus pulposus implant
No NP or implant material was exuded from the disc during mechanical testing. For the experimental group, the ROM following NP implantation was 0.72 mm, 17% lower than following discectomy (p < 0.05) and not significantly different from the intact condition (Figure 2). Both the compression stiffness and tension stiffness following NP implant treatment were significantly lower than intact condition and not significantly different from the discectomy condition (Figure 3 and 4). In contrast, for the sham control, with no implant, the ROM was 37% larger than the intact condition (p < 0.05) and not significantly different from the discectomy condition (Figure 2). The sham control compression and tension stiffness were not different from the discectomy condition in the absence of an NP implant (Figure 3 and 4).
Structural Observations
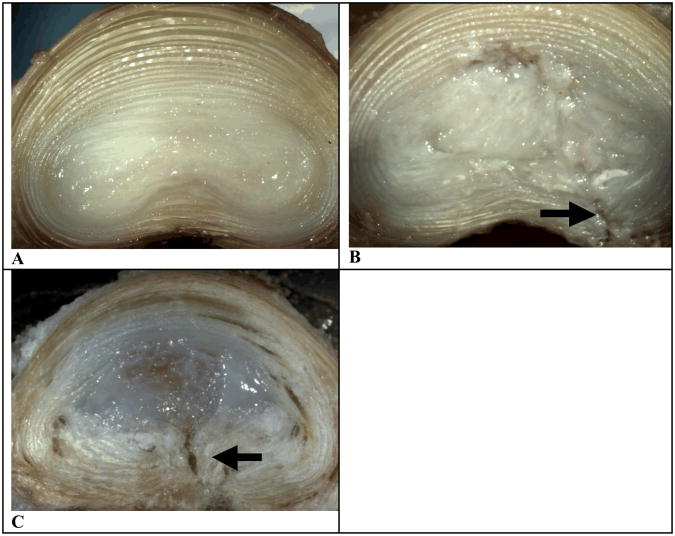
Gross morphology of the intact ovine disc shows concentric AF layers and a hydrated NP (Figure 5A). The experimental discs (Figure 5C) illustrated the successful filling of the NP cavity with implant. No implant material was ejected from the NP cavity. The control disc, where discectomy was performed and no implant placed, shows empty cavity/distribution of remaining NP material/any separation of AF lamella (Figure 5B). The site of annulotomy from discectomy can clearly be seen in both the treated and sham control discs (arrow, Figure 5B and 5C).
Figure 5.
Representative images of axially sectioned discs. A: Gross morphology of a healthy, intact ovine disc. B: Gross morphology of an ovine disc treated with discectomy. C: Gross morphology of an ovine disc treated with discectomy, followed by NP implant injection.
Discussion
The central findings of this study are two-fold. First, discectomy performed in vitro on the ovine disc results in a defined, statistically significant, decline in disc mechanical function that remains consistent following repeated testing. Secondly, an injectable oHA-gelatin NP implant can restore a key factor associated with NP degeneration: increased ROM. This study both demonstrates a successful study platform for assessing injectable NP implants and an effective implant prototype for normalizing NP mechanics. The implant designed to mimic native NP was able to return post-discectomy discs to intact range of motion (Figure 2). However, as expected, the compression and tension stiffness did not return upon the implant delivery due to the annular opening created during annulotomy, which was not treated (Figure 3 and 4).
Discectomy
Discectomy (or nucleotomy) in animal models and human cadaver studies demonstrates changes similar to those that occur in early human disc degeneration,4,35,36,37,38,39 supporting the use of this in vitro model system. Human cadaveric studies demonstrate that discectomy alters disc mechanics via decreased pressure, decreased disc height, increased deformation and flexibility, and increased AF bulging.4,35,36,37,38 Diminishing the amount of NP results in stepwise reduction of swelling and redistribution to support disc mechanics.5,35,36 The observed increase in ROM with discectomy (Figure 2) substantiates the notion that ROM relies heavily on the quantity of functional NP.19 In the sham control group, ROM appears to continually increase from its initial discectomy (p > 0.05 compared to discectomy). It is hypothesized that further cyclic loading in the discectomy group with no implant would lead to increased ROM from fatigue damage.
