Abstract
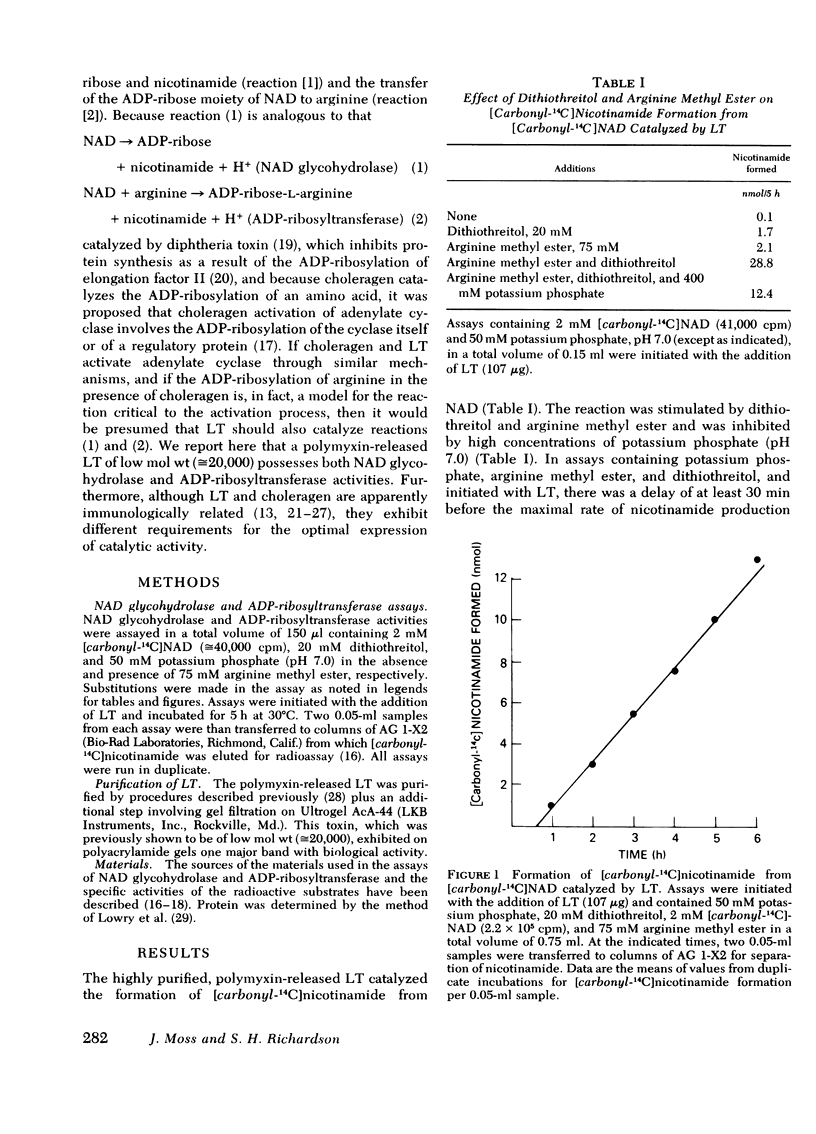
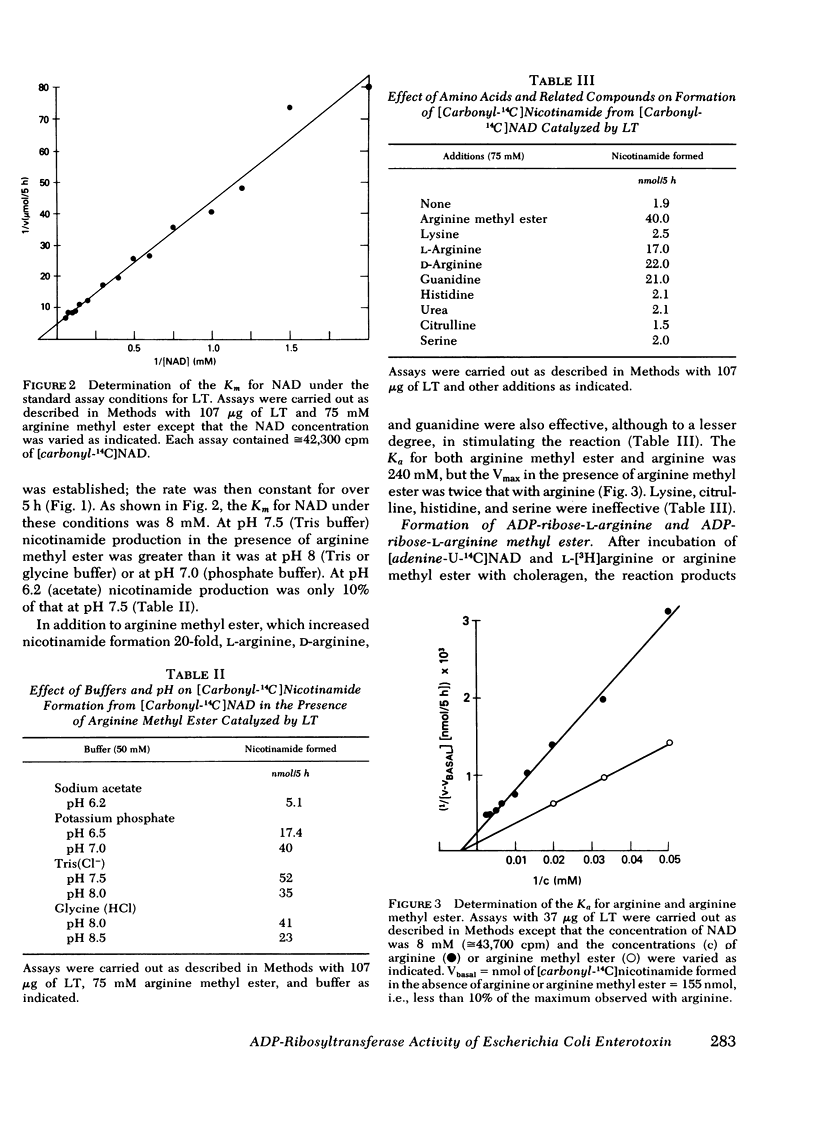
Highly purified, polymyxin-released, low molecular weight Escherichia coli heat-labile enterotoxin (LT) catalyzed the hydrolysis of NAD to ADP-ribose and nicotinamide. This NAD glycohydrolase activity was stimulated by dithiothreitol and was independent of cellular components. Nicotinamide formation was enhanced by arginine methyl ester > d-arginine ≅ l-arginine ≅ guanidine. A 20-fold increase in activity was noted with arginine methyl ester, and maximal activity again required dithiothreitol. When the reaction was initiated with toxin, a delay was observed before a constant rate was established. The reaction products found after incubation of [adenine-U-14C]NAD and l-[3H]arginine or unlabeled arginine methyl ester with the enterotoxin had mobilities on thin-layer chromatograms similar to the reaction products obtained after incubation of choleragen with these substrates and are consistent with the formation of ADP-ribose-l-arginine and ADP-ribose-l-arginine methyl ester, respectively. Both toxins, which catalyze the NAD-dependent activation of adenylate cyclase, thus appear to possess NAD glycohydrolase and ADP-ribosyltransferase activities. Although the activities of both toxins are dependent on dithiothreitol, Escherichia coli enterotoxin exhibited optimal activity in Tris (Cl−) (pH 7.5) and was inhibited by high concentrations of potassium phosphate (pH 7.0) or low pH (sodium acetate, pH 6.2). It appears that the optimal assay conditions as well as the kinetic constants for the reactants differ from those previously noted with choleragen. It is probable therefore that although the two toxins catalyze similar reactions, they differ in primary structure. The presence of transferase and glycohydrolase activities in structurally distinct toxins that activate adenylate cyclase strengthens our hypothesis that the ADP-ribosylation of arginine is a model for the NAD-dependent activation of adenylate cyclase; activation may result from ADP-ribosylation of the cyclase itself or of a protein that regulates its activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner F., Mayer P. Escherichia coli enterotoxin: stimulation of adenylate cyclase in broken-cell preparations. Infect Immun. 1975 Mar;11(3):429–435. doi: 10.1128/iai.11.3.429-435.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Chen L. C., Curlin G. T., Evans D. G. Stimulation of adenyl cyclase by Escherichia coli enterotoxin. Nat New Biol. 1972 Apr 5;236(66):137–138. doi: 10.1038/newbio236137a0. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Evan D. J., Jr, Evans D. G. Mechanism of activation adenylate cyclase in vitro by polymyxin-released, heat-labile enterotoxin of Escherichia coli. J Infect Dis. 1976 Mar;133 (Suppl):103–107. doi: 10.1093/infdis/133.supplement_1.s103. [DOI] [PubMed] [Google Scholar]
