ABSTRACT
International clinical trials have demonstrated compelling evidence on the prevention or delay of type 2 diabetes (T2D) by lifestyle change programs. Numerous studies have translated the Diabetes Prevention Program (DPP) protocol to “real-world” settings. The purpose of this paper is to review the translational research of the DPP protocol in adults at-risk for T2D. This study is a systematic review based on the guidelines from the Cochrane Handbook for Systematic Reviews. There were 16 studies that translated the DPP protocol in four distinct settings: (a) hospital outpatient, (b) primary care, (c) community, and (d) work and church. Settings varied considerably in terms of reach, efficacy, adoption, implementation, and maintenance. There were strengths and limitations to each setting. Better understanding of program adaptation and mediators and moderators to program efficacy are indicated. Future research also needs to continue to explore mechanisms to improve access and long-term outcomes.
KEYWORDS: Diabetes prevention program, Translation research
Type 2 diabetes (T2D) is one of the most rapidly increasing chronic illnesses worldwide and is associated with significant morbidity, mortality, and societal costs. Nearly 26 million adults in the USA have diabetes and 79 million have prediabetes. Ethnic minorities have a disproportionate risk and are twice as likely as non-Hispanic whites of similar age to develop T2D. The increasing prevalence of T2D is concerning due to the numerous complications associated with the disease. T2D is the 7th leading cause of death in the USA and contributes to increased risk for cardiovascular disease, renal failure, blindness, and nontraumatic amputation. In the USA, the costs of diabetes in 2007 were estimated to be 174 billion dollars [1]. Therefore, the greatest opportunity for addressing the personal and societal burden of T2D is to prevent the development of the disease.
Recent evidence clearly demonstrates that individuals at high risk for T2D can be identified and T2D delayed, if not prevented, through lifestyle change programs. International clinical trials, including the Diabetes Prevention Program (DPP), have demonstrated compelling evidence on the reduction of T2D for at-risk adults who participated in lifestyle change programs of weight reduction and physical activity compared to a control group [2–4]. In the DPP, lifestyle change resulted in a 58% reduction in T2D compared to a 31% reduction with metformin at 2.8 years of follow-up [3]. Recent evidence indicates that the prevention or delay of T2D can continue for at least 10 years [5]. Modest weight loss of 5–7% dramatically improves insulin resistance, a precursor to T2D [6, 7].
The benefit of lifestyle change has resulted in recommendations by the American Diabetes Association advocating for lifestyle change as the first line of treatment to prevent or delay T2D [8]. Results of a cost analysis of the DPP indicate a favorable cost-effective profile of the lifestyle program at any adult age [9].
The DPP was based on behavioral science evidence and included: a collaborative approach, education, behavioral support (i.e., goal setting, problem solving), and motivational interviewing. The primary goals of the DPP were for participants to lose 7% of initial body weight within 6 months and to participate in moderate physical activity for at least 150 min per week. The DPP consisted of a 16-week core curriculum provided individually to participants by trained health coaches followed by monthly group and/or individual meetings and a long-term maintenance program. In addition, motivational campaigns, a toolbox of additional strategies, and incentives were provided [10].
Having established efficacy in a large clinical trial, subsequent research on the DPP has been conducted on the translation of the DPP to typical or “real-world” settings. Since the completion of the DPP in 2002, numerous studies have been conducted translating the DPP to different settings with efforts to reach participants with low socioeconomic status and diverse race and ethnicity. Adaptation of programs to a new context has resulted in programs with different components, modes of delivery, length, providers, targeted population, and outcomes. Some studies have demonstrated outcomes similar to the DPP; others have reached highly diverse adults at risk for T2D with less weight loss demonstrated. Attrition in these studies has also varied considerably.
Several reviews have been conducted on diabetes prevention research, focusing on the international research [11, 12] or the comparative benefit of dietary change, physical activity, and/or weight loss [13, 14]. One review focused on community-based programs conducted in the USA prior to the DPP, with few manuscripts reporting on outcomes; rather, the process of intervention adaptation or lessons learned was reported [15]. Another review highlighted culturally relevant programs, targeting the Native American population, and proposed best practices for diabetes prevention in this population, one of which is based on the DPP [16]. One review focused on research specifically translating the DPP; however, the review was not exhaustive, and several of the programs targeted adults with type 2 diabetes [17]. Since this review, eight additional papers translating the DPP have been published. Therefore, the purpose of this paper is to systematically review the translational research on diabetes prevention programs for adults at-risk for T2D based on the DPP protocol.
Designing studies to test the translation of a research-based program (with established efficacy in clinical trials) into the healthcare or community setting requires consideration of broad processes and outcomes of care to improve dissemination. The Reach, Efficacy, Adoption, Implementation, Maintenance (RE-AIM) model is the organizing framework of this review as it was developed for use in evaluating the effectiveness of health behavior programs in terms of public health significance [18]. The major premise of the model is that public health impact of programs require more than efficacy (efficacy). Programs must also reach a diverse sample, representative of the population at-risk for T2D (reach). They must be appealing to health care providers and realistic to implement in specific practice settings (adoption). Programs must also be able to be delivered as intended (implementation). Lastly, programs must past the test of time and be sustained by both the individual and the clinical setting (maintenance). Cost will be evaluated as a proxy for maintenance in this review.
An important assumption of this model is that the characteristics that contribute to a program’s efficacy in a controlled clinical trial (i.e., intensive, complex, highly standardized) may be fundamentally different to the characteristics necessary for implementation in clinical practice (i.e., broad appeal, flexible, cost-effective). Thus, it is likely that programs with demonstrated efficacy in clinical trials will need to be modified when translating into a complex healthcare environment.
