ABSTRACT
Evidence supporting the benefits of exercise following the diagnosis of breast cancer is overwhelming and compelling. Exercise reduces the severity and number of treatment-related side effects, optimizes quality of life during and following treatment, and may optimize survival. Yet, exercise does not uniformly form part of the standards of care provided to women following a breast cancer diagnosis. This commentary summarizes the evidence in support of exercise as a form of adjuvant treatment and identifies and discusses potential issues preventing the formal integration of exercise into breast cancer care. Proposed within the commentary is a model of breast cancer care that incorporates exercise prescription as a key component but also integrates the need for surveillance and management for common breast cancer treatment-related morbidities, as well as education. While future research evaluating the potential cost savings through implementation of such a model is required, a committed, collaborative approach by clinicians, allied health professionals, and researchers will be instrumental in bridging the gap between research and practice.
KEYWORDS: Breast cancer, Exercise, Physical activity, Rehabilitation, Model of care
Evidence supporting the benefits of exercise following the diagnosis of breast cancer is overwhelming and compelling. Summarized in multiple meta-analyses and systematic reviews, the results from over 68 randomized, controlled trials involving exercise interventions in women with breast cancer have clearly demonstrated attenuation of treatment-related morbidity and multiple physical and psychosocial health benefits during and following treatment, all of which optimize quality of survival [1, 2]. Specifically, exercise interventions during and/or following breast cancer treatment can prevent decline and/or improve cardiorespiratory fitness, body composition (i.e., muscle mass and bone health), immune function, strength and flexibility, cognition, body image, self-esteem and mood, chemotherapy completion rates and allow for better adjustment to illness [3]. Exercise interventions have also been shown to reduce hospitalization duration, stress, depression, anxiety, and the number and severity of treatment side effects, including nausea, lymphedema, fatigue, and pain [3].
In addition, evidence from multiple cohort studies, summarized in a recent meta-analysis, demonstrates that post-diagnosis physical activity may be associated with 41% reduced all-cause mortality, 34% fewer breast cancer deaths, and 24% reduced risk of disease recurrence, with the effect independent of other prognostic factors [4]. Exercise also reduces risk of other comorbidities for which breast cancer survivors are at increased risk, including osteoporosis, diabetes, and heart disease [5]. This is especially important since breast cancer survivors are at least as likely to die of heart disease as of breast cancer [6, 7]. Importantly, the benefits accrued through participation in regular exercise extend well beyond the woman, influencing her role within the family, workplace, and broader society.
The global Exercise is Medicine® campaign (http://exerciseismedicine.org/) highlights exercise as an important mechanism for the prevention and treatment of chronic disease. Evidence in support of exercise as a form of adjuvant breast cancer treatment continues to mount. Importantly, all women diagnosed with breast cancer, irrespective of age, race, risk of morbidity, and stage of disease, can benefit from exercise during and following treatment [3]. Further, risk of adverse events and poorer quality of life may be higher in those who do not exercise [8, 9]. This is in contrast to other forms of adjuvant breast cancer treatment, such as chemotherapy, whereby survival benefits accrued are only available to women for whom this form of treatment is indicated (40–50% of breast cancer patients) [10], and are often accompanied by a myriad of side effects, some of which may persist for months or years [11]. This comparison is not being made to suggest exercise as a potential alternative to chemotherapy, but to help reinforce the evidence demonstrating the usefulness of exercise as an adjunct to breast cancer treatment, and one that is complementary to current standards of care. Yet, exercise is not a uniform component of breast cancer survivorship care. Potential issues influencing the integration of exercise as a form of adjuvant breast cancer treatment are proposed below:
CAPACITY FOR EXERCISE BEHAVIOR CHANGE
Approximately 85% of breast cancer survivors report being less physically active than recommended levels (150 min of moderate-intensity activity per week) [12], and women are more likely to reduce activity levels during and following treatment [13]. Not only do these women likely lack the knowledge and skills to become or stay sufficiently active but also breast cancer treatment for these women may result in physiologic and/or psychosocial changes that deepen the need for general and breast cancer-specific exercise know-how advice and support. For example, at least one in two women report upper-body morbidity and 40% experience upper-body function declines following breast cancer [14]. Common adverse treatment sequelae, such as fatigue, anxiety, and depression, also represent common exercise barriers, but ones that can be overcome through the health-promoting effects of exercise [1, 2, 15]. Further, even when self-reported pre-diagnosis physical activity levels are maintained, multiple cohort studies have shown that a significant minority of breast cancer survivors sustain long-term deficits in functional status compared to women who have not had breast cancer [16–18].
