Abstract
Other investigators have shown that fructose infusion in normal man and rats acutely depletes hepatic ATP and Pi and increases the rate of uric acid formation by the degradation of preformed nucleotides. We postulated that a similar mechanism of ATP depletion might be present in patients with glucose-6-phosphatase deficiency (GSD-I) as a result of ATP consumption during glycogenolysis and resulting excess glycolysis. The postulate was tested by measurement of: (a) hepatic content of ATP, glycogen, phosphorylated sugars, and phosphorylase activities before and after increasing glycolysis by glucagon infusion and (b) plasma urate levels and urate excretion before and after therapy designed to maintain blood glucose levels above 70 mg/dl and thus prevent excess glycogenolysis and glycolysis.
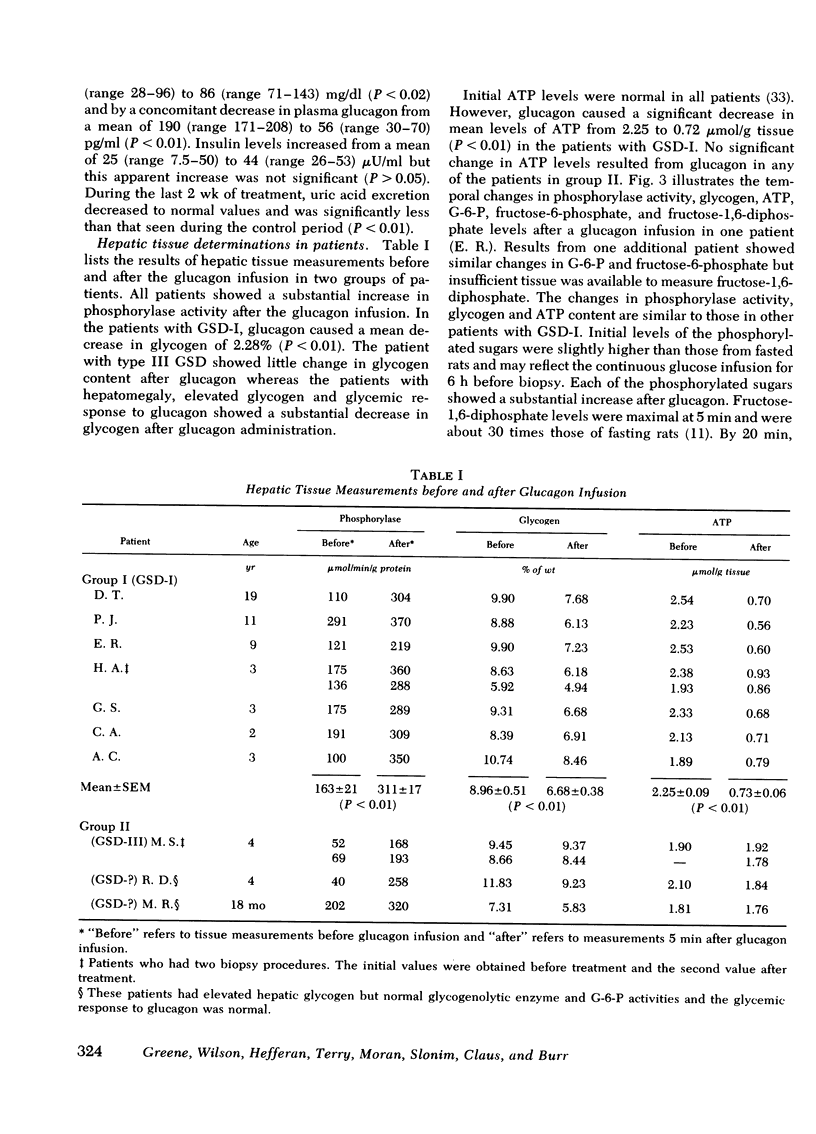
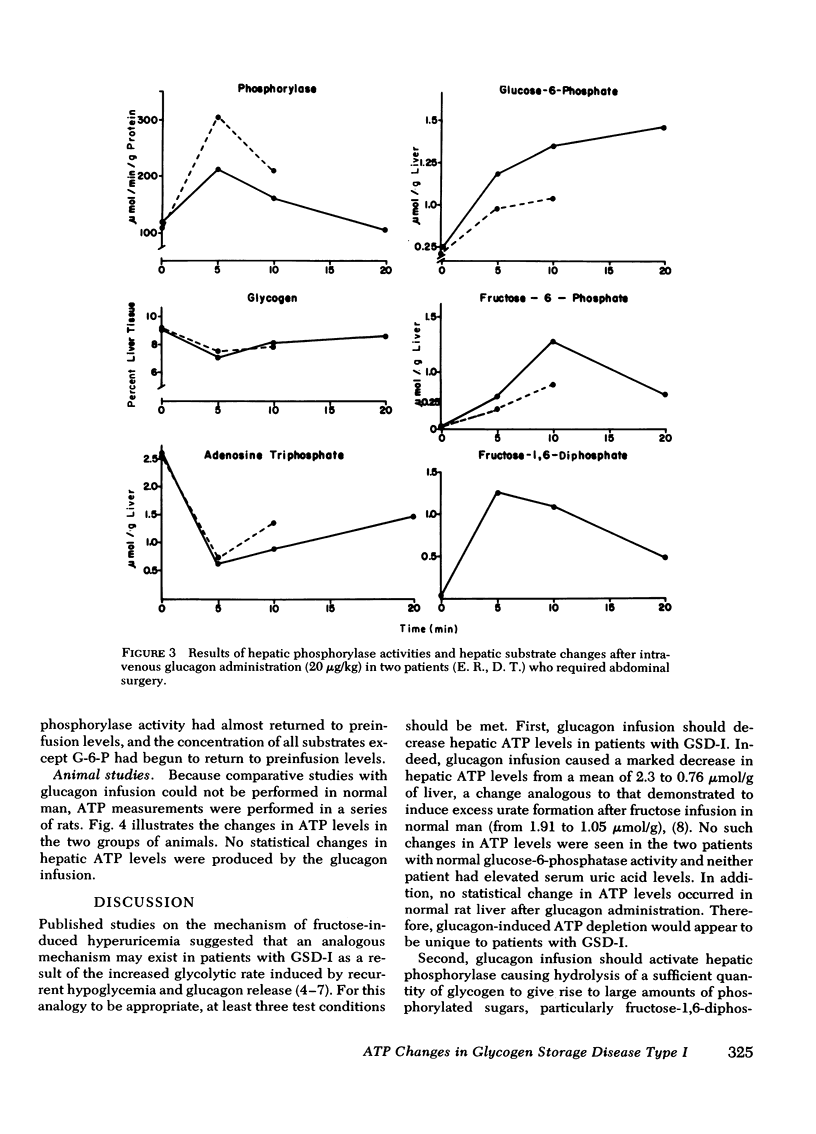
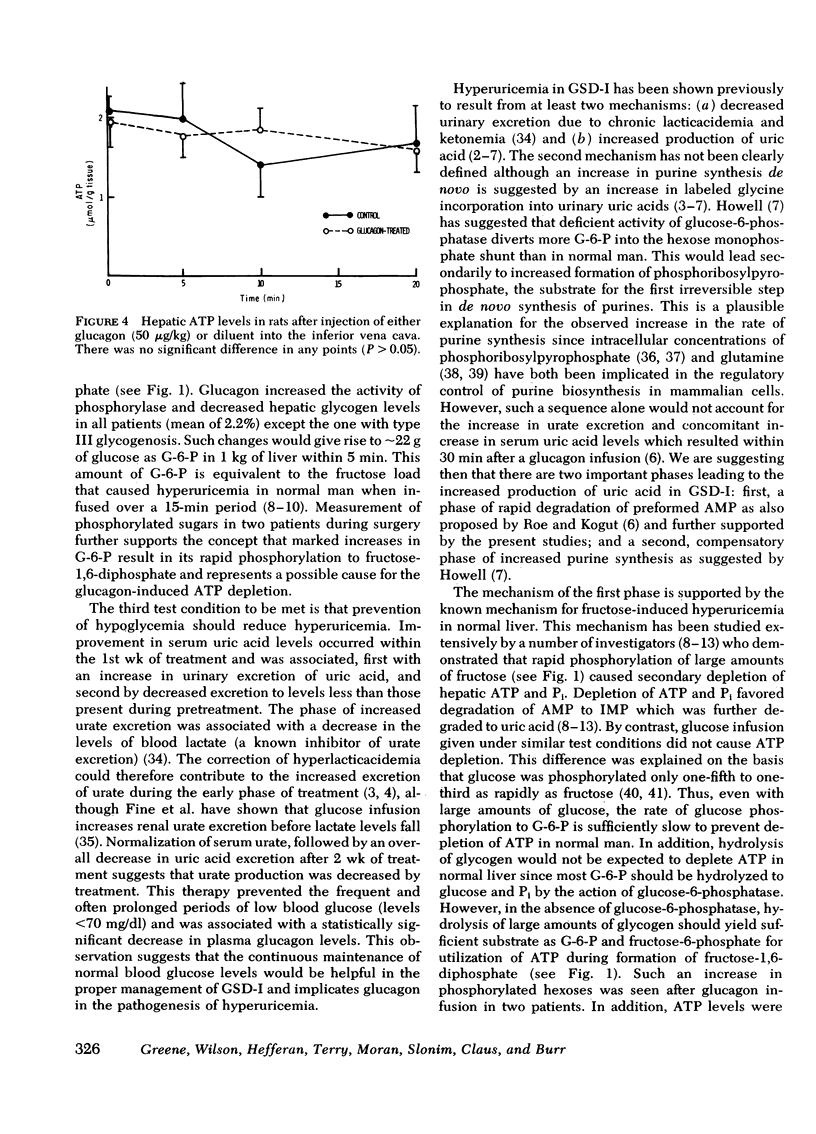
Glucagon infusion in seven patients with GSD-I caused a decrease in hepatic ATP from 2.25 ± 0.09 to 0.73 ± 0.06 μmol/g liver (P <0.01), within 5 min, persisting in one patient to 20 min (1.3 μmol/g). Three patients with GSD other than GSD-I (controls), and 10 normal rats, showed no change in ATP levels after glucagon infusion. Glucagon caused an increase in hepatic phosphorylase activity from 163 ± 21 to 311 ± 17 μmol/min per g protein (P <0.01), and a decrease in glycogen content from 8.96 ± 0.51 to 6.68 ± 0.38% weight (P <0.01). Hepatic content of phosphorylated hexoses measured in two patients, showed the following mean increases in response to glucagon; glucose-6-phosphate (from 0.25 to 0.98 μmol/g liver), fructose-6-phosphate (from 0.17 to 0.45 μmol/g liver), and fructose-1,6-diphosphate (from 0.09 to 1.28 μmol/g) within 5 min. These changes, except for glucose-6-phosphate, returned toward preinfusion levels within 20 min.
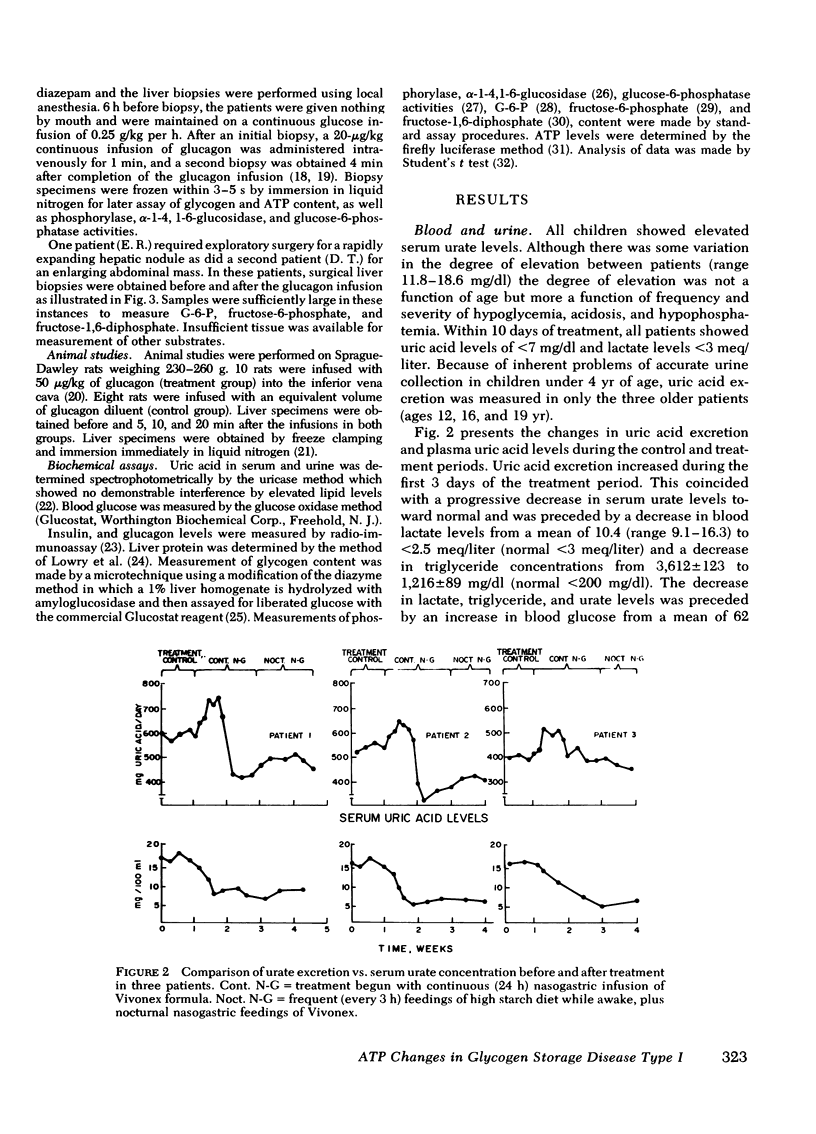