METHOD
A systematic review was undertaken using the guidelines from Cochrane’s Handbook for Systematic Reviews of Interventions for locating and analyzing studies [19]. A computer-assisted search was undertaken using the keywords of diabetes prevention and Diabetes Prevention Program in Medline and CINAHL from January 2002 to February 2011. Initial search results yielded 40 manuscripts. To be included, a study had to be a published report on the outcomes of a translation study evaluating a diabetes prevention program based on the DPP curriculum for adults at-risk for T2D. Manuscripts were excluded if programs were provided in schools or with cognitively impaired adults. Manuscripts reporting findings from subsamples of a larger study were excluded unless a unique intervention approach was used (i.e., telehealth). Published abstracts were excluded due to limited available data. Reference lists of all included papers were reviewed with no new manuscripts identified.
A total of 16 studies were identified that met the sampling inclusion criteria. Data were extracted from studies on study design, setting, sample, the program, how the DPP was modified, personnel who provided the program, attendance, attrition, outcomes, and data analysis procedures. Data display matrices were developed to display all of the coded information for each study by category. Graphs and charts were created to view data visually. Matrices and graphs were compared to narratively analyze and synthesize results. A meta-analysis was unable to be conducted due to variability in reporting the primary outcome of weight loss (kilograms, % body weight, and % of participants who met weight loss goals). Very few studies reported standard deviations for the outcome of kilogram weight loss.
RESULTS
Of the 16 studies, the majority were one-group designs (n = 13 studies; 81%) with varied length of follow-up ranging from 3 months to 2 years. Two studies were pilot clinical trials [20, 21] and one was a controlled cohort design [22]. Sample sizes in the studies ranged from 8 to 1,003 participants. Samples were predominately female (55–90%) and of varied race and ethnicity (0–100% non-white). Outcomes also varied considerably with weight loss at the longest point of follow-up varying between −1.0 and −8.6 kg. The percent of participants who met the 5% weight loss goal ranged from 11% to 64% across studies that reported this outcome. In studies that measured depressive symptoms, 33–35% of participants reported elevated depressive symptoms above a criterion score. Table 1 compares the DPP benchmarks and the translational research of this review. As can be seen, there is considerable variability in the diabetes prevention programs adapted for different settings with difficulty achieving the DPP benchmarks.
Table 1.
DPP benchmarks and translational research
| DPP | Translation research | |
|---|---|---|
| Sample size | N = 3,234 | N = 8–1,003 |
| Weight loss | −6.5 kg | −1.0 to −8.6 kg |
| Weight change | −6.9% | −2.7% to −6% |
| Percent who met 7% weight loss goal | 50% at 3 months | 18–49% at F/U (6–12 months) |
| 38% at F/U (2–4 years) | ||
| Percent who met 5% weight loss goal | 11–64% at F/U (3–12 months) | |
| Attendance | 95% | 57–96% |
| Attrition | 7% | 0–43% |
| Diversity | 46% non-White | 0–100% non-White |
| Depressive symptoms | 10% | 33–35% |
In order to compare the reach, efficacy, adoption, implementation, and maintenance across studies, the studies were categorized by setting. There were four distinct settings of diabetes prevention translational research: (a) hospital outpatient or diabetes education model of care [23–25] with one study a comparative effectiveness study comparing an on-site program to a telehealth program [26], (b) primary care [21, 22, 27] with one study combining primary care with an online program [28], (c) community settings (i.e., Young Men's Christian Association (YMCA)) [20, 29–31], and (d) church or workplace settings [32–35] (Tables 2, 3, 4, and 5).
Table 2.
DPP translation research—hospital outpatient
| McBride et al. [24] | Pagoto et al. [25] | Vadheim et al. [26] | Vanderwood et al. [23] | |
|---|---|---|---|---|
| Reach | ||||
| Sample size | N = 37 | N = 118 | N = 29 | N = 1003 |
| Mean age | 52 years | 48.8 years | 50 years in Telehealth group | 52.3 years |
| 53 years in onsite group | ||||
| Mean BMI at baseline | 37.4 | 43.3 | 38.7 | 36.3 |
| Gender (% female) | 60% | 72% | 93% in Telehealth group | 80% |
| 69% in onsite group | ||||
| Diversity (% non-White) | 0% | 9% | ||
| Adoption and implementation | ||||
| Intervention—core | 12 group sessions (1×/week) | 16 group sessions (1×/week) | 16 group sessions (1×/week) | 16 group sessions (1×/week) |
| Signed contracts | Charged fee | Signed contract | ||
| Charged fee (refunded) | Exercise classes | Charged fee | ||
| Exercise classes | Exercise classes | |||
| Intervention—maintenance | F/U-1×/month | F/U-1×/month | F/U-1×/month | F/U-1×/month |
| Personnel | Dietician, exercise physiologist (cardiac rehab) | Dietician, exercise physiologist, clinical psychologist (weight center) | Dietician (CDE) and exercise specialist | Dietician and exercise specialist |
| Attendance | Stated as “good” | 83% | 91% | 93% for core |
| 62% for after core | ||||
| Attrition | 11% at 12 months | 17% at 4 months | 12% at 6 months | 19% at 4 months |
| 42% at 12 months | ||||
| Efficacy | ||||
| Management of missing data | Analyzed completers | Last observation carried forward | Analyzed completers | Analyzed completers |
| Short-term weight loss (kg) (length of F/U) | 5.0 kg at 3 months* | 5.57 kg (SD 4.55) at 4 monthsa | 6.8 kg at 4 months*** | |
| Long term weight loss (kg) (length of F/U) | 4.5 kg at 12 months* | 6.7 kg (SD 3.7) in Telehealth group | 7.7 kg. at 12 months*** | |
| 6.5 kg (SD 3.1) in onsite group at 6 monthsb | ||||
| % Weight loss | 4.1% | 4.6% | ||
| % Subjects who met 5% weight loss | 64% at12 months | |||
| % Subjects who met 7% weight loss | 30% | 50% in Telehealth group | 49% at 12 months | |
| 46% in onsite group | ||||
| Other outcomes | Decrease in waist circumference*,% body fat*, BP*, fat intake*. Increase in METS*, fruit and vegetable intake*. Glucose and lipids not reported | Less weight loss compared to DPP*. Weight loss not significantly different from DPP when exclusion for comorbidity (type 2 diabetes, binge eating, depression) | No significant difference between telehelath and onsite group in weight loss, physical activity, or fat intake goal | Decrease in BP***, LDL cholesterol***, glucose***. Decrease in HDL*** after core, increased at 12 months*** |
| Maintenance | ||||
| Cost of program | $250 (charged) | $800 (charged) | ||
Blank cell data not reported
*p < .05, **p < .01, ***p < .001
aCompared outcomes to DPP benchmarks
bCompared Telehealth group to onsite group
Table 3.