A diagnosis of breast cancer has been considered a “teachable moment” for positive changes in exercise behavior [19]. Also, results from exercise intervention studies demonstrate high adherence rates (70–90%) to the exercise intervention [20], indicating that participation in exercise is feasible. Taken together, these findings suggest there is a need and capacity for change.
OPTIMAL TIMING AND DOSE OF EXERCISE
Exercise interventions have been initiated within 6 weeks post-surgery, during adjuvant therapy, and up to many years following treatment [1]. Exercise interventions during adjuvant therapy have been associated with prevention in declines in function and fewer and less severe treatment-related side effects [1, 21]. Exercise posttreatment assists to mitigate symptoms and to optimize recovery [1, 21]. Ultimately, when exercise is prescribed progressively and is individualized to accommodate each woman’s function and symptoms, exercise can be commenced at any time following a breast cancer diagnosis [3].
While the dose of exercise evaluated in the many randomized, controlled trials involving women with breast cancer varies significantly, findings from this work have been used to derive exercise prescription guidelines [3, 21, 22]. As a minimum, benefits have been observed in exercise interventions involving aerobic- and/or resistance-based exercise performed at least three sessions per week, for at least 30 min per session, at moderate intensity [3, 21]. When survival is the outcome of interest, there is some evidence derived from cohort studies to suggest a dose–response relationship exists [3], whereby more exercise is better than less, but possibly only up to levels meeting national recommendations (150 min of moderate-intensity activity per week). While the current evidence does not allow inferences to be made about the lower and upper thresholds of exercise required to achieve benefits, individualizing starting exercise levels and pace for progression is considered a crucial component of exercise prescription for women with breast cancer [3]. Exercise is like any other form of treatment—its efficacy is dependent on appropriate prescription of type, dosing, and timing that is specific to each woman.
SAFETY OF EXERCISE
Exercise in cancer survivors is considered to be safe. Among the studies that have reported adverse effects, they have been rare and mild in nature (e.g., plantar fasciitis from walking and other musculoskeletal injuries) [20, 21]. However, it is important to remember that a response bias exists in exercise intervention trials, with participants likely to be younger, healthier, and have a history of exercise participation, compared with the wider breast cancer cohort. It is therefore important to avoid complacency with exercise prescription, and also highlights the need for exercise to be prescribed by trained health professionals, such as exercise physiologists/kinesiologists, and undertaken in supervised environments until exercise safety has been established within clinical settings (beyond research settings).
POTENTIAL FOR INITIATION OR EXACERBATION OF TREATMENT-RELATED CONCERNS
Fatigue and lymphedema merit special attention in this regard as they represent symptoms that have previously been treated with rest. However, evidence demonstrates that it is too much sedentary behavior rather than participation in a progressive exercise program that is likely to exacerbate or contribute to the development of these conditions [9, 23]. Also, evidence from supervised exercise intervention trials demonstrates that at worst, exercise has no effect on existing fatigue and lymphedema, but may reduce symptom severity and exacerbation [15, 24, 25]. However, again, this evidence is derived from supervised programs under the direction of trained professionals during times when these symptoms are already present or were most likely to develop.
POTENTIAL COST OF INTEGRATING EXERCISE INTO BREAST CANCER CARE
This is a more difficult issue to deal with as the cost-effectiveness of exercise as a form of adjuvant breast cancer treatment is yet to be established, and there is a real need for more research in this area. Further, who pays will at least be partly dependent on the national health system in place for any given country. Many European countries have inpatient and/or outpatient cancer rehabilitation broadly available to cancer patients [26]. The extent to which this programming includes exercise is not clear. Given the concerns with costs, it is pertinent to highlight that exercise treatment has the potential to lead to significant short- and longer-term cost savings, by way of preventing disability, reducing symptom severity, or alleviating common treatment-related sequelae such as fatigue, lymphedema, and pain. Further, these potential cost savings will likely extend beyond the oncology treatment setting by influencing a woman’s ability to function effectively within her home, work environment, and community.