Treatment consisted of continuous intragastric feedings of a high glucose dietary mixture. Such treatment increased blood glucose from a mean level of 62 (range 28-96) to 86 (range 71-143) mg/dl (P <0.02), decreased plasma glucagon from a mean of 190 (range 171-208) to 56 (range 30-70) pg/ml (P <0.01), but caused no significant change in insulin levels. Urate output measured in three patients showed an initial increase, coinciding with a decrease in plasma lactate and triglyceride levels, then decreased to normal within 3 days after treatment. Normalization of urate excretion was associated with normalization of serum uric acid.
We suggest that the maintenance of blood glucose levels above 70 mg/dl is effective in reducing serum urate levels and that transient and recurrent depletion of hepatic ATP due to glycogenolysis is contributory in the genesis of hyperuricemia in untreated patients with GSD-I.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alepa F. P., Howell R. R., Klinenberg J. R., Seegmiller J. E. Relationships between glycogen storage disease and tophaceous gout. Am J Med. 1967 Jan;42(1):58–66. doi: 10.1016/0002-9343(67)90006-x. [DOI] [PubMed] [Google Scholar]
- Bode C., Schumacher H., Goebell H., Zelder O., Pelzel H. Fructose induced depletion of liver adenine nucleotides in man. Horm Metab Res. 1971 Jul;3(4):289–290. doi: 10.1055/s-0028-1096782. [DOI] [PubMed] [Google Scholar]
- Bode J. C., Zelder O., Rumpelt H. J., Wittkamp U. Depletion of liver adenosine phosphates and metabolic effects of intravenous infusion of fructose or sorbitol in man and in the rat. Eur J Clin Invest. 1973 Sep;3(5):436–441. doi: 10.1111/j.1365-2362.1973.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Burr I. M., O'Neill J. A., Karzon D. T., Howard L. J., Greene H. L. Comparison of the effects of total parenteral nutrition, continuous intragastric feeding, and portacaval shunt on a patient with type I glycogen storage disease. J Pediatr. 1974 Dec;85(6):792–795. doi: 10.1016/s0022-3476(74)80342-2. [DOI] [PubMed] [Google Scholar]