- Gill D. M. Involvement of nicotinamide adenine dinucleotide in the action of cholera toxin in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2064–2068. doi: 10.1073/pnas.72.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach S. L., Kean B. H., Evans D. G., Evans D. J., Jr, Bessudo D. Travelers' diarrhea and toxigenic Escherichia coli. N Engl J Med. 1975 May 1;292(18):933–936. doi: 10.1056/NEJM197505012921801. [DOI] [PubMed] [Google Scholar]
- Gyles C. L., Barnum D. A. A heat-labile enterotoxin from strains of Eschericha coli enteropathogenic for pigs. J Infect Dis. 1969 Oct;120(4):419–426. doi: 10.1093/infdis/120.4.419. [DOI] [PubMed] [Google Scholar]
- Gyles C. L. Immunological study of the heat-labile enterotoxins of Escherichia coli and Vibrio cholerae. Infect Immun. 1974 Mar;9(3):564–570. doi: 10.1128/iai.9.3.564-570.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyles C. L. Relationships among heat-labile enterotoxins of Escherichia coli and Vibrio cholerae. J Infect Dis. 1974 Mar;129(3):277–283. doi: 10.1093/infdis/129.3.277. [DOI] [PubMed] [Google Scholar]
- Hewlett E. L., Guerrant R. L., Evans D. J., Jr, Greenough W. B., 3rd Toxins of Vibrio cholerae and Escherichia coli stimulate adenyl cyclase in rat fat cells. Nature. 1974 May 24;249(455):371–373. doi: 10.1038/249371a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Söderlind O., Wadström T. Cross-reactivity between heat labile enterotoxins of Vibrio cholerae and Escherichia coli in neutralization tests in rabbit ileum and skin. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Dec;81(6):757–762. doi: 10.1111/j.1699-0463.1973.tb02272.x. [DOI] [PubMed] [Google Scholar]
- Honjo T., Nishizuka Y., Hayaishi O. Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis. J Biol Chem. 1968 Jun 25;243(12):3553–3555. [PubMed] [Google Scholar]
- Hynie S., Rasková H., Sechser T., Vanecek J., Matejovská D., Matejovská V., Treu M., Polák L. Stimulation of intestinal and liver adenyl cyclase by enterotoxin from strains of Escherichia coli enterpathogenic for calves. Toxicon. 1974 Mar;12(2):173–179. doi: 10.1016/0041-0101(74)90242-6. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel J., Collier R. J., Chung D. W. Interaction of fragment A from diphtheria toxin with nicotinamide adenine dinucleotide. J Biol Chem. 1974 Apr 10;249(7):2088–2097. [PubMed] [Google Scholar]
- Kantor H. S., Tao P., Gorbach S. L. Stimulation of intestinal adenyl cyclase by Escherichia coli enterotoxin: comparison of strains from an infant and an adult with diarrhea. J Infect Dis. 1974 Jan;129(1):1–9. doi: 10.1093/infdis/129.1.1. [DOI] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Immunological interrelationships between cholera toxin and the heat-labile and heat-stable enterotoxins of coliform bacteria. Infect Immun. 