ABSTRACT
Secondary stroke prevention is championed as guideline care; yet there are no systematic programs offered. We developed a stroke self-management program to address this gap and pilot test the program. We conducted a randomized controlled trial at two Veterans Administration (VA) hospital sites where we recruited patients with an acute stroke to receive either the stroke program or an attention-control protocol over a 12-week period following hospital discharge. The stroke program included six sessions that facilitated stroke self management focusing on increasing self-efficacy to recover from stroke and engage in secondary stroke risk factor management. We surveyed outcomes at baseline, 3 and 6 months. We conducted an intention to treat analysis comparing the intervention to the control group on changes of outcomes between baseline and follow-up modeled by a linear model with fixed effects for treatment, visit, and the treatment by visit interaction adjusting for baseline. We recruited 63 participants (33 control and 30 intervention) who were hospitalized with a primary diagnosis of ischemic stroke. We found trends in differences between groups on self-efficacy to communicate with physicians, weekly minutes spent in aerobic exercise, and on dimensions of stroke-specific quality of life. This pilot study demonstrated the feasibility of delivering a stroke self-management program to recent stroke survivors in a healthcare organization. The program also demonstrated improvements in patient self-efficacy, self-management behaviors, specific dimensions of stroke-specific quality of life compared to a group that received an attention placebo program.
KEYWORDS: Self-efficacy, Behavioral interventions, Stroke health‐related quality of life
INTRODUCTION
Stroke is a high-volume medical condition. On average, one United States citizen experiences a stroke every 45 s [1]. Stroke affects 700,000 persons each year in the United States, of which 200,000 are recurrent episodes [1]. Stroke is the third leading cause of death, produces the greatest number of hospitalizations for neurological disease [2], and is the leading cause of adult acute-onset and long-term disability in the United States [2–4]. Up to 50% of stroke survivors have some functional disability within 6 months of an ischemic stroke event [5–7]. Stroke is estimated to affect at least 15,000 veterans each year with estimated costs of $111 million for acute inpatient care, $75 million for post acute inpatient care, and $88 million for follow-up care over 6 months post-stroke [8].
Moreover, patients who have had a stroke or a transient ischemic attack (TIA) are at risk for recurrent stroke and death [1, 9, 10]. Over 12% of those with stroke or TIA will experience another event within a year [1, 9, 10]. A recent study of Canadian TIA survivors reported a 22% rate of recurrent vascular events [11]. Although some stroke risk factors are not modifiable (e.g., age) [1], many stroke risk factors are modifiable (e.g., hypertension, physical inactivity) [1]. Modifiable risk factors are most effectively managed through a combination of lifestyle and medication management and therefore require strategies that target and support behavioral modification and the uptake of prescribed therapies by clinical providers [12–14].
Stroke prevention efforts
Most patients who have had a stroke or TIA do not have adequate control of their stroke risk factors. Data from the national behavioral risk factor survey indicated only 3% of adults living in the US reported healthy lifestyle characteristics (e.g., nonsmoking, healthy weight, regular physical activity, and consumption of five fruits and vegetables per day) [1]. In a randomized trial of high-dose folate vitamin therapy in patients with recent stroke, stroke survivors in the trial continued lifestyle practices that elevated their stroke risk (e.g., cigarette smoking) [15]. Many studies have documented inadequate control of blood pressure after vascular events [16]. Despite knowledge of the methods and impact of risk reduction, providers may not aggressively counsel or treat patients with behavioral or medical interventions for stroke prevention [12].
Patient self-management programs
During the past several decades, patient self-management (PSM) programs were developed to foster self care among patients with chronic disease [17–21]. Variation exists in terms of the program components by disease (e.g., diabetes, arthritis), location of the program within the health care system (e.g., coordinated care, community-based) and staff involvement (e.g., clinical provider, peer leader) [22]. The delivery format also has varied across programs including delivery by mail, internet, group meetings, and telephone appointments. A recent Cochrane review stated that the most effective method for fostering PSM skills is unknown [56]. Experts define PSM as strategies that enable the patient’s ability to monitor and manage daily health and symptoms, to problem-solve to overcome barriers encountered, to modify lifestyle risk factors, and to communicate with clinical providers as active collaborators in defining and adhering to health and therapeutic goals [17, 18, 23–25]. The Chronic Disease Self-Management Program is a program that fosters such PSM strategies.
Social cognitive theory and patient self-management
We based our self-management intervention on the Stanford University Chronic Disease Self-Management Program, a program centered around enhancing patient self-efficacy to manage symptoms [17, 26, 27]. Self-efficacy, defined as the level of confidence in one’s ability to perform a specific behavior, is a concept within social cognitive theory and is the link between knowledge and action [28]. Patients may possess health knowledge, but may perceive themselves as incapable of performing health behaviors. From our formative evaluation with stroke survivors and their significant others, ischemic stroke survivors may lack self-efficacy to engage in self-management activities to foster recovery and risk factor management as they may question their abilities. (Patients surviving hemorrhagic stroke may be limited by the residual disability to participate in such programs.) According to the social cognitive theory, a person’s behavior is predicted by the confidence in ability to perform a specific behavior in a given situation and the outcome expectations of behavior performance [28, 29]. Bandura [28] suggests that we are constantly processing, evaluating, and reevaluating information about our strengths and weaknesses, which form the basis for perceptions of abilities (i.e., self-efficacy). We evaluate even more when we encounter problems or new situations. Self-efficacy, in turn, affects the behaviors we choose or avoid and the amount of effort put forth to perform those behaviors (e.g., treatment adherence).
Self-efficacy perceptions are influenced by a variety of cognitive determinants, including: (1) verbal persuasion; (2) past experiences or performance accomplishments; and (3) vicarious experiences or learning through observation of events or other people. We will specifically address these cognitive determinants in our self-management program [28, 29]. Physiological states (i.e., depression) also provide information to the person that may affect self-efficacy, so we include symptom awareness in the program.
The evidence on the effectiveness of PSM programs varies across chronic conditions. Randomized controlled trials of PSM programs have demonstrated improvements in outcomes for chronic medical conditions [26] including asthma [30], low back pain [31, 32], hypertension [33–35], arthritis [36, 37], and diabetes [38]. In addition, PSM programs offer patients options and strategies for coping with chronic medical conditions in order to function daily and maintain health-related quality of life (HRQOL). Patients with chronic disease are often left on their own to cope and manage their symptoms in between medical visits [39]. Similarly, patients recovering from stroke may participate in rehabilitation therapy during the acute stroke phase [40, 41], but are often left on their own to navigate coping with recovery. Post stroke, the survivor may be consuming several medications to manage risk factors, coping with physical and cognitive deficits, coping with post stroke anxiety and depression, and adjusting to new family, social and work roles.