- Faupel R. P., Seitz H. J., Tarnowski W., Thiemann V., Weiss C. The problem of tissue sampling from experimental animals with respect to freezing technique, anoxia, stress and narcosis. A new method for sampling rat liver tissue and the physiological values of glycolytic intermediates and related compounds. Arch Biochem Biophys. 1972 Feb;148(2):509–522. doi: 10.1016/0003-9861(72)90170-1. [DOI] [PubMed] [Google Scholar]
- Fine R. N., Strauss J., Donnell G. N. Hyperuricemia in glycogen-storage disease type 1. Am J Dis Child. 1966 Dec;112(6):572–576. doi: 10.1001/archpedi.1966.02090150116013. [DOI] [PubMed] [Google Scholar]
- Fine R. N., Strauss J., Donnell G. N. Hyperuricemia in glycogen-storage disease type 1. Am J Dis Child. 1966 Dec;112(6):572–576. doi: 10.1001/archpedi.1966.02090150116013. [DOI] [PubMed] [Google Scholar]
- Fine R. N., Strauss J., Donnell G. N. Hyperuricemia in glycogen-storage disease type 1. Am J Dis Child. 1966 Dec;112(6):572–576. doi: 10.1001/archpedi.1966.02090150116013. [DOI] [PubMed] [Google Scholar]
- Fink A. S., Hefferan P. M., Howell R. R. Enzymatic and biochemical characterization of the avian glycogen body. Comp Biochem Physiol B. 1975 Apr 15;50(4):525–530. doi: 10.1016/0305-0491(75)90082-6. [DOI] [PubMed] [Google Scholar]
- Greene H. L., Slonim A. E., O'Neill J. A., Jr, Burr I. M. Continuous nocturnal intragastric feeding for management of type 1 glycogen-storage disease. N Engl J Med. 1976 Feb 19;294(8):423–425. doi: 10.1056/NEJM197602192940805. [DOI] [PubMed] [Google Scholar]
- Greene H. L., Taunton O. D., Stifel F. B., Herman R. H. The rapid changes of hepatic glycolytic enzymes and fructose-1,6-diphosphatase activities after intravenous glucagon in humans. J Clin Invest. 1974 Jan;53(1):44–51. doi: 10.1172/JCI107557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene H. L., Wilson F. A., Glick A. D., Dunn G. D., Kilroy A. W. Hepatic ATP concentrations and glycolytic enzyme activities in Reye syndrome. J Pediatr. 1976 Nov;89(5):777–780. doi: 10.1016/s0022-3476(76)80804-9. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWELL R. R., ASHTON D. M., WYNGAARDEN J. B. Glucose-6-phosphatase deficiency glycogen storage disease. Studies on the interrelationships of carbohydrate, lipid, and purine abnormalities. Pediatrics. 1962 Apr;29:553–565. [PubMed] [Google Scholar]