PROPOSED MODEL OF BREAST CANCER COLLABORATIVE CARE THAT INCORPORATES EXERCISE
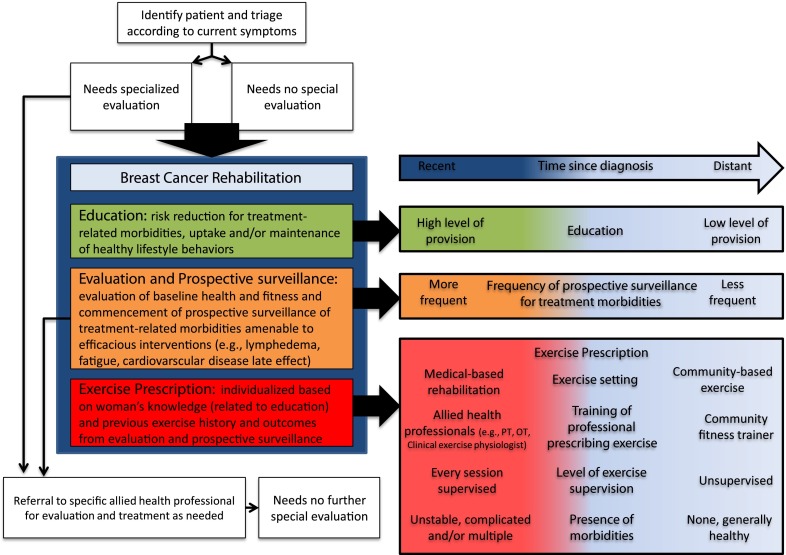
Figure 1 represents a proposed breast cancer rehabilitation model which incorporates exercise prescription as a core component but in doing so recognizes the importance of education, evaluation, and prospective surveillance of treatment-associated morbidity. This model draws on experiences learned in the cardiac rehabilitation setting [27] but integrates recommendations in a way that is relevant to the breast cancer setting. The model proposes that all women, irrespective of age, presence of comorbidities, stage of treatment, and presence of treatment-related sequelae, should be referred to breast cancer rehabilitation, which includes exercise prescription, although under certain circumstances, formal entry may be delayed until assessment and appropriate management of specific disease or treatment-related concerns can occur.
Fig 1.
Proposed breast cancer rehabilitation model which integrates surveillance, prevention education, and management of treatment-associated morbidity and exercise prescription
The model relies on triaging breast cancer patients for referral to appropriate allied health professionals according to need and referral to breast cancer rehabilitation. The appropriate medical professional to address this “triage” step might be the breast care nurses as they are now established internationally and play a key role in supporting women in coping with the impact of the disease and its treatment [28]. However, the specifics of this process will likely be dependent on the strengths and staffing of a particular setting.
Entry into breast cancer rehabilitation involves commencement of education, which over time will cover issues including recognition and risk-reduction strategies of treatment-related morbidities and uptake or maintenance of healthy lifestyle behaviors. Evaluation of baseline health and fitness and commencement of prospective surveillance of relevant morbidities should occur. Using information derived from the evaluation component, and building on education strategies, women should formally receive an exercise prescription. All three components of the model are integrated, informing each other, and in doing so will help to ensure that individual patient circumstances, such as accommodations needed for work, family, and societal roles, as well as changing symptoms during active treatment, are considered in the provision of care. For example, while exercise prescription will work to minimize risk of developing treatment-related concerns, integration of the model into breast cancer care will also enable early identification and management of treatment-related morbidities should they present. If morbidities do present, patients stay within the model but are also referred for more specific evaluation and treatment.
In the early phase of the proposed breast cancer rehabilitation model, level of education and evaluation is high, with more frequent surveillance of morbidities. Exercise is undertaken in supervised settings, under the direction of qualified health professionals, with exercise goals being short term in nature (focused on weekly exercise targets), with flexibility to accommodate change in presence and/or severity of treatment-related morbidities. However, over time, provision of information is reduced, prospective surveillance of morbidities declines, and exercise is undertaken in community-based settings, with or without formal prescription from a health professional. The later phase of breast cancer rehabilitation represents the time at which women have the capacity, skills, and know-how to independently monitor presence and change in persistent treatment-related symptoms and to know how to respond when adverse changes occur. It also represents the time when safety of participating in regular exercise, supervised or unsupervised, has been established, and exercise goals begin to have a longer-term focus (e.g., sustaining health behavior change in exercise habits). While the later phase of breast cancer rehabilitation may be well handled by broadly disseminated programs like those administered by breast cancer support groups or YMCA’s initiatives, it should not be dependent on the availability of such programs.