DPP translation research—primary care
| Kramer et al. [23] | McTigue et al. [22] | McTigue et al. [28] | Whittemore et al. [21] | |
|---|---|---|---|---|
| REACH | ||||
| Sample size | N = 93 (two studies) | N = 72 | N = 50 | N = 58 |
| Mean age | 53.1 years | 51.94 years | 47 years | |
| Mean BMI at baseline | 35.6 | 38.9 | 36.43 | 38.8 |
| Gender (% female) | 80% | 84% | 76% | 92% |
| Diversity (% non-White) | 22% | 30% | 14% | 55% |
| Adoption and implementation | ||||
| Intervention—core | 12 group sessions (1×/week) | 12 group sessions (1×/week) | 16 sessions online | 7 individual sessions, 5 phone sessions, 1 session with dietician (over 6 months) |
| Charged fee | E-coaching | |||
| Quarterly progress reports to provider | ||||
| Intervention—maintenance | F/U—1×/month | F/U—1×/month | F/U—1×/month | |
| personnel | Nurse, health educator, exercise specialist | Nurse educator | Nurse educator | Nurse practitioner, dietician |
| Attendance | 83% | 80% | 96% (in person) | |
| 37% (phone) | ||||
| Attrition | 22% at 3 months (2 studies) | 7% at 9–12 months | 10% at 12 months | 12% at 6–9 months |
| 28% at 12 months (1 study) | ||||
| Efficacy | ||||
| Management of missing data | Last observation carried forward (3 months) | Analyzed completers | Last observation carried forward | Last observation carried forward |
| Analyzed completers (12 months) | ||||
| Short term weight loss (kg) (length of F/U) | 3.4 kg at 3 months*** | |||
| Long term weight loss (kg) (length of F/U) | 4.99 at 12 months*** (one study) | 5.2 kg (SD 2.51) at 9–12 months***a | 4.79 kg (SD 2.56) at 12 months* | 8.7 kg at 6–9 monthsb |
| % Weight loss | 3.5% | |||
| % Subjects who met 5% weight loss | 52% | 30% | 25% | |
| % Subjects who met 7% weight loss | 23% | 27% | 18% | |
| Other outcomes | Decrease in waist circumference**, fasting glucose* (one study). Decrease in waist circumference***, cholesterol**, LDL**, and BP*** maintained at 12 months (except cholesterol) Increase HDL** at 12 months (one study) | Enrollees had 4.38 times the odds of clinically significant weight loss | Decrease in systolic BP* | Improvement in both groups for nutrition and exercise***. Trend for increase in exercise and HDL in DPP group. No significant difference in HOMA, glucose, LDL, cholesterol, or waist circumference |
| Maintenance | ||||
| Cost of program | $300 (estimated) | $150 (charged) | ||
Blank cell data not reported
*p < .05, **p < .01, ***p < .001
aCompared enrollees to non-enrollees
bCompared treatment and control group
Table 4.
DPP translation research—community
| Ackerman et al. [20] | Matvienko and Hoehns [29] | Mau et al. [30] | Seidel et al. [31] | |
|---|---|---|---|---|
| Reach | ||||
| Sample size | N = 92 | N = 31 | N = 239 | N = 88 |
| Mean age | 58 years | 55.8 years | 49 years | 54 years |
| Mean BMI at baseline | 31.5 | 36.1 | 39.1 | |
| Gender (% female) | 55% | 61% | 83% | 84% |
| Diversity (% non-White) | 18% | 6% | 98% | 27% |
| Adoption | ||||
| Intervention—core | 16 group sessions (1×/week) | 16 individual sessions (over 6 months) | 8 group sessions (over 12 weeks) | 12 group sessions (1×/week) |
| Exercise classes | Free YMCA membership | |||
| Intervention—maintenance | F/U-1×/month | F/U-1×/month (individual) | ||
| Monthly phone calls | ||||
| Personnel | YMCA staff (with health degree or equivalent experience) | Exercise science graduate students | Community peer educators | Dietician and exercise specialist, plus 2 lay health coaches |
| Attendance | 57% | 70% attended ≥ 75% of classes | ||
| Attrition | 16% at 4 months | 6% at 6 months | 29% at 3 months | 22% at 3 months |
| 33% at 12 months | 16% at 12 months | 43% at 6 months | ||
| Efficacy | ||||
| Management of missing data | Analyzed completers | Analyzed completers | Analyzed completers | Last observation carried forward |
| Short term weight loss (kg) (length of F/U) | 5.7 kg at 4 months***a | 6.1 kg at 6 month*** | 1.5 kg (SD 0.5) at 3 month* | |
| Long term weight loss (kg) (length of F/U) | 5.7 kg at 12 months**a | 6.1 kg at 12 months** | ||
| % Weight loss | 6% | |||
| % Subjects who met 5% weight loss | 39% at 6 months | 11% | 46% at 3 months (88% sustained at 6 months) | |
| 56% at 12 months | ||||
| % Subjects who met 7% weight loss | 25% at 6 months | 26% at 3 months | ||
| 32% at 12 months | (67% sustained at 6 months) | |||
| Other outcomes | Decrease in cholesterol at 4 months*** and 12 months** compared to control group. No difference in HbA1c, BP, or HDL | Decrease in waist circumference***, DBP*, cholesterol*, LDL*, HDL* at 6 months. No significant change in SBP. Decrease in waist circumference** and DBP* at 12 months | Decrease in BP*, dietary fat intake*. Increase in physical functioning*, physical activity* | 44% of participants improved in one metabolic parameter (i.e., BP) at 3 months |
| Maintenance | ||||
| Cost of program | $275–325 (estimated) | |||
Blank cell data not reported
*p < .05, **p < .01, ***p < .001
aCompared treatment and control group
Table 5.