- Hefferan P. M., Howell R. R. Genetic evidence for the common identity of glucose-6-phosphatase, pyrophosphate-glucose phosphotransferase, carbamyl phosphate-glucose phosphotransferase and inorganic pyrophosphatase. Biochim Biophys Acta. 1977 Feb 28;496(2):431–435. doi: 10.1016/0304-4165(77)90325-7. [DOI] [PubMed] [Google Scholar]
- Henderson J. F., Rosenbloom F. M., Kelley W. N., Seegmiller J. E. Variations in purine metabolism of cultured skin fibroblasts from patients with gout. J Clin Invest. 1968 Jul;47(7):1511–1516. doi: 10.1172/JCI105844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E. W., Wyngaarden J. B., Kelley W. N. Human glutamine phosphoribosylpyrophosphate amidotransferase. Two molecular forms interconvertible by purine ribonucleotides and phosphoribosylpyrophosphate. J Biol Chem. 1973 Sep 10;248(17):6035–6040. [PubMed] [Google Scholar]
- Holmsen H., Holmsen I., Bernhardsen A. Microdetermination of adenosine diphosphate and adenosine triphosphate in plasma with firefly luciferase system. Anal Biochem. 1966 Dec;17(3):456–473. doi: 10.1016/0003-2697(66)90181-3. [DOI] [PubMed] [Google Scholar]
- Howell R. R. Hyperuricemia in childhood. Fed Proc. 1968 Jul-Aug;27(4):1078–1082. [PubMed] [Google Scholar]
- Howell R. R. The interrelationship of glycogen storage disease and gout. Arthritis Rheum. 1965 Oct;8(5):780–785. doi: 10.1002/art.1780080441. [DOI] [PubMed] [Google Scholar]
- Hug G., Schubert W. K., Shwachman H. Imbalance of liver phosphorylase and accumulation of hepatic glycogen in a girl with progressive disease of the brain. J Pediatr. 1965 Nov;67(5):741–751. doi: 10.1016/s0022-3476(65)80362-6. [DOI] [PubMed] [Google Scholar]
- Hultman E., Nilsson L. H., Sahlin K. Adenine nucleotide content of human liver. Normal values and fructose-induced depletion. Scand J Clin Lab Invest. 1975 May;35(3):245–251. doi: 10.1080/00365517509095736. [DOI] [PubMed] [Google Scholar]