From the literature, we identified only one study underway comparing a stroke program entitled “Getting Your Life Back After Stroke” to the Chronic Disease Self-Management program for chronic disease [42]. This stroke program includes working with health professionals, fostering a healthy lifestyle, access to a case manager for home needs, and information on local support groups. Unfortunately, there are no systematic programs to address the coping and recovery needs of stroke survivors with demonstrated improvements in patient-centered outcomes.
We conducted a formative evaluation with key stroke stakeholders (i.e., stroke survivors, caregivers, and clinical providers involved in stroke care at the VA medical center) to best understand the pressing needs of stroke survivors. We incorporated consistent themes into the program. In addition, the stroke self management program applied theoretical concepts of Bandura’s Self-Efficacy to the intervention components: Verbal Persuasions (e.g., doctor recommendations), Social Modeling/Vicarious Experiences (e.g., learning from other stroke patients experiences), Past Achievements (e.g., smoking relapse), and Reinterpretation of Sensations/Physical State (e.g., use of distraction and relaxation methods to thwart depression and anxiety). The program elements mapped onto self-efficacy concepts are listed in Table 1.
Table 1.
Stroke self-management strategies mapped onto self-efficacy theoretical concepts
| Self-efficacy theoretical concepts | Mapped stroke self-management strategies |
|---|---|
| 1. Verbal persuasions | • Staff and lay leader explanations |
| • Promotion of physician and therapeutic recommendations | |
| 2. Social modeling/vicarious experiences | • Staff and lay leader demonstration of all strategies and new behaviors |
| • Peers practice behavior in group meetings and share vicarious experiences | |
| • View material depicting stroke patients similar to them | |
| 3. Past achievements/failures | • Set realistic, achievable behavioral goals |
| • Past failures may influence current level of effort so plan to overcome past failures | |
| • Gain an understanding of the course of stroke rehabilitation and realistic outcome expectations | |
| • Negotiate the effort placed into cardiovascular risk factor modification | |
| 4. Reinterpretation of sensations/physical state | • Discuss symptoms and how to diminish |
| • Employ distraction methods — mental imagery | |
| • Employ relaxation techniques |
The objective of this study was to pilot test the effect of our developed stroke specific, self-management intervention from our formative evaluation of stroke survivors and caregivers, and clinical providers of stroke patients to determine feasibility and estimate the effect on functioning and quality of life. Our primary hypothesis was veterans receiving the self-management intervention will demonstrate improvements in stroke specific, HRQOL, self-efficacy, and self-management behaviors compared to veterans receiving an attention placebo control group.
METHODS
Design
We employed a prospective, randomized trial to assess the impact of a self-management program vs. attention placebo control group among veteran stroke survivors at the two facilities. Outcomes were assessed by trained interviewers blinded to treatment assignment. Participants were identified during admission for ischemic stroke and were randomized in blocks of four, stratified by site and by receipt of inpatient rehabilitation (as a marker of stroke severity denoting more disability), to the PSM intervention or attention placebo control intervention. Of the total sample, nine (14%) subjects received inpatient rehabilitation and three were subsequently randomized to the intervention and six were randomized to the attention control group. Enrollment took place within a month of stroke discharge. Participants were consented using standard procedures in compliance with local IRB and VA guidelines. All reported procedures were approved by the local IRB and VA Research and Methodology Committees.
Intervention
The program included six biweekly telephone sessions to deliver the stroke self-management program or a placebo telephone call program that mimicked the intervention schedule. Sessions targeted outcomes of stroke self-management and self-efficacy to improve stroke specific quality of life. The self-management program followed a standardized manual (placebo call simply asked how the patient was doing), and interviews were conducted at 3 and 6 months with a booster call at 4.5 months. We based our self-management intervention on the Chronic Disease Self-Management Program, a program centered on enhancing patient self-efficacy to manage symptoms and fostering behavioral change given the low self-efficacy to perform such behaviors after stroke [26, 27].
Based upon the underlying theory and the results of our formative evaluation with key stakeholders of veterans and caregivers, and clinical providers of stroke care in the VA [39], we created a menu of stroke self-management topics that were incorporated into a six-session program with a written standardized manual delivered biweekly primarily by telephone (see Appendix 1). Less than 10% received the program in person due to hearing difficulties that made the telephone delivery difficult. The stroke specific portion of the program included an overview of stroke, typical changes caused by stroke, warning signs of stroke, recovery from stroke, dealing with fears of stroke survivors, rehabilitation services and recovery after stroke (keeping appointments and practicing prescribed home therapy), creating a daily schedule (to minimize complete inactivity as frequently reported both by stroke survivors and caregivers), adapting/coping with stroke related disability, modifying stroke risk factors, adapting new roles after stroke, reaching out to other stroke survivors and community resources for stroke related needs. Each session targeted building self-efficacy using goal setting and behavioral contracting. Each patient was coached to choose at least one specific goal to work on in each session, including the specifics of when, where, and for how long they would do the specific behavior. Patients rated their confidence in carrying out each behavior and were encouraged to only select behaviors that they rated a 7 out of a possible 10 (completely confident in doing). In the follow-up telephone calls, patients received individualized feedback about their progress toward their selected goal(s) and were encouraged to continue to work on the chosen behavior or to select or add a new behavior goal for the next 2 weeks. The average length of a session was approximately 20 min and the instructors were a nurse, a physician assistant, and a master’s level social scientist. All had received 18 h of standardized training prior to patient instruction.
Enrollment, consent and other patient variables were collected at baseline (prior to hospital discharge or within 4 weeks of discharge). Outcomes were collected via telephone at 3 months (primary outcome) and 6 months (sustainability) post-enrollment. Patients assigned to usual care received stroke education pamphlets and a general stroke education session at enrollment, and phone calls to assess stroke symptoms (with no self-management training) at identical time points as intervention patients as an active attention control.
Telephone calls
The purpose of the telephone calls in the self-management program was twofold. First, half of the self-management intervention sessions were delivered by telephone during the first 6 months given that transportation and mobility barriers hindered participation in a program delivered by group sessions. Second, we provided feedback and assisted with goal setting through telephone follow-up biweekly during the first 6 months. Calls addressed the chosen behavior goal, self-management strategies, and assessed progress. We helped the patient problem-solve and identify successes, and modify goals and strategies appropriately. At all calls, we asked patients to describe their current practice of chosen self-management behaviors by type, frequency and duration. The staff recorded and tracked these practices in our database.