Movement within the components of education, surveillance, and exercise prescription may occur at different paces. For example, a woman who has a history of exercising regularly may require comparatively less exercise prescription support and advice following her breast cancer diagnosis but may require high levels of education relating to understanding common treatment-related concerns and ways to identify them. Also, some women may systematically progress through breast cancer rehabilitation towards low levels of education, surveillance, and exercise prescription support and advice (e.g., a woman who has had a lumpectomy with no adjuvant therapy and who has not experienced any treatment-related side effects). Others may find they progress and regress along the breast cancer rehabilitation continuum (e.g., a woman who develops lymphedema at 6 months post-diagnosis may move back to more highly supervised, medically prescribed exercise for a period, despite being on an independent exercise in a community-based setting prior to her lymphedema diagnosis). Nonetheless, it is anticipated that the vast majority of women will have entered the later phases of breast cancer rehabilitation, categorized by low levels of education, less frequent symptom evaluation, and minimal (to no) levels of supervised exercise advice and support by 12 months post-diagnosis. The flexible nature of the proposed model seeks to optimize efficiency of the model and minimize associated costs.
The model acknowledges that for many treating centers, in particular centers that sit within well-resourced health systems, there already exists a breast cancer care model that integrates a multidisciplinary team in the management of women with breast cancer. However, the current efficiencies of any given system vary widely. Even when a model that integrates a multidisciplinary team exists, it is plausible that at times, if not frequently, it operates on a “squeaky wheel” approach. That is, those of higher socioeconomic status who have the capacity to follow-up change in treatment-related symptoms will be more likely referred, evaluated, and treated, compared with those of poorer socioeconomic status [29]. This is a system which contributes to the health disparities commonly observed across socioeconomic status and ethnicity for breast cancer-related morbidities and mortality [30, 31].
The model proposed here seeks to utilize, formalize, and expand on existing infrastructure, health professionals, and resources and to do so in a way that is advantageous to all women with breast cancer. Specifically, the model will not only enable early integration of exercise as a form of adjuvant treatment but will aid prevention, early detection, and management of treatment-related symptoms [32]. We acknowledge that this is not the first time a breast cancer rehabilitation model has been proposed; nor are we the first to advocate for the integration of exercise into survivorship care for women with breast cancer. It may also be perceived as an idealist model and one that would certainly be implemented if there exists the infrastructure and resources to support such a model. That time seems to be approaching. As indicated earlier, guidelines for exercise prescription for women with breast cancer already exist [3, 21, 22], as do tertiary-trained health professionals who have the skills and experience in prescribing such treatment. Further, a number of programs have been developed and disseminated at the community level that could be utilized as part of the later phase of breast cancer rehabilitation, such as the YWCA Encore program in the USA and Australia, the LIVESTRONG at the YMCA program in the USA, or the Swedish physical activity referral scheme [33]. Nonetheless, there will likely be some “tipping point” that will lead to breast cancer rehabilitative and exercise programming becoming part of “standard of care.” With cardiac rehabilitation, there was never a single definitive randomized trial that established that exercise reduced recurrence or cardiac mortality, though a meta-analysis finally established this causal link in 1988 [34]. There was, however, evidence that exercise rehabilitation could get patients out of the hospital sooner and assist patients in returning to their roles within family and society [35]. For breast cancer rehabilitation, there is yet to be a randomized controlled trial of the effects of exercise on survival and breast cancer recurrence and/or one that confirms cost-effectiveness, which would improve insurance reimbursement of these programs. However, if the cardiac rehabilitation story teaches us anything, it may be that we need not wait for findings derived from such trials to push toward a model of breast cancer rehabilitation that incorporates exercise prescription. Whatever the tipping point may be, it is clear that a change in practice will require collaborative efforts between those involved with the management of women with breast cancer, with a particular emphasis on active support for integrating exercise as a form of adjuvant therapy from clinicians. Together, researchers, clinicians, and allied health professionals can help bridge the gap between what is fast becoming public knowledge about the important role of exercise for a variety of health outcomes following a breast cancer diagnosis and the provision of high-quality rehabilitative exercise programming to all breast cancer patients as standard care.