DPP translation research—work/church
| Aldana et al. [32] | Boltri et al. [33] | Davis-Smith et al. [34] | Dodani and Fields [35] | |
|---|---|---|---|---|
| Reach | ||||
| Sample size | N = 37 | N = 8 | N = 10 | N = 40 |
| Mean age | 52 years | 46 years | ||
| Mean BMI at baseline | 30.95 | 31.6 | 35.7 | (49% obese, 32% morbidly obese) |
| Gender (% female) | 64% | 70% | 85% | |
| Diversity (% non-White) | 50% | 100% | 100% | 100% |
| Adoption and implementation | ||||
| Intervention—core | 24 group sessions (1×/week) + 4 individual sessions and when requested | 16 group sessions (1×/week) | 6 group sessions (1×/week) | 12 group sessions |
| Free gym membership | ||||
| Intervention—maintenance | F/U-1×/month | |||
| Personnel | Nurse, certified health educator | Volunteer health professional with diabetes prevention experience | Volunteer health professional | Volunteer health professionals |
| Attendance | 65% | 78% | ||
| Attrition | 6% at 12 months | 0% at 12 months | 10% at 12 months | 12% at 3 months |
| Efficacy | ||||
| Management of missing data | Last observation carried forward | Last observation carried forward | Analyzed completers | |
| Short term weight loss (kg) (length of F/U) | 2.94 kg at 6 months* | 2.6 kg at 6 months* | 4 kg at 3 months* | |
| Long term weight loss (kg) (length of F/U) | 3.3 kg at 12 months* | 0.5 kg at 12 months | 4.8 kg at 12 months* | |
| % Weight loss | 3.6% | |||
| % Subjects who met 5% weight loss | 46% | 48% at 3 months | ||
| % Subjects who met 7% weight loss | 26% at 3 months | |||
| Other outcomes | Decrease in waist circumference*, glucose,* insulin*, cholesterol*, triglyceride*, at 6 months. Decrease in glucose* and triglyceride* at 12 months. Increase in aerobic fitness* at 6 and 12 months | Decrease in glucose*, BP* at 6 and 12 months | Decrease in glucose*, BP* at 4 and 12 months | |
| Maintenance | ||||
| Cost of program | $108 (estimated) | |||
Blank cell data not reported
*p < .05, **p < .01, ***p < .001
Reach
All of the DPP translational studies had minimal exclusion criteria, reflecting a more heterogeneous population at-risk for T2D. Inclusion criteria were also expanded in many studies to include adults at-risk for T2D with and without prediabetes. Several studies did include some participants with T2D; however, this group represented a small proportion of the total sample (<50%) [24, 25, 28, 29]. Sample sizes varied considerably across studies from eight participants in a church-based program [33] to 1,003 in a diabetes education model of care provided in a community setting [23]. Overall, the work/church setting had the smallest sample sizes. The mean age across all studies in which age was reported (n = 13) was 51.7 years, slightly older in comparison to the DPP study of 50.6 years. Mean body mass index (BMI) across studies in which BMI was reported (n = 14) was 36.5 kg/m2, considerably higher than the mean BMI of the DPP study of 30.5 kg/m2. The majority of participants in diabetes translation programs were female (74%; n = 15 studies reported gender), which is higher than in the DPP study (68% female). In studies that evaluated depressive symptoms, psychosocial comorbidity was much higher than in the DPP [21, 25].
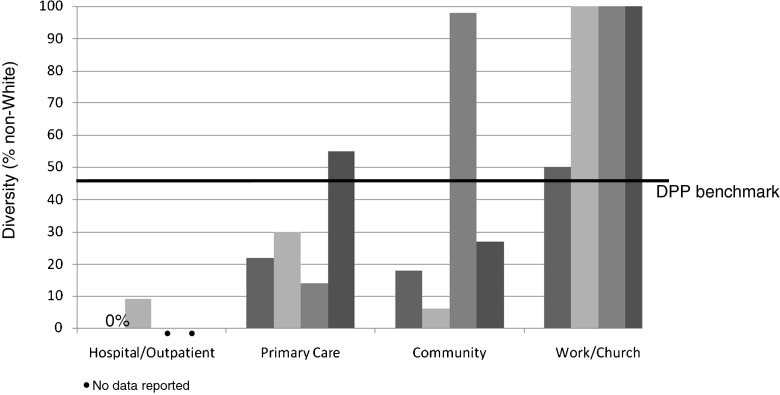
Diversity in this review was determined by calculating the % of non-White participants. Diversity varied by setting from 0% to 100%, with greater diversity in programs provided at work or church, followed by community settings, primary care, and lastly hospital outpatient or diabetes education models of care (n = 14 studies reported on diversity; Fig. 1). Two of the hospital outpatient studies did not have access to data on race or ethnicity [23, 26].
Fig 1.
Diversity of the sample across settings
Efficacy
Weight loss was the primary outcome evaluated across all studies. Some studies reported weight loss in kilograms, others in percent body weight change, and/or the percent of participants who met a 5% or 7% weight loss goal (Tables 2, 3, 4, and 5). Evaluating weight loss was also complicated by follow-up time, which ranged from 3 to 12 months. With respect to weight loss in kilograms and at the longest point of follow-up, hospital outpatient settings achieved the most weight loss, followed by primary care, community settings, and work or church settings (Fig. 2). As can be seen, weight loss in some studies was comparable to the DPP benchmark.
Fig 2.