- Jakovcic S., Sorensen L. B. Studies of uric acid metabolism in glycogen storage disease associated with gouty arthritis. Arthritis Rheum. 1967 Apr;10(2):129–134. doi: 10.1002/art.1780100207. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. A specific enzyme defect in gout associated with overproduction of uric acid. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1735–1739. doi: 10.1073/pnas.57.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIDDLE L., SEEGMILLER J. E., LASTER L. The enzymatic spectrophotometric method for determination of uric acid. J Lab Clin Med. 1959 Dec;54:903–913. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mäenpä P. H., Raivio K. O., Kekomäki M. P. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968 Sep 20;161(3847):1253–1254. doi: 10.1126/science.161.3847.1253. [DOI] [PubMed] [Google Scholar]
- NIERLICH D. P., MAGASANIK B. REGULATION OF PURINE RIBONUCLEOTIDE SYNTHESIS BY END PRODUCT INHIBITION. THE EFFECT OF ADENINE AND GUANINE RIBONUCLEOTIDES ON THE 5'-PHOSPHORIBOSYL-PYROPHOSPHATE AMIDOTRANSFERASE OF AEROBACTER AEROGENES. J Biol Chem. 1965 Jan;240:358–365. [PubMed] [Google Scholar]
- Ockerman P. A. Assay by a spectrofluorimetric method of glucose-6-phosphate in the liver in glycogen storage disease type 1. Clin Chim Acta. 1965 Oct;12(4):445–452. doi: 10.1016/0009-8981(65)90135-x. [DOI] [PubMed] [Google Scholar]
- Passonneau J. V., Lauderdale V. R. A comparison of three methods of glycogen measurement in tissues. Anal Biochem. 1974 Aug;60(2):405–412. doi: 10.1016/0003-2697(74)90248-6. [DOI] [PubMed] [Google Scholar]
- RENOLD A. E., HASTINGS A. B., NESBETT F. B. Studies on carbohydrate metabolism in rat liver slices. III. Utilization of glucose and fructose by liver from normal and diabetic animals. J Biol Chem. 1954 Aug;209(2):687–696. [PubMed] [Google Scholar]
- Raivio K. O., Becker 7. A., Meyer L. J., Greene M. L., Nuki G., Seegmiller J. E. Stimulation of human purine synthesis de novo by fructose infusion. Metabolism. 1975 Jul;24(7):861–869. doi: 10.1016/0026-0495(75)90133-x. [DOI] [PubMed] [Google Scholar]