While NP plays a central role in ROM, compression and tension stiffness rely on the AF function.40,41 This has been clearly shown by using a trans-endplate nucleotomy to isolate the NP function without annular disruption.5 With trans-endplate nucleotomy the ROM is increased, while the compression and tension stiffness are unaltered,5 thus delineating the roles of NP and AF in functional mechanics. In this present study, the ROM increased with discectomy, consistent with the trans-endplate nucleotomy. Therefore, it is concluded that decreased compression and tension stiffness is due to annulotomy to access the NP. This decline in traits related to AF function was expected in this model because the annular opening created during discectomy was not repaired or treated. In addition, decreased compression and tension stiffness from annulotomy also may be due to significantly smaller ovine disc area (∼500 mm2) compared to human discs (∼1900mm2). This effect will be improved if human cadaveric model is used.
Treatment with nucleus pulposus implant
In the setting of degeneration, or discectomy, load transfer changes occur via a loss of NP pressure, which results in increased ROM and subsequent AF bulging, which may initiate and propagate AF tears.2,3,4,9,35,41,42 Restoration of ROM is a key factor for treatment strategies designed for painful disc degeneration.4,5 Since much of disc degeneration begins in the NP, and subsequently results in altered ROM, a critical goal of implant development is the ability to restore ROM. ROM, subsequent to discectomy, returned to normal after treatment with the NP implant, while ROM in discs without an implant remained 33% greater than intact values (Figure 2). Return to normal ROM subsequent to implantation demonstrates that an injectable implant can serve as a proxy for healthy NP in this crucial mechanical role. The current study reported no NP implant herniation or extrusion upon implantation via qualitative analysis. However, future studies will address potential for herniation more rigorously. In addition, future studies will also investigate how the implant functions when subjected to high number of axial tension-compression cycles via fatigue testing.
While many treatment paradigms for disc disease exist, it is increasingly apparent that less invasive, less destructive, motion preserving alternatives are needed. Many spine arthroplasty technologies are currently being tested with the intent of relieving pain and maintaining motion while preserving the health of adjacent segments.43 Arthroplasty options include total disc replacement (TDR) and subtotal disc replacement or nucleoplasty.13,44 TDR, while permitting some motion preservation, is destructive to the native joint and requires highly invasive implantation techniques. Nucleoplasty via injectable implant has the advantages of being less invasive in deployment and does not preclude future surgical alternatives, including TDR or spinal fusion.15 The advantage of injectable implants include, inter-digitation with native NP and potential to recreate the natural NP function with uniform stress distribution and shock absorption capability.15 Contrary to injectable implants, solid mechanical implants are not able to maintain even stress distribution and lack of the ability to absorb shock.15 A further division between the types of NP implants is delivery approach. Prefabricated mechanical implants necessitate surgical approaches associated with some joint destruction. With motion preservation and minimal tissue destruction as a focus, injectable implants will restore short term and long term function of the disc.14,44,45,46
This study was performed in a non-human in vitro model that permits collection of healthy baseline mechanical data in the same animal model intended for future in vivo pre-clinical testing. There are limitations in the use of sheep, which has a small disc height compared to the human that resulted in large discectomy-related changes to the AF. However, the use of a sham control group and repeated testing on the same sample enabled separation of implant-related treatment effects and discectomy-related damage. Moreover, there is a key advantage to use of the same model for in vitro and in vivo phases by providing baseline mechanical effects prior to biological remodeling in vivo. Another limitation is that the damping properties of the disc were not considered. However, viscoelastic effects are highly dependent on the relative size and fluid flow pathways, which cannot be appropriately determined in the ovine model. Future studies using cadaveric human discs will investigate these properties.
The conclusion of this work is that an injectable implant will return ROM, a crucial parameter of disc mechanical function, to baseline. While the current study established a number of preliminary requirements for the current material to be developed for an NP implant, future work will comprehensively address material characterization, biocompatibility assessment, and safety evaluation. For the millions of patients each year not responding to conservative therapy and also not meeting criteria for surgical intervention there is currently no proven treatment modality. An NP injectable implant may be a new treatment for disc disease to reach these patients.