1977 Oct;18(1):110–117. doi: 10.1128/iai.18.1.110-117.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan C. N., Wishnow R. M. Escherichia coli enterotoxin-induced steroidogenesis in cultured adrenal tumor cells. Infect Immun. 1974 Jul;10(1):146–151. doi: 10.1128/iai.10.1.146-151.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mashiter K., Mashiter G. D., Hauger R. L., Field J. B. Effects of cholera and E. coli enterotoxins on cyclic adenosine 3',5'-monophosphate levels and intermediary metabolism in the thyroid. Endocrinology. 1973 Feb;92(2):541–549. doi: 10.1210/endo-92-2-541. [DOI] [PubMed] [Google Scholar]
- Merson M. H., Morris G. K., Sack D. A., Wells J. G., Feeley J. C., Sack R. B., Creech W. B., Kapikian A. Z., Gangarosa E. J. Travelers' diarrhea in Mexico. A prospective study of physicians and family members attending a congress. N Engl J Med. 1976 Jun 10;294(24):1299–1305. doi: 10.1056/NEJM197606102942401. [DOI] [PubMed] [Google Scholar]
- Moss J., Manganiello V. C., Vaughan M. Hydrolysis of nicotinamide adenine dinucleotide by choleragen and its A protomer: possible role in the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4424–4427. doi: 10.1073/pnas.73.12.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Osborne J. C., Jr, Fishman P. H., Brewer H. B., Jr, Vaughan M., Brady R. O. Effect of gangliosides and substrate analogues on the hydrolysis of nicotinamide adenine dinucleotide by choleragen. Proc Natl Acad Sci U S A. 1977 Jan;74(1):74–78. doi: 10.1073/pnas.74.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- Rowe B., Taylor J., Bettelheim K. A. An investigation of traveller's diarrhoea. Lancet. 1970 Jan 3;1(7636):1–5. doi: 10.1016/s0140-6736(70)90520-9. [DOI] [PubMed] [Google Scholar]
- Sack D. A., Kaminsky D. C., Sack R. B., Wamola I. A., Orskov F., Orskov I., Slack R. C., Arthur R. R., Kapikian A. Z. Enterotoxigenic Escherichia coli diarrhea of travelers: a prospective study of American Peace Corps volunteers. Johns Hopkins Med J. 1977 Aug;141(2):63–70. [PubMed] [Google Scholar]
- Shore E. G., Dean A. G., Holik K. J., Davis B. R. Enterotoxin-producing Escherichia coli and diarrheal disease in adult travelers: a prospective study. J Infect Dis. 1974 May;129(5):577–582. doi: 10.1093/infdis/129.5.577. [DOI] [PubMed] [Google Scholar]
- Smith N. W., Sack R. B. Immunologic cross-reactions of enterotoxins from Escherichia coli and Vibrio cholerae. J Infect Dis. 1973 Feb;127(2):164–170. doi: 10.1093/infdis/127.2.164. [DOI] [PubMed] [Google Scholar]
- Zenser T. V., Metzger J. F. Comparison of the action of Escherichia coli enterotoxin on the thymocyte adenylate cyclase-cyclic adenosine monophosphate system to that of cholera toxin and prostaglandin E1. Infect Immun. 1974 Sep;10(3):503–509. doi: 10.1128/iai.10.3.503-509.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