Weight loss across settings at longest point of follow-up
When comparing reach (in terms of diversity) and efficacy (in terms of weight loss) across settings, an opposite trend is apparent. In hospital outpatient settings that demonstrate greater weight loss, there is less diversity. In settings with more diversity, there is less weight loss; although the majority of studies with diverse samples demonstrated statistically significant weight loss. As mentioned, two studies conducted in the hospital outpatient setting may have had increased diversity; however, this data was not available. In addition, studies with diverse samples may have had other implementation factors that affected efficacy (i.e., type and skill of interventionist, protocol fidelity, and sample size).
Some studies also demonstrated significant improvements in other clinical indicators (i.e., fasting glucose, waist circumference, blood pressure, cholesterol) and behavioral indicators (i.e., dietary intake, and physical activity; Tables 2, 3, 4, and 5).
Adoption
As previously mentioned, diabetes prevention translation programs have been conducted in different settings. Despite this variability, the majority of programs have utilized health care providers (health coaches, dieticians, exercise physiologists, certified diabetes educators, nurses, and nurse practitioners) as the interventionist (n = 12). Three programs provided in the church setting trained volunteer health professionals to provide the program. Only one program was provided by trained community peer educators [30] which reached a highly diverse and large sample (n = 293), provided the fewest sessions (n = 6), yet demonstrated little weight loss (1.5 kg at 3 months). One program employed a community health worker to assist health professionals [31].
Implementation
Translational research typically involves some modification to adapt the program for the targeted population and setting. The majority of DPP translational studies modified the program to be provided in a group setting (81%). Programs not provided as a group included individual sessions [21, 29], a combination of group sessions and individual sessions [32], and a combination of group online sessions and individual sessions [28]. All programs eliminated the intensive toolbox strategies of the DPP (i.e., free sneakers), substituting less expensive or no strategies.
All programs utilized the DPP curriculum. Some programs provided all 16 sessions of the original DPP core curriculum; however, the majority modified the curriculum to decrease the number of classes and some of the content. Most studies used a collaborative approach with providers to adapt the program to the local context; only two studies used a community-based participatory research process involving health professionals and members of the community [30, 35]. When programs were adapted to decrease the number of sessions, most reports did not identify which sessions were eliminated and/or combined. With the exception of hospital outpatient programs that provided a 16-session curriculum, there was not any consistent pattern with respect to the number of sessions and outcomes across settings.
All programs provided education on healthy eating and physical activity. In addition, all programs provided behavioral support in terms of goal setting, problem solving, and relapse prevention. There was little evidence that motivational interviewing was a major component of any of the programs with the exception of one study provided as individual sessions in the primary care setting [21]. Only two studies provided evidence of intervention fidelity, both of which were randomized pilot trials [20, 21].
Three of the four hospital outpatient programs required participants to sign a contract indicating readiness or motivation to engage in the program and make lifestyle changes [23–25]. One of these programs conducted a thorough assessment of all eligible participants to determine and enhance motivation; only those motivated were enrolled in this study. In addition, this program charged a fee ($800.00) for the program [25]. The two other hospital outpatient programs that required contracts charged a fee for the program ($250.00 in one, not reported in the other) that were reimbursed for “good attendance” or upon completion of the program [23, 24]. There was one primary care program that also charged a fee ($150.00) [22].
Other indicators of implementation include attendance and attrition. Hospital outpatient programs and primary care had the highest attendance (80–96%), followed by the work/church setting (65–78%), and community settings (57%). With respect to attrition, the work/church setting had the lowest attrition (0–12%), followed by primary care settings (7–28%), hospital outpatient (11–42%), and community settings (16–43%). The comparison of attrition by setting is provided in Fig. 3.
Fig 3.
Attrition across settings at longest point of follow-up
Maintenance
The majority of studies were pilot or feasibility studies, evaluating the core curriculum of the DPP. Ten studies followed participants for 1 year, with eight of these studies providing a maintenance program. Programs with a maintenance component demonstrated weight loss at 1 year ranging from 3.3 to 7.7 kg [20, 22–24, 27–29, 32]. The other two studies with 12 month follow-up were in a church setting and did not provide a maintenance component; one demonstrated weight loss maintenance at 1 year [34], the other program did not [33].
The ability to maintain programs in “real-world” settings, particularly low-resource settings can also be evaluated by examining the cost of the program. Few studies evaluated cost. One program provided in primary care estimated the cost of the program at $300.00 per participant [27]. Another program, provided in the YMCA, cost approximately $275.00–325.00 per participant [20]. A program provided in a church with donated space and volunteer personnel estimated a cost of $108.00 for supplies for 10 participants [34]. These estimates are considerably lower than the cost of the DPP at approximately $1400.00 [36].
DISCUSSION
Limitations in this review include studies with primarily one-group designs, small sample sizes, variable outcomes reported, variable follow-up timing, low to moderate methodological quality, and the potential for publication bias. Despite these limitations, there are important clinical and research implications of this translational research. Diabetes prevention programs that have translated the DPP protocol to a “real-world” setting do have the potential to achieve positive outcomes in terms of the reach, efficacy, adoption, implementation, and maintenance of programs. However, there were strengths and limitations of each type of setting in which these programs were translated.
Hospital outpatient or diabetes education models of care are excellent settings to provide a modified DPP protocol with weight loss demonstrated in all four studies. Adoption and implementation were also high in these settings as health care professionals who provide diabetes self-management programs are highly qualified to provide a DPP. In addition, facilities where the program can be delivered and access to electronic systems to facilitate scheduling and maintenance of records are readily available. Billing for select services can also be accomplished (i.e., dietary counseling for adults with metabolic syndrome); however, hospital outpatient settings may not reach adults of diverse race or ethnicity who are at-risk for T2D due to cost or transportation challenges. Of the two studies in this setting that reported on diversity, diversity of samples was 0% and 9% [24, 25]. In addition, diabetes prevention programs in these settings reached a highly motivated sample, committed to making lifestyle change.