Key Points.
A significant increase in ROM resulting from discectomy supports the notion that ROM depends on the quantity of functional NP.
Return to normal ROM following an NP implant suggests that the injectable implant can serve as a proxy for healthy NP in this crucial mechanical role.
Decline in properties related to AF function (tension and compression stiffness) is expected in this model because the annular opening created during discectomy was not repaired.
The conclusion of this work is that an injectable implant will return ROM to normal, a crucial parameter of NP mechanical function.
Acknowledgments
This research was funded by the Neurosurgery Research and Education Foundation.
The manuscript submitted does not contain information about medical device(s)/drug(s). Neurosurgery Research and Education Foundation funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1.Bibby SR, Jones DA, Lee RB, et al. The pathophysiology of the intervertebral disc. Joint Bone Spine. 2001;68:537–542. doi: 10.1016/s1297-319x(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 2.Meakin JR, Hukins DW. Effect of removing the nucleus pulposus on the deformation of the annulus fibrosus during compression of the intervertebral disc. J Biomech. 2000;33:575–580. doi: 10.1016/s0021-9290(99)00215-8. [DOI] [PubMed] [Google Scholar]
- 3.Panjabi M, Brown M, Lindahl S, et al. Intrinsic disc pressure as a measure of integrity of the lumbar spine. Spine. 1988;13:913–917. doi: 10.1097/00007632-198808000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Seroussi RE, Krag MH, Muller DL, et al. Internal deformations of intact and denucleated human lumbar discs subjected to compression, flexion, and extension loads. J Orthop Res. 1989;7:122–131. doi: 10.1002/jor.1100070117. [DOI] [PubMed] [Google Scholar]
- 5.Johannessen W, Cloyd JM, O'Connell GD, et al. Trans-endplate nucleotomy increases deformation and creep response in axial loading. Ann Biomed Eng. 2006;34:687–696. doi: 10.1007/s10439-005-9070-8. [DOI] [PubMed] [Google Scholar]
- 6.Kuroki H, Goel VK, Holekamp SA, et al. Contributions of flexion-extension cyclic loads to the lumbar spinal segment stability following different discectomy procedures. Spine. 2004;29:E39–46. doi: 10.1097/01.brs.0000106683.84600.e5. [DOI] [PubMed] [Google Scholar]
- 7.Wilke HJ, Kavanagh S, Neller S, et al. Effect of a prosthetic disc nucleus on the mobility and disc height of the L4-5 intervertebral disc postnucleotomy. J Neurosurg. 2001;95:208–214. doi: 10.3171/spi.2001.95.2.0208. [DOI] [PubMed] [Google Scholar]
- 8.Boyd LM, Carter AJ. Injectable biomaterials and vertebral endplate treatment for repair and regeneration of the intervertebral disc. Eur Spine J. 2006;15:414–421. doi: 10.1007/s00586-006-0172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Martino A, Vaccaro AR, Lee JY, et al. Nucleus pulposus replacement: basic science and indications for clinical use. Spine. 2005;30:S16–22. doi: 10.1097/01.brs.0000174530.88585.32. [DOI] [PubMed] [Google Scholar]
- 10.Huang RC, Wright TM, Panjabi MM, et al. Biomechanics of nonfusion implants. Orthop Clin North Am. 2005;36:271–280. doi: 10.1016/j.ocl.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Joshi A, Mehta S, Vresilovic E, et al. Nucleus implant parameters significantly change the compressive stiffness of the human lumbar intervertebral disc. J Biomech Eng. 2005;127:536–540. doi: 10.1115/1.1894369. [DOI] [PubMed] [Google Scholar]
- 12.Klara PM, Ray CD. Artificial nucleus replacement: clinical experience. Spine. 2002;27:1374–1377. doi: 10.1097/00007632-200206150-00022. [DOI] [PubMed] [Google Scholar]