Footnotes
Implications
Researchers: Future work must seek to address the limitations in existing exercise and breast cancer literature, with a particular emphasis on recruiting a representative sample of women with breast cancer.
Practitioners: Sufficient evidence exists to encourage patients to follow broad physical activity guidelines during and following treatment for breast cancer (that is, “something is better than nothing, more is better than less, but progression should be slow and based on symptom response”) and to advocate for the integration of more formal exercise prescription into their care plan.
Policymakers: Consideration must be given to the potential short- and longer-term cost savings at the individual and public health level by integrating exercise prescription into the care plan of women with breast cancer.
References
- 1.Speck R, Courneya KS, Masse L, Duval S, Schmitz K. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Journal of Cancer Survivorship: Research and Practice. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiology, Biomarkers & Prevention. 2005;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 3.Hayes S, Spence R, Galvao D, Newton R. Australian Association for Exercise and Sport Science position stand: optimising cancer outcomes through exercise. Journal of Science and Medicine in Sport. 2009;12:428–434. doi: 10.1016/j.jsams.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim, E., & Al-Homaidh, A. (2010). Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Medical Oncology. doi:10.1007/s12032-010-9536-x. [DOI] [PubMed]
- 5.Physical Activity Guidelines Advisory Committee Report. Washington, DC: US Department of Health and Human Services; 2008. [Google Scholar]
- 6.Hanrahan E, Gonzalez-Angulo A, Giordano S, et al. Overall survival and cause-specific mortality of patients with stage T1a, bN0M0 breast carcinoma. Journal of Clinical Oncology. 2007;25(31):4952–4960. doi: 10.1200/JCO.2006.08.0499. [DOI] [PubMed] [Google Scholar]
- 7.Reeves K, Faulkner K, Modugno F, et al. Body mass index and mortality among older breast cancer survivors in the Study of Osteoporotic Fractures. Cancer Epidemiology, Biomarkers & Prevention. 2007;16(7):1468–1473. doi: 10.1158/1055-9965.EPI-07-0051. [DOI] [PubMed] [Google Scholar]
- 8.DiSipio T, Hayes S, Newman B, Janda M. What determines the health-related quality of life among regional and rural breast cancer survivors. Australian and New Zealand Journal of Public Health. 2009;33(6):534–539. doi: 10.1111/j.1753-6405.2009.00449.x. [DOI] [PubMed] [Google Scholar]
- 9.Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphoedema following breast cancer: incidence, risk factors and effect on upper body function. Journal of Clinical Oncology. 2008;26(21):3536–3542. doi: 10.1200/JCO.2007.14.4899. [DOI] [PubMed] [Google Scholar]
- 10.DiSipio T, Hayes S, Newman B, Aitken J, Janda M. Does quality of life among breast cancer survivors one year after diagnosis differ depending on urban and non-urban residence? A comparative study. Health and Quality of Life Outcomes. 2010;8:3. doi: 10.1186/1477-7525-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hewitt, M., Greenfield, S., & Stovall, E. (2006). From cancer patient to cancer survivor: lost in transition. In Committee on Cancer Survivorship: Improving Care and Quality of Life, Institute of Medicine and National Research Council (Eds.). The National Academic Press: Washington DC
- 12.Smith S, Chagpar A. Adherence to physical activity guidelines in breast cancer survivors. The American Surgeon. 2010;76(9):962–965. [PubMed] [Google Scholar]
- 13.Irwin ML, Crumley D, McTiernan A, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97:1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes, S., Rye, S., Battistutta, D., DiSipio, T., & Newman, B. (2010). Upper-body morbidity following breast cancer treatment is common, may persist longer-term and adversely influences quality of life. Health and Quality of Life Outcomes, 8(92). [DOI] [PMC free article] [PubMed]
- 15.McNeely M, Courneya K. Exercise programs for cancer-related fatigue: evidence and clinical guidelines. Journal of the National Comprehensive Cancer Network. 2010;8(8):945–953. doi: 10.6004/jnccn.2010.0069. [DOI] [PubMed] [Google Scholar]
- 16.Sweeney C, Schmitz K, Lazovich D, Virnig B, Wallace R, Folsom A. Functional limitations in elderly female cancer survivors. Journal of the National Cancer Institute. 2006;98(8):521–529. doi: 10.1093/jnci/djj130. [DOI] [PubMed] [Google Scholar]