Quality control
One of the study team investigators observed a random sample of the stroke self management program sessions by all instructors to insure the fidelity of the program. For the secondary site sessions, we utilized teleconferencing with the participant’s permission to monitor the call during the session for quality control purposes. Feedback was provided to the instructors after the calls.
Attention place control group
Stroke survivors randomized to the control group received written patient education materials on the stroke warning signs and pamphlets from the American Stroke Association on preventing secondary strokes. In addition, to control for the added attention, our case manager provided telephone calls on the same schedule as the intervention but did not deliver the intervention program. Instead participants were asked how they were doing that day.
Subjects
Prospective subject identification
We prospectively recruited patients from two Veterans Health Administration sites (Indianapolis, IN, and Gainesville, FL). Subjects were identified during their stroke admission and Emergency Room visits. For all potential study subjects, we obtained permission to discuss the study with the patient from the attending physician prior to recruitment. Study personnel at each site conducted daily screenings of neurology, emergency service, medicine, and rehabilitation admissions (including review of electronic medical record and/or written admission logs and verbal contact with providers) to identify all patients with acute ischemic stroke/TIA. Patients who did not wish to participate in this study were excluded.
Patient eligibility criteria
Patient eligibility criteria included: (1) age 18 years or older; (2) having a diagnosis of ischemic stroke within past month; (3) able to speak and understand English; (4) no severe cognitive impairment; (5) access to a telephone; (6) willing to follow up in VA outpatient care; and (7) life expectancy of at least 12 months as determined by the physician. Our language and cognitive screening strategy was identical to that which we have successfully employed in our NIH-funded post-stroke depression study [43] and was designed to allow patients with moderate cognitive and language effects of stroke to be included in the study. We used the National Institutes of Health Stroke Scale (NIHSS) [44] to screen language commands and questions. Patients with significant language comprehension (commands score >0) or receptive language deficits (aphasia score ≥2) were excluded. Cognitive screening was completed with the Short Portable Mental Status Questionnaire [45, 46], which assesses short- and long-term memory and orientation. This screener has been validated in a study of older community dwelling adults and we have also successfully used this screener in our VA study of post stroke depression. All patients with a score >6 were enrolled, as this score has previously identified patients without severe dementia, which would decrease reliability of self-reported symptoms and satisfaction, while allowing the inclusion of patients with cognitive effects of stroke.
Consent and enrollment
We obtained written consent and enrolled patients into the randomized controlled trial using standard procedures in compliance with local Institutional Review Board, HIPAA guidelines and VA R&D committees. We did not provide any participation incentives.
Sample
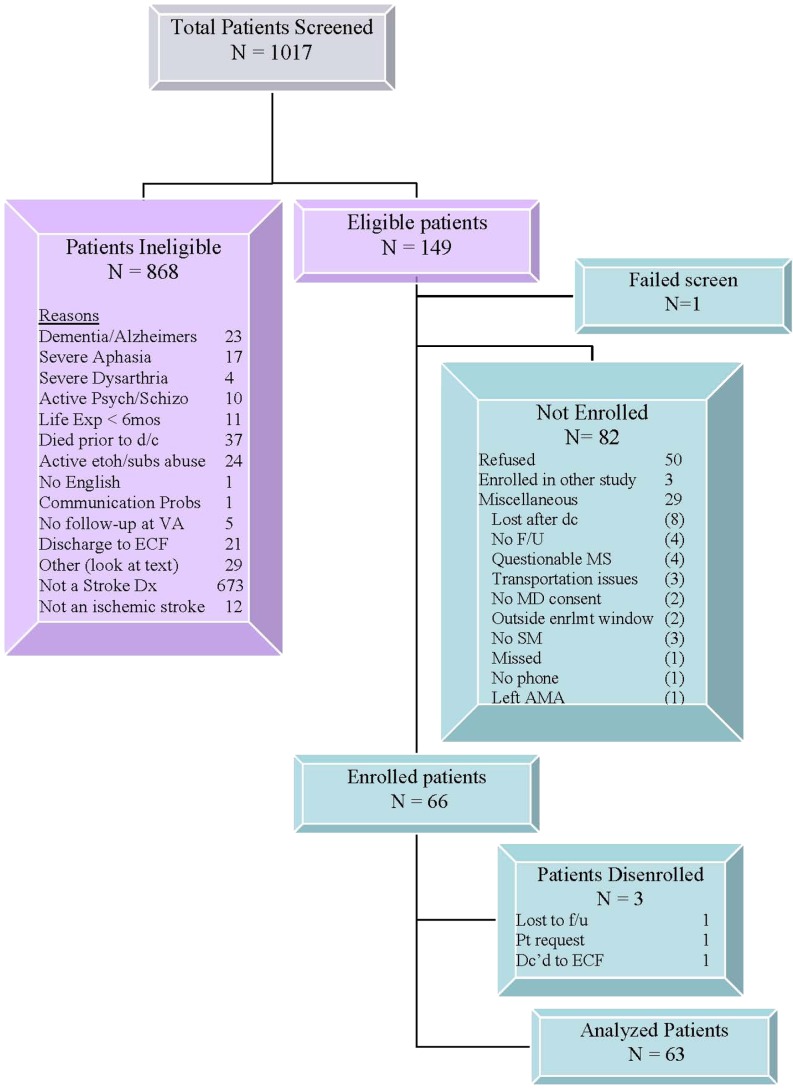
We screened 1017 patients admitted to the Roudebush and Randall VAMC (see Fig. 1). Of those screened, 868 were ineligible with not having a stroke (78%) as the most frequent reason for ineligibility. Among the 149 eligible patients, one became ineligible, 82 (55%) did not enroll with patient refusal listed as the most frequent reason (61%). We enrolled 66 participants (45% of the eligible patients), of which three were disenrolled, leaving a final sample into our pilot program of 63 veterans with stroke (41 from the Indianapolis VAMC and 22 from the Gainesville VAMC) over 18 months. The sample was 20.6% African-American, 69.8% White and approximately 10% with race unknown. All but one (1.6%) were men.
Fig 1.