- 13.Bertagnoli R, Sabatino CT, Edwards JT, et al. Mechanical testing of a novel hydrogel nucleus replacement implant. Spine J. 2005;5:672–681. doi: 10.1016/j.spinee.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Joshi A, Fussell G, Thomas J, et al. Functional compressive mechanics of a PVA/PVP nucleus pulposus replacement. Biomaterials. 2006;27:176–184. doi: 10.1016/j.biomaterials.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Coric D, Mummaneni PV. Nucleus replacement technologies. J Neurosurg Spine. 2008;8:115–120. doi: 10.3171/SPI/2008/8/2/115. [DOI] [PubMed] [Google Scholar]
- 16.Cloyd JM, Malhotra NR, Weng L, et al. Material properties in unconfined compression of human nucleus pulposus, injectable hyaluronic acid-based hydrogels and tissue engineering scaffolds. Eur Spine J. 2007;16:1892–1898. doi: 10.1007/s00586-007-0443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cloyd JM, Malhotra NR, Weng L, et al. Modulus and Poisson's Ratio in Unconfined Compression for Human Nucleus Pulposus and Potential Implants to Treat Disc Degeneration. Eur Spine J. In Press. [Google Scholar]
- 18.Weng L, Pan H, Chen W. Self-crosslinkable hydrogels composed of partially oxidized hyaluronan and gelatin: In vitro and in vivo responses. J Biomed Mat Res A. 2008;85:352–365. doi: 10.1002/jbm.a.31491. [DOI] [PubMed] [Google Scholar]
- 19.Wilke HJ, Heuer F, Neidlinger-Wilke C, et al. Is a collagen scaffold for a tissue engineered nucleus replacement capable of restoring disc height and stability in an animal model? Eur Spine J. 2006;15(15):433–438. doi: 10.1007/s00586-006-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckstein JC, Sen S, Schaer TP, et al. Comparison of animal discs used in disc research to human lumbar disc axial compression mechanics and glycosaminoglycan content. Spine. 2008;33:E166–173. doi: 10.1097/BRS.0b013e318166e001. [DOI] [PubMed] [Google Scholar]
- 21.O'Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine. 2007;32:328–333. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- 22.Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines? Spine. 1997;22:2365–2374. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]
- 23.Wilke HJ, Kettler A, Wenger KH, et al. Anatomy of the sheep spine and its comparison to the human spine. Anat Rec. 1997;247:542–555. doi: 10.1002/(SICI)1097-0185(199704)247:4<542::AID-AR13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 24.Kadoya K, Kotani Y, Abumi K, et al. Biomechanical and morphologic evaluation of a three-dimensional fabric sheep artificial intervertebral disc: in vitro and in vivo analysis. Spine. 2001;26:1562–1569. doi: 10.1097/00007632-200107150-00012. [DOI] [PubMed] [Google Scholar]
- 25.Meakin JR, Hukins DW. Effect of removing the nucleus pulposus on the deformation of the annulus fibrosus during compression of the intervertebral disc. J Biomech. 2000;33:575–580. doi: 10.1016/s0021-9290(99)00215-8. [DOI] [PubMed] [Google Scholar]
- 26.Smit TH. The use of a quadruped as an in vivo model for the study of the spine - biomechanical considerations. Eur Spine J. 2002;11:137–144. doi: 10.1007/s005860100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahlgren BD, Lui W, Herkowitz HN, et al. Effect of anular repair on the healing strength of the intervertebral disc: a sheep model. Spine. 2000;25:2165–2170. doi: 10.1097/00007632-200009010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Gunzburg R, Fraser RD, Moore R, et al. An experimental study comparing percutaneous discectomy with chemonucleolysis. Spine. 1993;18:218–226. doi: 10.1097/00007632-199302000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Martini L, Fini M, Giavaresi G, et al. Sheep model in orthopedic research: a literature review. Comp Med. 2001;51:292–299. [PubMed] [Google Scholar]
- 30.Melrose J, Ghosh P, Taylor TK, et al. A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibrosus. J Orthop Res. 1992;10:665–676. doi: 10.1002/jor.1100100509. [DOI] [PubMed] [Google Scholar]