The primary care setting does have the potential to reach adults of diverse race and ethnicity, be efficacious (less than the DPP), adopted, implemented, and maintained. A potential benefit of the primary care setting is that participants of the program have established relationships with providers, which may enhance intervention efficacy and implementation as well as decrease attrition. In addition, primary care settings can also manage co-morbidities that frequently occur in adults at-risk for T2D (i.e., hypertension). The major challenge of implementing a DPP in primary care is the need to provide adequate components of the protocol while simultaneously addressing efficiency in terms of who provides the intervention and how the program is implemented. Not all primary care practices have access to health educators, nurses, or dieticians, and implementing group-based interventions in primary care is challenging due to scheduling and space. One program in this review provided individual sessions supplemented with phone sessions and home reading, although phone sessions were challenging to complete [21]. Another program provided the DPP curriculum via the internet and supplemented this with monthly group sessions and e-counseling [28].
Community and work/church settings have the greatest potential to reach adults of diverse race and ethnicity at-risk for T2D; however, these programs had the greatest variability with respect to other RE-AIM indicators. Some programs were efficacious; other programs were not. Some programs had considerable implementation challenges. While further research is indicated, it appears that programs linked to existing structures of care (i.e., YMCA) or social structures (i.e., work/church) may enhance adoption, implementation, and maintenance of programs. It also appears that community health workers may not be optimal providers for a DPP. Additional research is indicated as a preliminary report of a DPP provided by health professionals and community health workers with T2D has demonstrated promising results [37, 38]. The effectiveness of community health workers in other behavioral interventions has been variable, depending on their role and the population served. Community health workers can aid in increasing access to care in underserved populations and can improve the reach and relevance of health promotion programs as part of professional teams [39].
All of the programs translating the DPP protocol to a different setting included a core curriculum with sessions provided frequently over 3–6 months. Several programs included a maintenance program of monthly sessions up to 1 year. The hospital outpatient setting or diabetes education model of care was able to translate the DPP with little adaptation from the original protocol. Other programs demonstrated varying degrees of adaptation. In adapted programs, optimal components of core and maintenance programs, as well as dose, have yet to be determined. One challenge of translational research is to retain essential elements of the original protocol while adapting to a local context. Highly structured protocols may be impossible to implement [40], and highly adapted protocols may not include key components of the protocol [41]; both of which may impact outcomes. Therefore, protocol adaptation needs to be systematic, carefully considered, and adequately described [42]. In addition, program fidelity needs to be systematically evaluated in translational research, particularly with complex behavioral interventions [43]. Program adaptation and fidelity were not adequately described in most studies of this review. For example, motivational interviewing was a major component of the DPP; however, it is not clear if and how this behavioral counseling was applied in the majority of programs included in this review, particularly those provided with a group or media-based approach. The primary purpose of motivational interviewing is to assist adults to enhance motivation to change health behaviors and to resolve ambivalence to change [44]. Therefore, motivational interviewing may be indicated when an individual demonstrates low readiness or motivation to change and/or inability to meet incremental behavioral goals. Hospital outpatient programs required participants to demonstrate motivation to change health behaviors, which may have limited the need for motivational interviewing.
Future translational research also needs to include an adequate description of sample characteristics, including characteristics or conditions that may interfere with the ability to make lifestyle change, such as race and ethnicity, income, and depressive symptoms. For example, attitudes and beliefs about physical activity vary by race and ethnicity, which may influence physical activity [45]. Low socioeconomic neighborhoods have been shown to have less access to healthy food [46]. Increased depressive symptoms in adults at risk of T2D have been reported, ranging from 33% to 48% [21, 25, 47]. Depression has been shown to negatively impact self-management in T2D [48], which may be similar in adults at-risk for T2D. Pagoto et al. [25] demonstrated that participants with depression, binge eating disorder, or T2D were less likely to improve weight outcomes and suggested that participants with these conditions may require a more intensive or different approach to lifestyle change. Other potential moderators to program outcomes include age, baseline BMI, and physical functioning [21, 25, 31]. Expanding the reach of diabetes translational research to more heterogenous samples enhances generalizability but has the potential to confound the interpretation of results. Statistical control or examination of moderators is indicated.
Mediators to program efficacy also need to be examined. Previous research has supported that participants of weight loss or diabetes prevention programs who consistently self-monitor diet and physical activity behavior, and who are able to meet behavioral goals, demonstrate greater weight loss [49, 50]. Health literacy, self-efficacy, adherence to protocol, and stress may be other mediators to consider, particularly with adults at risk for T2D with low education and/or socioeconomic status.
Greater consistency in how weight loss outcomes are reported and analyzed is also indicated in future diabetes prevention translational research. In order to compare studies, data on mean weight loss in kilograms, mean percent of body weight loss, and the percent of participants meeting a 5% and 7% weight loss goal all need to be reported. Mean values can be biased by outliers who have extreme weight loss or gain. Participants meeting weight loss goals can be biased by attrition, with weight loss over-estimated when there is systematic attrition. To address these issues, assumptions of statistical analyses, treatment of outliers, and data management for program attendance and study completion should be specified. Analyses comparing completers to noncompleters on baseline weight, weight loss, and demographic characteristics will allow for determination of systematic attrition. Power analysis recalculations can determine if the final sample has adequate power to test the primary hypothesis. Careful consideration of missing data also needs to be undertaken as per protocol analyses or data imputations with the last observation carried forward may overestimate treatment effects. Statistical models accounting for incomplete data (i.e., mixed linear model, GEE model) are recommended [51]. To further improve methodological quality, more randomized trials and pilot studies need to be conducted.
Future research also needs to continue to explore mechanisms to improve access and outcomes. Financial incentive-based approaches for weight-loss, based on behavioral economics, are beginning to be explored [52] and may be particularly successful in persons with limited resources. Technology-based programs (i.e., telehealth, internet, smart phones), while potentially expensive to develop, may be a cost-effective intervention extender. Multilevel, multifaceted approaches involving individuals, families, communities, and policy have also been advocated for curtailing the rising prevalence of T2D. Lastly, research with larger sample sizes and more males as well as research examining cost-effectiveness, are also indicated.