Participant recruitment flow diagram
Measures
Primary outcomes: stroke-specific, health-related, quality of life
We assessed stroke-specific quality of life with the SS-QOL [47, 48]. This 62-item instrument assessed 12 domains relevant to stroke patients on a 5-point Likert response scale including energy, mobility, work, upper extremity function, ADLs, family roles, social roles, vision, language, thinking, mood, and personality during the past week and in comparison to current state prior to the stroke event. Our work has shown that the SS-QOL has good psychometric properties and that it provides a more meaningful assessment of overall post-stroke HRQOL than the SF-36 [47, 48]. In addition to the separate domains, an overall score was calculated. The overall Cronbach alpha reliability coefficient reported in this sample was 0.96 reflecting high reliability. Excluding the vision subscale (alpha coefficient = 0.48), the remaining subscales’ Cronbach alpha reliability coefficients ranged from 0.70 to 0.90 denoting good reliability.
Secondary outcomes
We assessed Self-Management Behavior Frequency using validated scales designed to measure the frequency during the past week the patient engaged in the specific behaviors including exercise (Cronbach alpha coefficient = 0.80), social and recreation activities (Cronbach alpha coefficient = 0.88), and cognitive/mental relaxation (Cronbach alpha coefficient = 0.68) [49]. Response options for self-management behaviors included total weekly time spent in activity or the weekly frequency of the specific behavior. We used these scales previously among patients with stroke and with chronic medical conditions [31, 32, 50]. We measured self-efficacy using established scales designed to measure a patient’s confidence level to manage stroke self symptoms (Cronbach alpha coefficient = 0.82), communicate with their providers (Cronbach alpha coefficient =0.93) [49]. As in our previous research [31, 32, 50], patients rated their self-efficacy on a 1 to 10 scale where 10 denotes high self-efficacy.
Demographics and health
Age and race/ethnicity were assessed during the patient baseline interview. In addition, our we trained our research staff on assessing a retrospective NIHSS for stroke patients to estimate stroke severity using the patient medical record within 24 h of admission [43, 44]. Dr. Williams established the validity of this methodology. This scale captures stroke deficits in several domains (consciousness, vision, extraocular movement, facial control, limb strength, ataxia, sensation, and speech and language).
Depression symptoms
Given that post stroke depression may affect self-management engagement, we measured depression symptoms using the valid Patient Health Questionnaire (PHQ-9) [51, 52], a nine-item depression screener tool that rates the frequency of depression symptoms over the past 2 weeks on a 0 to 3 Likert response scale and perform well as a depression screener in primary care and in post-stroke patients [31, 43]. A cutoff score of ≥10 reflects moderate depression.
Statistical analyses
Patient characteristics including age, sex, race, living alone vs. with others, caregiver present, NIHSS (e.g., stroke severity), depression symptoms, and outcome measurements SSQoL scores, self-efficacy and self-management behaviors were compared between the control and intervention arm at baseline (Table 2). For continuous outcome variables, we used two-sample t-tests for comparisons and for categorical variables, we conducted χ2 and Fisher’s exact tests.
Table 2.
Baseline subject characteristics: by site and group
| Indianapolis | Gainesville | All | |||||
|---|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | Control (n = 33) | Intervention (n = 30) | Test statistic, p valuea | |
| Age Mean ± SD (median: range) | 63.7 ± 9.3 (65.0: 42.1–3.7) | 63.7 ± 12.7 (64.2: 40.1–86.9) | 64.6 ± 6.8 (63.4 : 57.2–79.3) | 73.8 ± 8.2 (76.7: 60.7–81.4) | 64 ± 8.4 (64.9: 42.1–83.7) | 67.3 ± 12.4 (67.5: 40.1–86.9) | t(59) = −1.24, p≤0.22 |
| Sex | |||||||
| Male | 21 (95.4%) | 19 (100.0%) | 11 (100%) | 11 (100%) | 32 (97.0%) | 30 (100%) | 0.99 |
| Female | 1 (4.6%) | 1 (3.0%) | |||||
| Race | |||||||
| Black | 6 (27.3%) | 5 (26.3%) | 1 (9.1%) | 1 (9.1%) | 7 (21.2%) | 6 (20.0%) | 0.31 |
| White | 14 (63.6%) | 13 (68.4%) | 7 (63.6%) | 10 (90.9%) | 21 (63.6%) | 23 (76.7%) | |
| Unknown | 2 (9.1%) | 1 (5.3%) | 3 (27.3%) | 5 (15.2%) | 1 (3.3%) | ||
| Living status | |||||||
| Alone | 6 (27.3%) | 6 (31.6%) | 5 (45.4%) | 1 (9.1%) | 11 (33.3%) | 7 (23.3%) | χ2 (2, n = 63) |
| With only 1 other person | 11 (50.0%) | 7 (36.8%) | 6 (54.6%) | 8 (72.7%) | 17 (51.5%) | 15 (50.0%) | =1.57, |
| With 2+ | 5 (22.7%) | 6 (31.6%) | 0 (0.0) | 2 (18.2%) | 5 (15.2%) | 8 (26.7%) | p≤0.46 |
| Caregiver present | 7 (31.8%) | 8 (44.4%) | 5 (45.4%) | 3 (27.3%) | 12 (36.4%) | 11 (37.9%) | χ2 (1, n = 63) =0.02, p≤0.90 |
| NIHSS Mean ± SD (median: range) | 3.1 ± 3.2 (2.0: 0.0–10.0) | 3.7 ± 3.1 (3.0: 0.0–11.0) | 3.7 ± 3.8 (3.0: 0.0–11.0) | 2.5 ± 1.9 (3.0: 0.0–5.0) | 3.33 ± 3.37 (2.0 : 0.0–11.0) | 3.27 ± 2.72 (3.0: 0.0–11.0) | t(61) = 0.09, p≤0.93 |
aMeans of age and NIHSS (National Institute of Health Stroke Scale) were compared by two-sample t-tests. Categorical variables of sex and race were tested by using Fisher’s exact test, and living status and caregiver present were tested by using χ2 tests
The intervention effect was evaluated by comparing the changes from baseline between the two study arms. Only subjects with at least one follow-up visit were included in this analysis. If the response for the second follow-up visit was missing (n = 12), then the response at the first follow-up visit was carried forward to the second. Change from baseline was calculated using these imputed data. Baseline means and means of change from baseline were compared between groups by two sample t-tests. Plots of means at each visit—baseline, first (e.g., 3 months) and second follow-up (e.g., 6 months)—were presented. Change from baseline was modeled by a linear mixed-effect model with fixed effects for treatment, visit, and the treatment by visit interaction. A random subject effect was included in order to accommodate the correlation induced by repeated measurements of the same subject. If the two-sample t-test of baseline means showed that treatment groups were significantly different at the 0.1 level, then the linear mixed-effects model also adjusted for the baseline score. No other covariates were adjusted. All analyses were performed using SAS® 9.2 (Cary, NC).