- 31.Moore RJ, Latham JM, Vernon-Roberts B, et al. Does plate fixation prevent disc degeneration after a lateral anulus tear? Spine. 1994;19:2787–2790. doi: 10.1097/00007632-199412150-00010. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki M, Takahashi T, Miyahara K, et al. Effects of chondroitinase ABC on intradiscal pressure in sheep: an in vivo study. Spine. 2001;26:463–468. doi: 10.1097/00007632-200103010-00008. [DOI] [PubMed] [Google Scholar]
- 33.Argoubi M, Shirazi-Adl A. Poroelastic creep response analysis of a lumbar motion segment in compression. J Biomech. 1996;29:1331–1339. doi: 10.1016/0021-9290(96)00035-8. [DOI] [PubMed] [Google Scholar]
- 34.Elliott DM, Sarver JJ. Young investigator award winner: validation of the mouse and rat disc as mechanical models of the human lumbar disc. Spine. 2004;29:713–722. doi: 10.1097/01.brs.0000116982.19331.ea. [DOI] [PubMed] [Google Scholar]
- 35.Brinckmann P, Grootenboer H. Change of disc height, radial disc bulge, and intradiscal pressure from discectomy. An in vitro investigation on human lumbar discs. Spine. 1991;16:641–646. doi: 10.1097/00007632-199106000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Goel VK, Nishiyama K, Weinstein JN, et al. Mechanical properties of lumbar spinal motion segments as affected by partial disc removal. Spine. 1986;11:1008–1012. doi: 10.1097/00007632-198612000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Keller TS, Hansson TH, Holm SH, et al. In vivo creep behavior of the normal and degenerated porcine intervertebral disk: a preliminary report. J Spinal Disord. 1988;1:267–278. [PubMed] [Google Scholar]
- 38.Panjabi MM, Krag MH, Chung TQ. Effects of disc injury on mechanical behavior of the human spine. Spine. 1984;9:707–713. doi: 10.1097/00007632-198410000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Shea M, Takeuchi TY, Wittenberg RH, et al. A comparison of the effects of automated percutaneous diskectomy and conventional diskectomy on intradiscal pressure, disk geometry, and stiffness. J Spinal Disord. 1994;7:317–325. [PubMed] [Google Scholar]
- 40.Hickey DS, Hukins DW. Relation between the structure of the annulus fibrosus and the function and failure of the intervertebral disc. Spine. 1980;5:106–116. doi: 10.1097/00007632-198003000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Shirazi-Adl A. Finite-element simulation of changes in the fluid content of human lumbar discs. Mechanical and clinical implications Spine. 1992;17:206–212. [PubMed] [Google Scholar]
- 42.Meakin JR, Redpath TW, Hukins DW. The effect of partial removal of the nucleus pulposus from the intervertebral disc on the response of the human annulus fibrosus to compression. Clin Biomech. 2001;16:121–128. doi: 10.1016/s0268-0033(00)00075-9. [DOI] [PubMed] [Google Scholar]
- 43.Moumene M, Geisler FH. Comparison of biomechanical function at ideal and varied surgical placement for two lumbar artificial disc implant designs: mobilecore versus fixed-core. Spine. 2007;32:1840–1851. doi: 10.1097/BRS.0b013e31811ec29c. [DOI] [PubMed] [Google Scholar]
- 44.Bao QB, Yuan HA. New technologies in spine: nucleus replacement. Spine. 2002;27:1245–1247. doi: 10.1097/00007632-200206010-00020. [DOI] [PubMed] [Google Scholar]
- 45.Alini M, Roughley PJ, Antoniou J, et al. A biological approach to treating disc degeneration: not for today, but maybe for tomorrow. Eur Spine J. 2002;11(2):S215–220. doi: 10.1007/s00586-002-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diwan AD, Parvataneni HK, Khan SN, et al. Current concepts in intervertebral disc restoration. Orthop Clin North Am. 2000;31:453–464. doi: 10.1016/s0030-5898(05)70163-2. [DOI] [PubMed] [Google Scholar]