Abstract
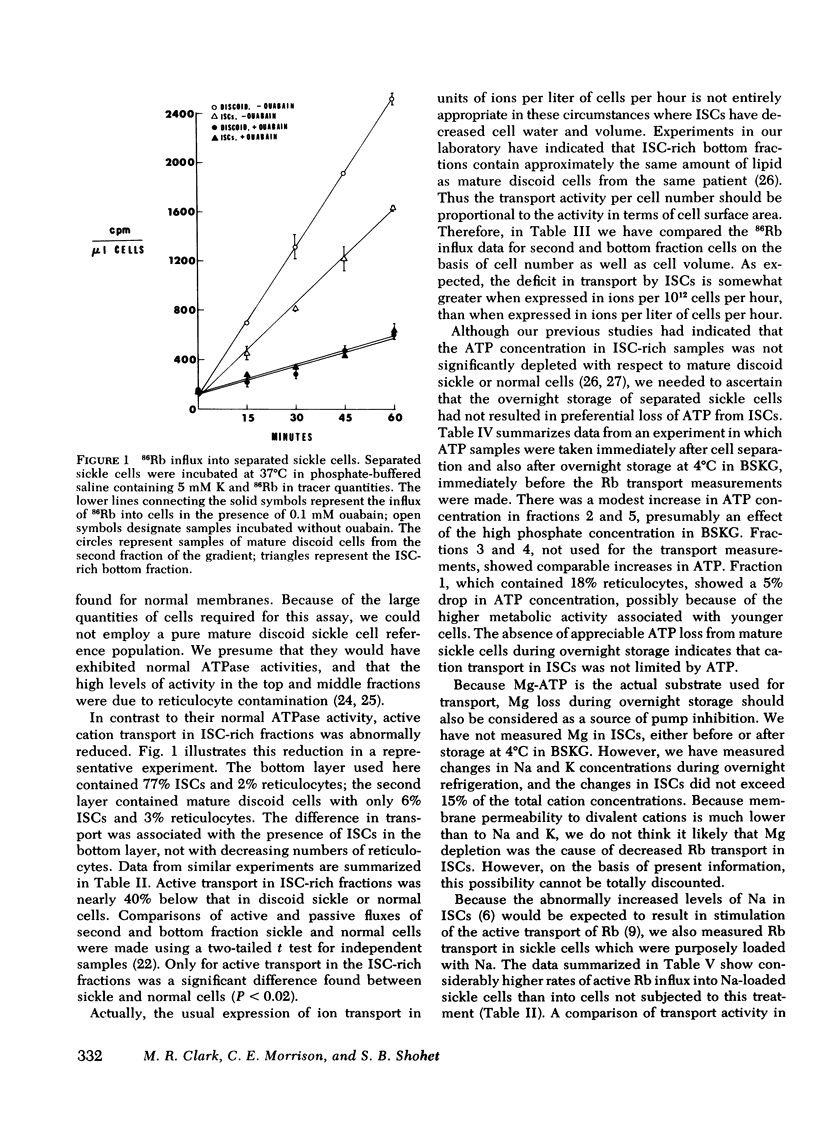
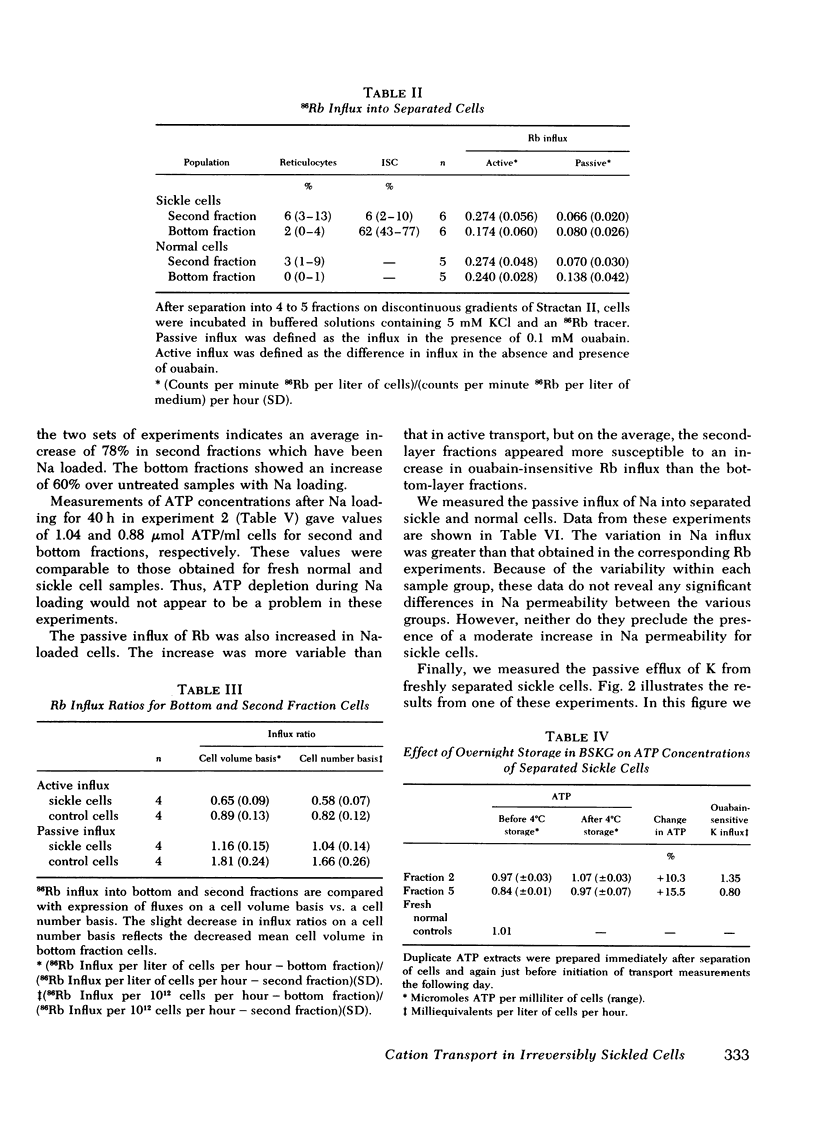
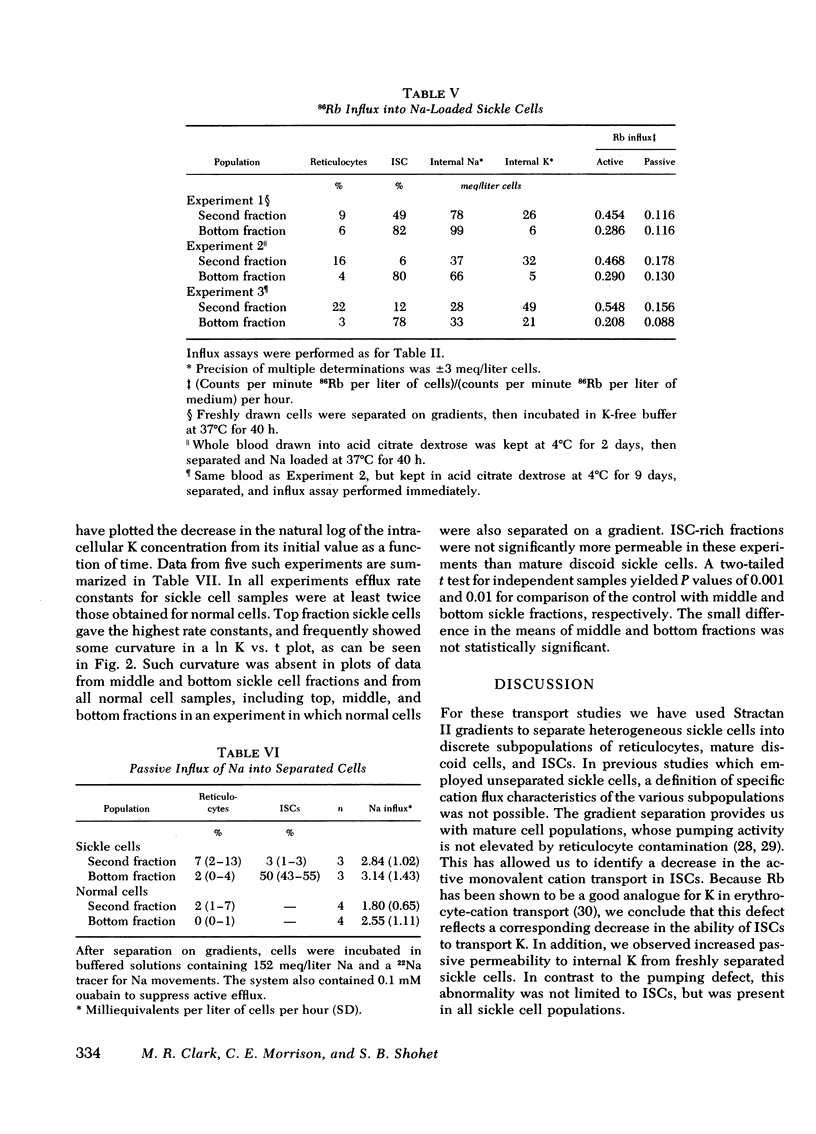
Using discontinuous density gradients of Stractan II, we have separated sickle cell blood into discrete subpopulations of reticulocytes, mature discoid cells, and irreversibly sickled cells (ISCs). We have measured active and passive fluxes of monovalent cations in mature discoid cells, ISCs, and normal control cells, also separated upon density gradients. These measurements revealed a decreased active cation transport in ISC-rich populations. However, parallel measurements of Na, K-ATPase activity showed normal ouabain-sensitive ATPase activity in ISCs. Passive permeability to external Rb was also normal in ISCs. The observation of depressed pump activity in intact ISCs, contrasted with normal ATPase activity in ISC membranes, suggests the presence of factors in the intact cell which inhibit the active transport of Na and K in ISCs.