RESULTS
Sample characteristics
The intervention and control groups did not differ significantly on age (t(59) = −1.24, p ≤ 0.22), race, sex, or on proportion living alone (see Table 1). The groups also did not differ on the admission NIHSS (3.27 intervention vs. 3.33 control) (t(61) = 0.09, p ≤ 0.93) indicating the participants had on average minor stroke severity. Although not statistically different, the intervention group had slightly higher baseline depression symptom scores than the control group (mean PHQ9 6.5 vs. 4.2) (t(56) = −1.92, p ≤ 0.06, effect size = −0.51).
Stroke self-management intervention processes
Of the six planned sessions, the average number of sessions completed by the intervention group was 5.0 and the average number of attention sessions completed by the control group was 5.7. The session rates completed did not differ by site. The most frequent activities reported in the behavioral plans by those in the intervention were the following: became active around the home, walked in the community, took pills as doctor recommended, ate healthy foods and eliminated unhealthy foods, practiced other physical activity and listened to a relaxation compact disc.
Self-efficacy
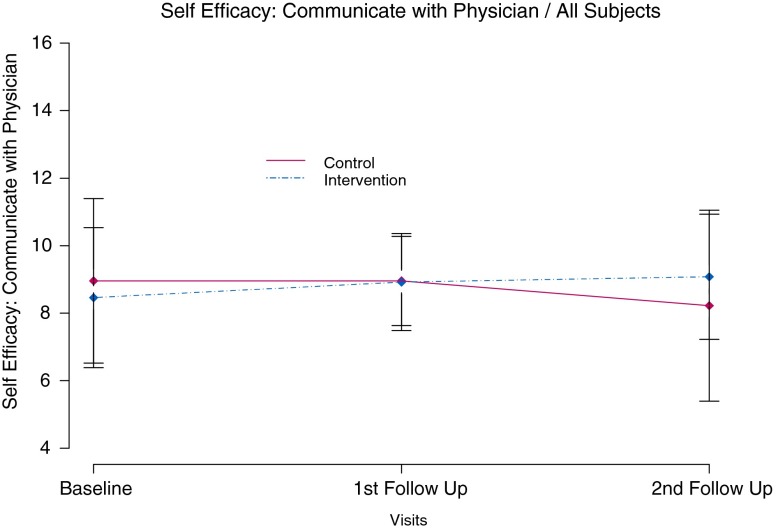
Baseline mean score for the self efficacy measure of Communicating with Physician in the intervention group was 8.5 vs. 8.9 in the control group (t(55) = 0.68, p ≤ 0.50, effect size = 0.18). Mean change from baseline was significantly different between groups at the second follow-up visit (intervention group with mean increase in confidence of 0.5 points vs. control group mean decline of 0.8 points (t(52) = −2.14, p ≤ 0.04, effect size = −0.59). Model based estimates controlling for intervention group and visit also showed a significant difference in mean self efficacy of communicating with a physician at the second follow-up visit (t(50) = 2.34, p ≤ 0.02) (see Fig. 2).
Fig 2.
Self-efficacy to communicate with physician over 6 months
Self-management behaviors
Participants in the intervention group reported an increase in time spent in aerobic activity (baseline 78.5 weekly min) compared to the control group (baseline 107.4 weekly min) (t(55) = 1.18, p ≤ 0.24, effect size = 0.31). At 3 months, the intervention group demonstrated a mean increase in 47.6 min in intervention compared to a mean decrement of 3 min in the control group (t(51) = −1.56, p ≤ 0.13, effect size = −0.43); this effect was sustained at 6 months (increase in 24.4 min in the intervention group compared to increase of 4 min in control group, (t(52) = −0.69, p ≤ 0.50, effect size = −0.19), although these differences were not statistically significant. For cognitive self-management practices, the groups did not differ significantly at baseline or at follow-up.
Stroke-specific quality of life
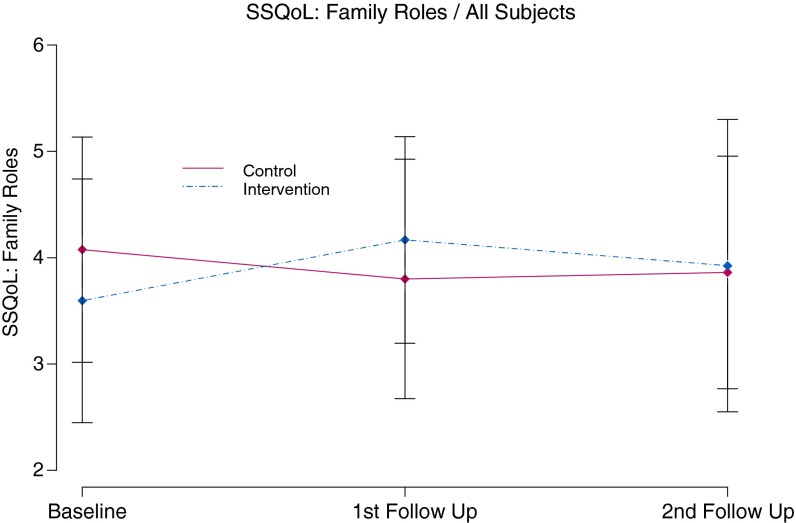
At baseline, subjects in the intervention group had significantly lower (worse) mean scores for several SS-QOL scales including Mobility, Thinking, Energy, and Work as well as SSQoL overall score. Mean change from baseline in SSQoL Family roles (baseline intervention mean score = 3.6 and control mean = 4.2) (t(56) = 1.91, p ≤ 0.06, effect size = 0.51) was significantly different between groups at the 3-month follow-up visit. On average, subjects in the Intervention group showed an improvement of 0.5 points, while subjects in the control group showed a decline of 0.3 points (t(54) = −2.90, p ≤ 0.01, effect size = −0.78). Model-based comparisons at this time point were also different; however, this difference was only marginally significant (t(54) = 1.98, p ≤ 0.05) (see Fig. 3).
Fig 3.