- Raivio K. O., Kekomäki M. P., Mäenpä P. H. Depletion of liver adenine nucleotides induced by D-fructose. Dose-dependence and specificity of the fructose effect. Biochem Pharmacol. 1969 Oct;18(10):2615–2624. doi: 10.1016/0006-2952(69)90192-0. [DOI] [PubMed] [Google Scholar]
- Raivio K. O., Seegmiller J. E. Role of glutamine in purine synthesis and in guanine nucleotide formation in normal fibroblasts and in fibroblasts deficient in hypoxanthine phosphoribosyltransferase activity. Biochim Biophys Acta. 1973 Mar 19;299(2):283–292. doi: 10.1016/0005-2787(73)90351-1. [DOI] [PubMed] [Google Scholar]
- Roe T. F., Kogut M. D. The pathogenesis of hyperuricemia in glycogen storage disease, type I. Pediatr Res. 1977 May;11(5):664–669. doi: 10.1203/00006450-197705000-00008. [DOI] [PubMed] [Google Scholar]
- Sadeghi-Nejad A., Presente E., Binkiewicz A., Senior B. Studies in type I glycogenosis of the liver. The genesis and disposition of lactate. J Pediatr. 1974 Jul;85(1):49–54. doi: 10.1016/s0022-3476(74)80284-2. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Putnam C. W., Porter K. A., Halgrimson C. G., Corman J., Brown B. I., Gotlin R. W., Rodgerson D. O., Greene H. L. Portal diversion for the treatment of glycogen storage disease in humans. Ann Surg. 1973 Oct;178(4):525–539. doi: 10.1097/00000658-197310000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taunton O. D., Stifel F. B., Greene H. L., Herman R. H. Rapid reciprocal changes in rat hepatic glycolytic enzyme and fructose diphosphatase activities following insulin and glucagon injection. J Biol Chem. 1974 Nov 25;249(22):7228–7239. [PubMed] [Google Scholar]
- WYNGAARDEN J. B., ASHTON D. M. The regulation of activity of phosphoribosyl-pyrophosphate amidotransferase by purine ribonacleotides: a potential feedback control of purine biosyntoesis. J Biol Chem. 1959 Jun;234(6):1492–1496. [PubMed] [Google Scholar]
- Woods H. F., Eggleston L. V., Krebs H. A. The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J. 1970 Sep;119(3):501–510. doi: 10.1042/bj1190501. [DOI] [PMC free article] [PubMed] [Google Scholar]