Full text
PDF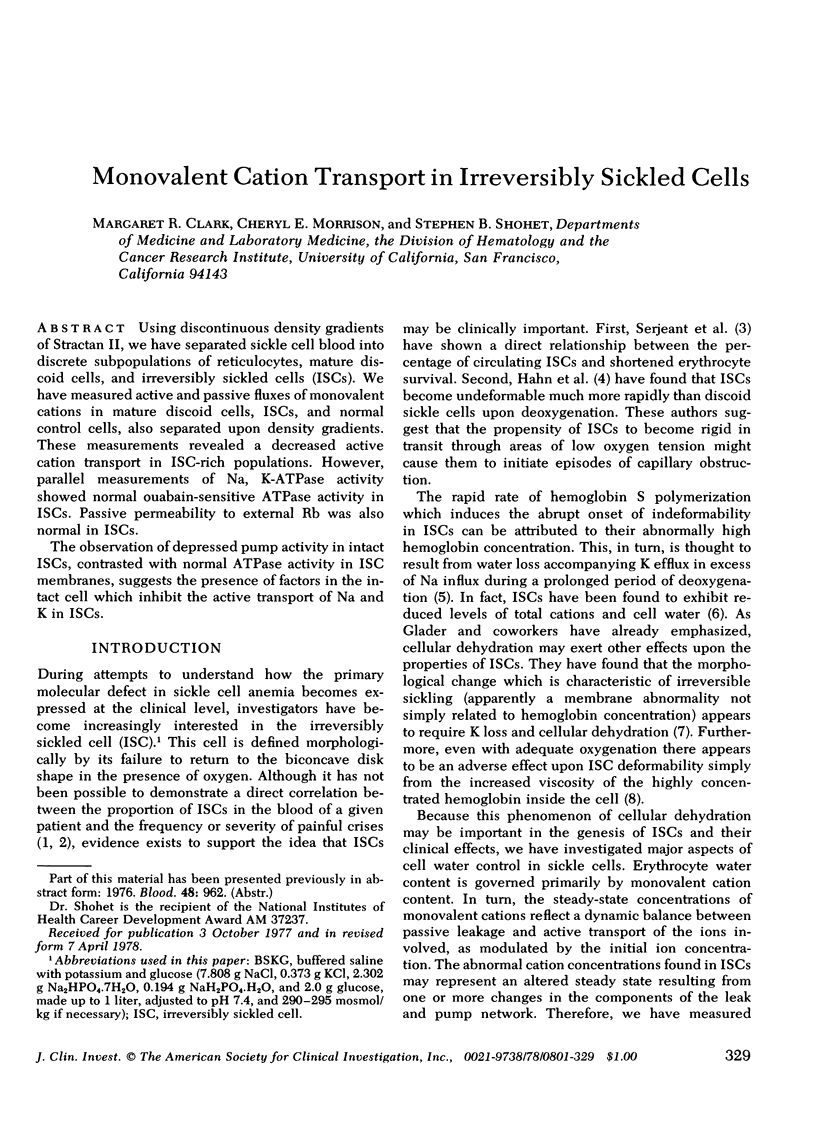




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEUTLER E., BALUDA M. C. SIMPLIFIED DETERMINATION OF BLOOD ADENOSINE TRIPHOSPHATE USING THE FIREFLY SYSTEM. Blood. 1964 May;23:688–698. [PubMed] [Google Scholar]
- BRECHER G. New methylene blue as a reticulocyte stain. Am J Clin Pathol. 1949 Sep;19(9):895–895. [PubMed] [Google Scholar]
- Bernstein J. C., Israel Y. Active transport of Rb86 in human red cells and rat brain slices. J Pharmacol Exp Ther. 1970 Aug;174(2):323–329. [PubMed] [Google Scholar]
- Blostein R. Relationships between erythrocyte membrane phosphorylation and adenosine triphosphate hydrolysis. J Biol Chem. 1968 Apr 25;243(8):1957–1965. [PubMed] [Google Scholar]
- Brewer G. J., Eaton J. W., Beck C. C., Feitler L., Shreffler D. C. Sodium-potssum tmulated ATPase activity of mammalian hemolysates: clinical observations and ominance of ATPase deficiency in the potassium polymorphism of sheep. J Lab Clin Med. 1968 May;71(5):744–753. [PubMed] [Google Scholar]
- CRANE R. K., LIPMANN F. The effect of arsenate on aerobic phosphorylation. J Biol Chem. 1953 Mar;201(1):235–243. [PubMed] [Google Scholar]
- Chien S., Usami S., Bertles J. F. Abnormal rheology of oxygenated blood in sickle cell anemia. J Clin Invest. 1970 Apr;49(4):623–634. doi: 10.1172/JCI106273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. R., Greenquist A. C., Shohet S. B. Stabilization of the shape of sickled cells by calcium and A23187. Blood. 1976 Dec;48(6):899–909. [PubMed] [Google Scholar]
- Clark M. R., Unger R. C., Shohet S. B. Monovalent cation composition and ATP and lipid content of irreversibly sickled cells. Blood. 1978 Jun;51(6):1169–1178. [PubMed] [Google Scholar]
- Corash L. M., Piomelli S., Chen H. C., Seaman C., Gross E. Separation of erythrocytes according to age on a simplified density gradient. J Lab Clin Med. 1974 Jul;84(1):147–151. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dunn M. J. Red blood cell calcium and magnesium: effects upon sodium and potassium transport and cellular morphology. Biochim Biophys Acta. 1974 May 30;352(1):97–116. doi: 10.1016/0005-2736(74)90182-5. [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Skelton T. D., Swofford H. S., Kolpin C. E., Jacob H. S. Elevated erythrocyte calcium in sickle cell disease. Nature. 1973 Nov 9;246(5428):105–106. doi: 10.1038/246105a0. [DOI] [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glader B. E., Fortier N., Albala M. M., Nathan D. G. Congenital hemolytic anemia associated with dehydrated erythrocytes and increased potassium loss. N Engl J Med. 1974 Sep 5;291(10):491–496. doi: 10.1056/NEJM197409052911003. [DOI] [PubMed] [Google Scholar]