Stroke specific quality of life social role functioning over 6 months
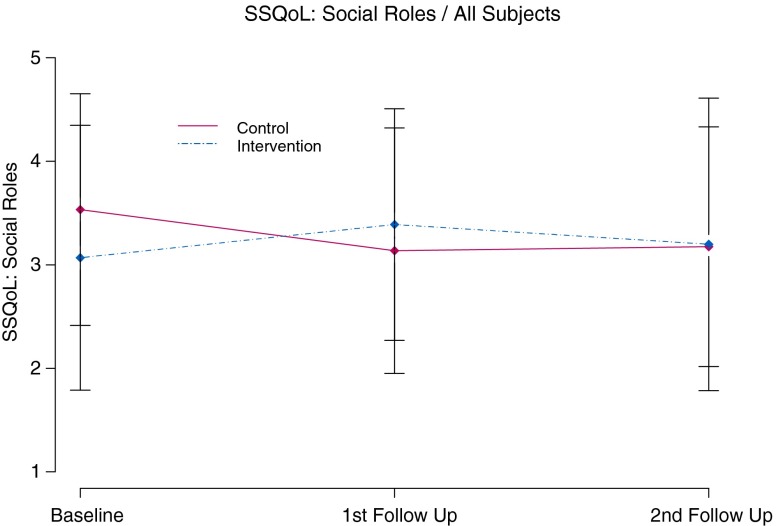
Mean change from baseline for SSQoL Social roles scores (baseline intervention mean score = 3.1 and control mean = 3.6) (t(56) = 1.66, p ≤ 0.10, effect size = 0.44) was significantly different between groups at the first follow-up visit (intervention group improved by 0.3 points vs. control group decreased by 0.4 points) (t(55) = −2.04, p ≤ 0.05, effect size = −0.54). Model based comparisons also showed a similar difference at the first follow-up visit with p = 0.04 (t(55) = 2.13, p ≤ 0.04) (see Fig. 4). Similar estimates of mean change from baseline (t(51) = 2.41, p ≤ 0.02, effect size = 0.66) of SSQoL Work scores were also significantly different between groups at the 6-month visit.
Fig 4.
Stroke specific quality of life family role functioning over 6 months
DISCUSSION
Self-management intervention impact and context
This study is one of the first to test the efficacy of a stroke self-management program that was developed with input from key stakeholders (i.e., veterans with stroke, caregivers, clinical providers of stroke care) for stroke specific contents while incorporating general self-management principles from a chronic disease program similar to the methods used in a recent development of the Hepatitis C Self-Management Program [55]. Currently, there are no other published results from a stroke specific self-management program for comparison. Our process measures indicate that offering a stroke self-management program after stroke is feasible. However, recruiting strictly from the acute stroke phase can limit the reach of this program into the veteran population, especially for VA facilities that provide less acute inpatient stroke care but may provide significant stroke rehabilitation or follow-up care. During recruitment, we found stroke patients who stated that they needed time to adjust to the acute event and could not commit to participating in our program. Future programming may include tailored components to address the patient’s needs based on the length of time since the acute stroke event.
Veterans stroke survivors assigned to the attention control group received more contact with our instructors than those assigned to the intervention. Although we did not query the control group participants directly about this topic, many expressed their appreciation for the phone call as a reminder that someone was concerned about their health. Given that the majority of our sample did not have a caregiver present, the supportive program facilitator appears to be a key component of the program.
Although our sample size limited our statistical power to detect intervention effects, we observed consistent differences in patient self-efficacy and stroke specific quality of life suggesting that the program has the potential to improve the quality of life of veterans with stroke. The use of the telephone to conduct sessions was sometimes a cost barrier to participation. Enhancing access is a key implementation issue for subsequent self-management interventions.
There are several limitations of this study. First, this veteran sample was all male. Female stroke survivors may have unique needs. We plan to include a community hospital with a female stroke population in our next evaluation to determine any sex differences to the program. Second, we included patients with either an ischemic stroke or TIA. Patients with other types of stroke may experience more severe stroke related disability and may be limited in their ability to learn self-management skills. Nonetheless, there may be a wide variation in disability and deficits among stroke survivors and self-management programs may be tailored to include more rehabilitation therapies during the post acute phase to foster recovery and to eventually improve PSM skills. Finally, this study was conducted within the Veterans Health Administration (VHA). The implementation of stroke self-management programs in other healthcare organizations may need additional navigation through its system. Within the VHA, there are opportunities for care coordination between specialty and primary care services to include the fostering of stroke self-management skill building.
CONCLUSIONS
Participating in a stroke self-management intervention may improve a stroke survivor’s self-efficacy to communicate with providers, improve time spent in exercise (e.g., walking) and improve stroke specific quality of life. The impact of the program and the relationships between these patient centered domains need further examination. Moreover, we need to understand how best to increase the reach of post-stroke programs to veterans during stroke recovery. Our next steps in development and testing of this intervention for veteran stroke survivors will include a multi-site prospective efficacy trial that increases the recruitment window and increases the period of program support to sustain the short term gains. Moreover, we plan to target the intervention on veterans’ social and family roles to provide greater impact on stroke specific quality of life and to target the development of additional tools to support the increase in aerobic activity (e.g., walking) after stroke.
The results of this study demonstrate feasibility in participating in a self-management program for veteran stroke survivors. Such programs may be efficacious in improving the quality of life of veterans with stroke. Veterans with stroke may have some unique needs for self management support for some issues including feeling confident and comfortable enough to leave the home and participate in the community after stroke. A lack of transportation services (some stroke survivors are unable to drive) and difficulty with reading and writing after stroke may produce challenges in the process of learning self-management. Thus, alternative delivery methods are warranted to meet the delivery needs of the veteran and foster improvements in post-stroke self-management.
Acknowledgements
This project was supported by a merit review grant from the VA Health Services Research and Development IMV#04-096. We thank Ms. Gloria Nicholas and Ms. Randi Cameron for providing case management to the study participants and Ms. Danielle Sager for providing research support. In addition, we thank the veterans and the clinical staff from the two VA facilities (Roudebush and Randall VAMCs) for their time and participation.