CONCLUSION
Considerable research has translated the DPP protocol to different settings for adults at risk with T2D, with promising results. Ongoing development of innovative programs for diverse adults with low health literacy and low socioeconomic status are indicated. More rigorous evaluation of program reach, adoption, implementation, and maintenance is needed.
Acknowledgment
The author thanks Heather Jacobs, MPH for assistance in manuscript preparation
Footnotes
Implications
Practice: To obtain optimal reach, efficacy, adoption, implementation, and maintenance of diabetes prevention translational programs, a variety of programs, settings, and providers are necessary.
Policy: Resources for diabetes prevention programs are needed to enhance the ability to reach diverse adults at-risk for type 2 diabetes and to implement diabetes prevention programs in clinical and community settings.
Research: Future research needs to examine mechanisms for dissemination and implementation of diabetes prevention programs in clinical and community settings as well as examine mechanisms for efficient coordination of programs with follow-up.
References
- 1.Centers for Disease Control and Prevention (2011) National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States.
- 2.Eriksson KF, Lingarde F. Prevention of type 2 diabetes mellitus by diet and physical exercise: The 6-year Malmo feasibility study. Diabetologia. 1991;34:891–898. doi: 10.1007/BF00400196. [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Prevention Program Research Group 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;14:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colman E, Katzel LI, Rogus E, Coon P, Muller D, Goldberg AP. Weight loss reduces abdominal fat and improves insulin action in middle-aged and older men with impaired glucose tolerance. Metabolism. 1995;44(11):1502–1508. doi: 10.1016/0026-0495(95)90153-1. [DOI] [PubMed] [Google Scholar]
- 7.Rana JS, Li TY, Manson JE, Hu FB. Adiposity compared with physical inactivity and risk of type 2 diabetes in women. Diabetes Care. 2007;30(1):53–58. doi: 10.2337/dc06-1456. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association Standards of care in diabetes. Diabetes Care. 2009;32:S1–S61. doi: 10.2337/dc09-S001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman WH, Hoerger TJ, Brandle M, et al. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Annals of Internal Medicine. 2005;142(5):323–332. doi: 10.7326/0003-4819-142-5-200503010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Prevention Research Group Design and methods for a clinical trial in the prevention of T2D. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain A, Claussen B, Ramachandran A, Williams R. Prevention of type 2 diabetes: A review. Diabetes Research and Clinical Practice. 2007;76:317–326. doi: 10.1016/j.diabres.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Yamaoka K, Tango T. Efficacy of lifestyle education to prevent type 2 diabetes. Diabetes Care. 2005;28:2780–2786. doi: 10.2337/diacare.28.11.2780. [DOI] [PubMed] [Google Scholar]
- 13.Nield L, Summerbell CD, Hooper L, Whittaker V, Moore H. Dietary advice for the prevention of type 2 diabetes mellitus in adults. Cochrane Database Systematic Review. 2008;16(3):CD005102. doi: 10.1002/14651858.CD005102.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Orozco LJ, Buchieitner AM, Gimenez-Perez G, Roque I, Figuls M, Richter B, Mauricio D. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database Systematic Review. 2008;16(3):CD003054. doi: 10.1002/14651858.CD003054.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Satterfield DW, Volansky M, Caspersen CJ, et al. Community-based lifestyle interventions to prevent type 2 diabetes. Diabetes Care. 2003;26:2643–2652. doi: 10.2337/diacare.26.9.2643. [DOI] [PubMed] [Google Scholar]
- 16.Edwards K, Patchell B. State of the science: A cultural view of Native Americans and diabetes prevention. Journal of Cultural Diversity. 2009;16(1):32–35. [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson L. Translating the diabetes prevention program into practice: A review of community interventions. The Diabetes Educator. 2009;35:309–320. doi: 10.1177/0145721708330153. [DOI] [PubMed] [Google Scholar]
- 18.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. American Journal of Public Health. 1999;89:1322–1327. doi: 10.2105/AJPH.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. 2009;Version 5.0.2.
- 20.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. American Journal of Preventive Medicine. 2008;35(4):357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whittemore R, Melkus G, Wagner J, Dziura J, Northrup V, Grey M. Translating the diabetes prevention program to primary care: A pilot study. Nursing Research. 2009;58(1):2–12. doi: 10.1097/NNR.0b013e31818fcef3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McTigue KM, Conroy MB, Bigi L, Murphy C, McNeil M. Weight loss through living well: Translating an effective lifestyle intervention into clinical practice. Diabetes Educ. 2009;35(2):199–204. doi: 10.1177/0145721709332815. [DOI] [PubMed] [Google Scholar]
- 23.Vanderwood KK, Hall TO, Harwell TS, Butcher MK, Helgerson SD. Montana Cardiovascular Disease and Diabetes Prevention Program Workgroup. Implementing a state-based cardiovascular disease and diabetes prevention program. Diabetes Care. 2010;33(12):2543–2545. doi: 10.2337/dc10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBride PE, Einerson JA, Grant H, et al. Putting the Diabetes Prevention Program into practice: A program for weight loss and cardiovascular risk reduction for patients with metabolic syndrome or type 2 diabetes mellitus. The Journal of Nutrition, Health & Aging. 2008;12(10):745S–749S. doi: 10.1007/BF03028624. [DOI] [PubMed] [Google Scholar]
- 25.Pagoto SL, Kantor L, Bodenlos JS, Gitkind M, Ma Y. Translating the diabetes prevention program into a hospital-based weight loss program. Health Psychology. 2008;27(1 Suppl):S91–8. doi: 10.1037/0278-6133.27.1.S91. [DOI] [PubMed] [Google Scholar]