- 17.Michael Y, Kawachi I, Berkman L, Holmes M, Colditz G. The persistent impact of breast carcinoma on functional health status: prospective evidence from the Nurses' Health Study. Cancer. 2000;89(11):2176–2186. doi: 10.1002/1097-0142(20001201)89:11<2176::AID-CNCR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Ness K, Wall M, Oakes J, Robinson L, Gurney J. Physical performance limitations and participation restrictions among cancer survivors: a population-based study. Annals of Epidemiology. 2006;16(3):197–205. doi: 10.1016/j.annepidem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Demark-Wahnefried W, Aziz N, Rowland J, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. Journal of Clinical Oncology. 2005;23(24):5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Courneya JRMS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Canadian Medical Association Journal. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitz K, Courneya K, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine and Science in Sports and Exercise. 2010;43(1):195. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 22.Campbell A, Foster J, Stevinson C. The importance of physical activity for people living with and beyond cancer: a concise evidence review. London: Macmillan Cancer Support; 2011. [Google Scholar]
- 23.Johansson K, Ohlsson K, Ingvar C, Albertsson M, Ekdahl C. Factors associated with the development of arm lymphedema following breast cancer treatment: a match pair case–control study. Lymphology. 2002;35(2):59–71. [PubMed] [Google Scholar]
- 24.Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer-related lymphoedema. The New England Journal of Medicine. 2009;361(7):661–673. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz K, Ahmed R, Troxel A, et al. Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA: Journal of the American Medical Association. 2010;304(24):2699–2705. doi: 10.1001/jama.2010.1837. [DOI] [PubMed] [Google Scholar]
- 26.Hellbom M, Bergelt C, Bergenmar M, et al. Cancer rehabilitation: a Nordic and European perspective. Acta Oncologica. 2011;50(2):179–186. doi: 10.3109/0284186X.2010.533194. [DOI] [PubMed] [Google Scholar]
- 27.Leon AS, Franklin BA, Costa F, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2005;111(3):369–376. doi: 10.1161/01.CIR.0000151788.08740.5C. [DOI] [PubMed] [Google Scholar]
- 28.Cruickshank, S., Kennedy, C., Lockhart, K., Dosser, I., & Dallas, L. (2008). Specialist breast care nurses for supportive care of women with breast cancer. The Cochrane Database of Systematic Reviews Jan 23(1), CD005634. [DOI] [PubMed]
- 29.Cheville AL, Beck LA, Petersen TL, Marks RS, Gamble GL. The detection and treatment of cancer-related functional problems in an outpatient setting. Supportive Care in Cancer. 2009;17(1):61–67. doi: 10.1007/s00520-008-0461-x. [DOI] [PubMed] [Google Scholar]
- 30.Deimling G, Sterns S, Bowman K, Kahana B. The health of older-adult, long-term cancer survivors. Cancer Nursing. 2005;28(6):415–424. doi: 10.1097/00002820-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Meeske K, Sullivan-Halley J, Smith A, et al. Risk factors for arm lymphedema following breast cancer diagnosis in Black women and White women. Breast Cancer Research and Treatment. 2009;113(2):383–391. doi: 10.1007/s10549-008-9940-5. [DOI] [PubMed] [Google Scholar]
- 32.Johansson K, Branje E. Arm lymphoedema in a cohort of breast cancer survivors 10 years after diagnosis. Acta Oncologica. 2010;49(2):166–173. doi: 10.3109/02841860903483676. [DOI] [PubMed] [Google Scholar]
- 33.Leijon M, Bendtsen P, Nilsen P, Festin K, Stahle A. Does a physical activity referral scheme improve the physical activity among routine primary health care patients. The Scandinavian Journal of Medicine and Science in Sports. 2009;19(5):627–636. doi: 10.1111/j.1600-0838.2008.00820.x. [DOI] [PubMed] [Google Scholar]
- 34.Oldridge N, Guyatt G, Fishcher M, Rimm A. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA: Journal of the American Medical Association. 1988;260(7):945–950. doi: 10.1001/jama.1988.03410070073031. [DOI] [PubMed] [Google Scholar]
- 35.Wenger N. Rehabilitation after myocardial infarction. JAMA: Journal of the American Medical Association. 1979;242(26):2879–2881. doi: 10.1001/jama.1979.03300260049030. [DOI] [PubMed] [Google Scholar]