APPENDIX 1: STROKE SELF-MANAGEMENT PROGRAM (SSMP) OVERVIEW
The SSMP was delivered over six sessions delivered both in person (three sessions) and by telephone (three sessions) over a 3-month period. Additional telephone contacts focused on reinforcing, monitoring, and adjusting the goals and self-management strategies (biweekly calls) over the following 3 months. We have developed a detailed standardized program manual for all six sessions, which is available upon request. Below is the course overview as delivered over the six sessions.
| Course Overview | Session | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Overview of stroke/TIA and self-management | × | |||||
| Changes caused by stroke | × | |||||
| Warning signs of stroke | × | |||||
| Expectations for recovery after stroke | × | |||||
| Dealing with fears | × | |||||
| Recognizing the Symptoms of Depression | × | × | × | |||
| Rehabilitation — keeping appointments and following prescribed exercise at home | × | × | × | × | × | × |
| Planning/Creating a Schedule | × | × | × | × | × | × |
| Feedback and problem-solving | × | × | × | × | × | |
| Dealing with negative emotions | × | |||||
| Getting Active at Home | × | × | ||||
| Medication adherence | × | |||||
| Adapting/Coping with Disabilities (stroke related) | × | × | × | × | × | |
| Walking for health | × | × | × | |||
| Modifying your Diet (stroke risk factor management) | × | × | ||||
| Relaxation — Deep breathing, Progressive Muscle Relaxation | × | × | × | × | × | |
| Changing your outlook with positive thinking | × | |||||
| Adapting to a new role after stroke — creating a schedule | × | |||||
| Reducing Cholesterol, Hypertension (stroke risk factor management) | × | |||||
| Finding a buddy (talking to stroke survivors) | × | |||||
| Community resources — where to get help for stroke related issues and social needs | × | |||||
| Working with health care providers and caregivers | × | |||||
| Stop smoking | × | |||||
| Reducing your alcohol | × | |||||
Footnotes
Implications
Practice: To obtain optimal post stroke, specific quality of life, interventions fostering patient self management should occur at the level of recovery and secondary risk factor management for individuals.
Policy: Dedicating coordinated clinical resources to foster stroke self-management during recovery may increase patient access and participation.
Research: This report demonstrates the feasibility for delivering a stroke self-management program within a healthcare organization as well as the feasibility that patients with neurological deficits can access and participate.
References
- 1.American Heart Association. (2011). American Heart Association. Heart Disease and Stroke Statistics — 2011 Update.
- 2.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.NHLBI Death Rates for Cerebrovascular Diseases, by Sex, Race, Hispanic Origin, and Age: United States, Selected years 1950–2004 (16894). Centers for Disease Control and Prevention (CDC) National Center for Health Statistics 2006. Available at: http://www.cdc.gov/nchs/data/hus/hus06.pdf#page222.
- 4.Duncan PW, Samsa GP, Weinberger M, et al. Health status of individuals with mild stroke. Stroke. 1997;28(4):740–745. doi: 10.1161/01.STR.28.4.740. [DOI] [PubMed] [Google Scholar]
- 5.Wolf PA, Clagett GP, Easton JD, et al. Preventing ischemic stroke in patients with prior stroke and transient ischemic attack: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 1999;30(9):1991–1994. doi: 10.1161/01.STR.30.9.1991. [DOI] [PubMed] [Google Scholar]
- 6.CDC Prevalence of disabilities and associated health conditions among adults — United States, 1999. Morbidity and Mortality Weekly Review. 1999;50:120–5. [PubMed] [Google Scholar]
- 7.Dombovy ML, Basford JR, Whisnant JP, Bergstralh EJ. Disability and use of rehabilitation services following stroke in Rochester, Minnesota, 1975–1979. Stroke. 1987;18(5):830–836. doi: 10.1161/01.STR.18.5.830. [DOI] [PubMed] [Google Scholar]
- 8.Reker, D. (2005). The VA Stroke QUERI Strategic Plan.
- 9.Johnston S, Gress D, Browner W, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284:2901–2906. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]
- 10.Rothwell P, Johnston S. Transient ischemic attacks, stratifying risk. Stroke. 2006;37(2):320–322. doi: 10.1161/01.STR.0000200555.89117.d2. [DOI] [PubMed] [Google Scholar]
- 11.Hill MD, Yiannakoulias N, Jeerakathil T, et al. The high risk of stroke immediately after transient ischemic attack. A population-based study. Neurology. 2004;62:2015–2020. doi: 10.1212/01.wnl.0000129482.70315.2f. [DOI] [PubMed] [Google Scholar]
- 12.Hanley DF. The challenge of stroke prevention. JAMA. 2004;291(5):621–622. doi: 10.1001/jama.291.5.621. [DOI] [PubMed] [Google Scholar]
- 13.Lai SM, Alter M, Friday G, Sobel E. A multifactorial analysis of risk factors for recurrence of ischemic stroke. Stroke. 1994;25:958–962. doi: 10.1161/01.STR.25.5.958. [DOI] [PubMed] [Google Scholar]
- 14.Kenner ME, Kelley RE. Update on stroke prevention. Comprehensive Therapy. 2005;31(2):113–118. doi: 10.1007/s12019-005-0006-x. [DOI] [PubMed] [Google Scholar]
- 15.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291(5):565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 16.Haijar I, Kotchen TA. Trends in prevalence, awareness, treatment and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 17.Lorig K. Self-management education: more than a nice extra. Medical Care. 2003;41:699–701. doi: 10.1097/01.MLR.0000072811.54551.38. [DOI] [PubMed] [Google Scholar]
- 18.Glasgow RE, Christiansen S, Smith KS, Stevens VJ, Toobert DJ. Development and implementation of an integrated, multi-modality, user-centered interactive dietary change program. Health Education Research. 2009;24:461–471. doi: 10.1093/her/cyn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 20.Stepnowsky CJ, Palau JJ, Gifford AL, Ancoli-Israel S. A self-management approach to improving continuous positive airway pressure adherence and outcomes. Behavioral Sleep Medicine. 2007;5:131–46. doi: 10.1080/15402000701190622. [DOI] [PubMed] [Google Scholar]
- 21.Bodenheimer, T., Wagner, E. H., & Grumbach, K. (2002). Improving primary care for patients with chronic illness. JAMA, 1775–1779. [DOI] [PubMed]
- 22.Patient Self-Management Support Programs: an evaluation. Final contract report. AHRQ publication No. 08–0011, November 2007. Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/qual/ptmgmt/.