- 26.Vadheim LM, McPherson C, Kassner DR, et al. Adapted diabetes prevention program lifestyle intervention can be effectively delivered through telehealth. The Diabetes Educator. 2010;36(4):651–656. doi: 10.1177/0145721710372811. [DOI] [PubMed] [Google Scholar]
- 27.Kramer MK, Kriska AM, Venditti EM, et al. Translating the Diabetes Prevention Program: A comprehensive model for prevention training and program delivery. American Journal of Preventive Medicine. 2009;37(6):505–511. doi: 10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 28.McTigue KM, Conroy MB, Hess R, et al. Using the internet to translate an evidence-based lifestyle intervention into practice. Telemed J E Health. 2009;15(9):851–858. doi: 10.1089/tmj.2009.0036. [DOI] [PubMed] [Google Scholar]
- 29.Matvienko OA, Hoehns JD. A lifestyle intervention study in patients with diabetes or impaired glucose tolerance: Translation of a research intervention into practice. Journal of the American Board of Family Medicine. 2009;22(5):535–543. doi: 10.3122/jabfm.2009.05.090012. [DOI] [PubMed] [Google Scholar]
- 30.Mau MK, Keawe'aimoku Kaholokula J, West MR, et al. Translating diabetes prevention into native Hawaiian and Pacific Islander communities: The PILI 'Ohana Pilot project. Progress in Community Health Partnerships. 2010;4(1):7–16. doi: 10.1353/cpr.0.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seidel MC, Powell RO, Zgibor JC, Siminerio LM, Piatt GA. Translating the Diabetes Prevention Program into an urban medically underserved community: A nonrandomized prospective intervention study. Diabetes Care. 2008;31(4):684–689. doi: 10.2337/dc07-1869. [DOI] [PubMed] [Google Scholar]
- 32.Aldana S, Barlow M, Smith R, et al. A worksite diabetes prevention program: Two-year impact on employee health. AAOHN Journal. 2006;54(9):389–395. doi: 10.1177/216507990605400902. [DOI] [PubMed] [Google Scholar]
- 33.Boltri JM, Davis-Smith YM, Seale JP, Shellenberger S, Okosun IS, Cornelius ME. Diabetes prevention in a faith-based setting: Results of translational research. Journal of Public Health Management and Practice. 2008;14(1):29–32. doi: 10.1097/01.PHH.0000303410.66485.91. [DOI] [PubMed] [Google Scholar]
- 34.Davis-Smith YM, Boltri JM, Seale JP, Shellenberger S, Blalock T, Tobin B. Implementing a diabetes prevention program in a rural African–American church. Journal of the National Medical Association. 2007;99(4):440–446. [PMC free article] [PubMed] [Google Scholar]
- 35.Dodani S, Fields JZ. Implementation of the fit body and soul, a church-based life style program for diabetes prevention in high-risk African Americans: a feasibility study. The Diabetes Educator. 2010;36(3):465–472. doi: 10.1177/0145721710366756. [DOI] [PubMed] [Google Scholar]
- 36.Diabetes Prevention Program Research Group Costs associated with the primary prevention of type 2 diabetes in the Diabetes Prevention Program. Diabetes Care. 2003;26:36–47. doi: 10.2337/diacare.26.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katula JA, Vitolins MZ, Espeland M, et al. Translating diabetes prevention: Healthy living partnerships to prevent diabetes (HELP PD). Diabetes. 2010:1739-P. [DOI] [PMC free article] [PubMed]
- 38.Katula JA, Vitolins MZ, Rosenberger EL, et al. Healthy Living Partnerships to Prevent Diabetes (HELP PD): design and methods. Contemporary Clinical Trials. 2010;31(1):71–81. doi: 10.1016/j.cct.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albright A, Araujo R, Brownson C, et al. AADE position statement: Community health workers in diabetes management and prevention. The Diabetes Educator. 2009;35:48S–52S. doi: 10.1177/0145721709339140. [DOI] [Google Scholar]
- 40.Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annual Review of Public Health. 2007;28:413–433. doi: 10.1146/annurev.publhealth.28.021406.144145. [DOI] [PubMed] [Google Scholar]
- 41.Oakley A, Strange V, Bonell C, Allen E, Stephenson J. Process evaluation in randomized controlled trials of complex interventions. British Medical Journal. 2006;332:413–416. doi: 10.1136/bmj.332.7538.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castro FG, Barrera M, Martinez CR. The cultural adaptation of prevention interventions: Resolving tensions between fit and fidelity. Prevention Science. 2004;5:41–45. doi: 10.1023/B:PREV.0000013980.12412.cd. [DOI] [PubMed] [Google Scholar]
- 43.Santacroce SJ, Maccarelli LM, Grey M. Intervention fidelity. Nursing Research. 2004;53:63–66. doi: 10.1097/00006199-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Rollnick S, Miller WR, Butler CC. Motivational interviewing in health care. NY: Guilford Press; 2007. [Google Scholar]
- 45.Im EO, Lee B, Hwang H, et al. "A waste of time": Hispanic women's attitudes toward physical activity. Women & Health. 2010;50(6):563–579. doi: 10.1080/03630242.2010.510387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Treuhaft S, Karpyn A. The grocery gap: Who has access to healthy food and why it matters. 2010. http://www.policylink.org/site/c.lkIXLbMNJrE/b.5860321/k.A5BD/The_Grocery_Gap.htm.
- 47.Merriam PA, Tellez TL, Rosal M, et al. Methodology of a diabetes prevention translational research project utilizing a community–academic partnership for implementation in an underserved Latino community. BMC Medical Research Methodology. 2009;9:20. doi: 10.1186/1471-2288-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin EHB, Katon W, VonKorff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 49.Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obesity Review. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 50.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. The New England Journal of Medicine. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 51.Baron G, Ravaud P, Samson A, Giraudeau B. Missing data in randomized controlled trials of rheumatoid arthritis with radiographic outcomes: A simulation study. Arthritis and Rheumatism. 2008;59(1):25–31. doi: 10.1002/art.23253. [DOI] [PubMed] [Google Scholar]
- 52.Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: A randomized trial. Journal of the American Medical Association. 2008;300(22):2631–2637. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]