- 23.Von Korff M, Gruman J, Schaefer J, Curry SJ, Wagner EH. Collaborative management of chronic illness. Annals of Internal Medicine. 1997;127:1097–102. doi: 10.7326/0003-4819-127-12-199712150-00008. [DOI] [PubMed] [Google Scholar]
- 24.Clark NM, Gong M, Kaciroti N. A model of self-regulation for control of chronic disease. Health Education & Behavior. 2001;28(6):769–782. doi: 10.1177/109019810102800608. [DOI] [PubMed] [Google Scholar]
- 25.Clark NM, Becker MH, Janz NK, Lorig K, Rakowski W, Anderson L. Self-management of chronic disease by older adults. Journal of Aging & Health. 1991;3:3–27. doi: 10.1177/089826439100300101. [DOI] [Google Scholar]
- 26.Lorig KR, Ritter P, Stewart AL, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Medical Care. 2001;39(11):217–223. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Medical Care. 1999;37(1):5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs: Prentice-Hall; 1986. [Google Scholar]
- 29.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychological Review. 1977;84:191–215. doi: 10.1037/0033-295X.84.2.191. [DOI] [PubMed] [Google Scholar]
- 30.van der Meer V, Bakker MJ, van den Hout WB, et al. SMASHING (Self-management in asthma supported by hospitals, ICT, nurses and general practitioners) Study Group. Internet-based self-management plus education compared with usual care in asthma: a randomized trial. Annals of Internal Medicine. 2009;151:110–120. doi: 10.7326/0003-4819-151-2-200907210-00008. [DOI] [PubMed] [Google Scholar]
- 31.Kroenke K, Bair MJ, Damush TM, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA. 2009;301:2099–2110. doi: 10.1001/jama.2009.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damush TM, Weinberger, Perkins SM, Rao JK, Tierney WM, Rong Q, et al. The long term effects of a self-management program for inner-city, primary care patients with acute low back pain. Archives of Internal Medicine. 2003;163:2632–2638. doi: 10.1001/archinte.163.21.2632. [DOI] [PubMed] [Google Scholar]
- 33.Bosworth HB, Olsen MK, Dudley T, Orr M, Goldstein MK, Datta SK, et al. Patient education and provider decision support to control blood pressure in primary care: a cluster randomized trial. American Heart Journal. 2009;157:450–456. doi: 10.1016/j.ahj.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Bosworth HB, Olsen MK, Goldstein MK, et al. The veterans’ study to improve the control of hypertension (V-STITCH): Design and methodology. Contemporary Clinical Trials. 2005;26:155–168. doi: 10.1016/j.cct.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Bosworth HB, Olsen MK, Dudley T, et al. The Take Control of Your Blood pressure (TCYB) study: study design and methodology. Contemporary Clinical Trials. 2007;28:33–47. doi: 10.1016/j.cct.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Yip YB, Sit WJ, Karin KY, et al. Impact of an arthritis self-management programme with an added exercise component for osteoarthritic knee sufferers on improving pain, functional outcomes, and use of health care services: an experimental study. Patient Education and Counseling. 2007;65:113–121. doi: 10.1016/j.pec.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Lorig KR, Ritter PL, Laurent DD, Plant K. The internet-based arthritis self-management program: a one year randomized trial for patients with arthritis or fibromyalgia. Arthritis & Rheumatism. 2008;59:1009–1017. doi: 10.1002/art.23817. [DOI] [PubMed] [Google Scholar]
- 38.Glasgow RE, Toobert DJ, Hampson SE, Brown JE, Lewisohn PM, Donnelly J. Improving self-care among older patients with type II diabetes: the Sixty something study. Patient Education and Counseling. 1992;19:61–74. doi: 10.1016/0738-3991(92)90102-O. [DOI] [PubMed] [Google Scholar]
- 39.Damush TM, Plue L, Bakas T, Schmid A, Williams LS. Barriers and facilitators to exercise among stroke survivors. Rehabilitation Nursing. 2007;32(6):253–260. doi: 10.1002/j.2048-7940.2007.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 40.Guidetti, S., Ytterberg, C. (2010). A randomized controlled trial of a client-centered self-care intervention after stroke: a longitudinal pilot. Disability & Rehabilitation, Jul 2 [Epub]. [DOI] [PubMed]
- 41.Chumbler NR, Rose DK, Griffiths P, et al. Study protocol: home-based telehealth stroke care: a randomized trial for veterans. Trials. 2010;11:74. doi: 10.1186/1745-6215-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Battersby M, Hoffman S, Cadilhac D, Osborne R, Lalor E, Lindley R. Getting your life back on track after stroke. International Journal of Stroke. 2009;4(2):137–44. doi: 10.1111/j.1747-4949.2009.00261.x. [DOI] [PubMed] [Google Scholar]
- 43.Williams LS, Kroenke K, Bakas T, et al. Care management of poststroke depression: a randomized, controlled trial. Stroke. 2007;38(3):998–1003. doi: 10.1161/01.STR.0000257319.14023.61. [DOI] [PubMed] [Google Scholar]
- 44.Lyden P, Raman R, Liu L, Emr M, Warren M, Marler J. National Institutes of Health Stroke Scale certification is reliable across multiple venues. Stroke. 2009;40(7):2507–2511. doi: 10.1161/STROKEAHA.108.532069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welch DC, West RL. The short portable mental status questionnaire: assessing cognitive ability in nursing home residents. Nursing Research. 1999;48(6):329–332. doi: 10.1097/00006199-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Medical Care. 2002;40(9):771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Williams L, Weinberger M, Harris L, Clark D, Biller J. Development of a stroke-specific quality of life (SS-QOL) scale. Stroke. 1999;30:1362–1369. doi: 10.1161/01.STR.30.7.1362. [DOI] [PubMed] [Google Scholar]
- 48.Williams LS, Weinberger M, Harris LE, Biller J. Measuring quality of life in a way that is meaningful to stroke patients. Neurology. 1999;53:1839–1843. doi: 10.1212/wnl.53.8.1839. [DOI] [PubMed] [Google Scholar]
- 49.Lorig K, Stewart AL, Ritter P, Gonzalez VM, Laurent D, Lynch J. Outcome measures for health education and other health care interventions. Thousand Oaks: Sage; 1996. [Google Scholar]
- 50.Damush, T. M., Nicholas, G., Plue, L., Ofner, S., Beyth, R., Zhangsheng, Y., et al. (2010). Behavioral lifestyle change strategies after stroke in veterans: data from the VA Stroke QUERI. Paper presented at the International Stroke Meeting, San Antonio, TX, February 25, 2010.
- 51.Williams, L. S., Brizendine, E. J., Plue, L., et al. (2005). Performance of the PHQ-9 as a Screening Tool for Depression After Stroke. Stroke, January 27. [DOI] [PubMed]
- 52.Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression. Two questions are as good as many. Journal of General Internal Medicine. 1997;12(7):439–445. doi: 10.1046/j.1525-1497.1997.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 55.Groessl E, Weingart KR, Gifford AL, Asch SM, Ho SD. Development of the Hepatitis C self-management program. Patient Education and Counseling. 2011;83:252–255. doi: 10.1016/j.pec.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Deakin T, McShane C, Cade J, Williams R. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database System Review. 2005;18(2):CD003417. doi: 10.1002/14651858.CD003417.pub2. [DOI] [PubMed] [Google Scholar]