- Glader B. E., Nathan D. G. Cation permeability alterations during sickling: relationship to cation composition and cellular hydration of irreversibly sickled cells. Blood. 1978 May;51(5):983–989. [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J. A., Messer M. J., Bradley T. B. Ultrastructure of sickling and unsickling in time-lapse studies. Br J Haematol. 1976 Dec;34(4):559–565. doi: 10.1111/j.1365-2141.1976.tb03601.x. [DOI] [PubMed] [Google Scholar]
- JOYCE C. R. Uptake of potassium and sodium by parts of packed human blood cell column. Q J Exp Physiol Cogn Med Sci. 1958 Jul;43(3):299–309. doi: 10.1113/expphysiol.1958.sp001333. [DOI] [PubMed] [Google Scholar]
- Kurantsin-Mills J., Kudo M., Addae S. K. Cation content and transport characteristics of the sickle-cell erythrocyte and their relationship with structural changes in the membrane. Clin Sci Mol Med. 1974 Jun;46(6):679–692. doi: 10.1042/cs0460679. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LaCelle P. L., Rothsteto A. The passive permeability of the red blood cell in cations. J Gen Physiol. 1966 Sep;50(1):171–188. doi: 10.1085/jgp.50.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D. G., Shohet S. B. Erythrocyte ion transport defects and hemolytic anemia: "hydrocytosis" and "desiccytosis". Semin Hematol. 1970 Oct;7(4):381–408. [PubMed] [Google Scholar]
- Palek J., Thomae M., Ozog D. Red cell calcium content and transmembrane calcium movements in sickle cell anemia. J Lab Clin Med. 1977 Jun;89(6):1365–1374. [PubMed] [Google Scholar]
- Rieber E. E., Veliz G., Pollack S. Red cells in sickle cell crisis: observations on the pathophysiology of crisis. Blood. 1977 Jun;49(6):967–979. [PubMed] [Google Scholar]
- Rimington C. Haemoglobinometry. Br Med J. 1942 Feb 7;1(4231):177–178. doi: 10.1136/bmj.1.4231.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serjeant G. R., Serjeant B. E., Milner P. F. The irreversibly sickled cell; a determinant of haemolysis in sickle cell anaemia. Br J Haematol. 1969 Dec;17(6):527–533. doi: 10.1111/j.1365-2141.1969.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Steinberg M. H., Dreiling B. J., Lovell W. J. Sickle cell anemia: erythrokinetics, blood volumes, and a study of possible determinants of severity. Am J Hematol. 1977;2(1):17–23. doi: 10.1002/ajh.2830020103. [DOI] [PubMed] [Google Scholar]
- WELT L. G., SACHS J. R., MCMANUS T. J. AN ION TRANSPORT DEFECT IN ERYTHROCYTES FROM UREMIC PATIENTS. Trans Assoc Am Physicians. 1964;77:169–181. [PubMed] [Google Scholar]
- Yunis A. A., Arimura G. K. Sodium-potassium dependent adenosine triphosphatase of mammalian reticulocytes and mature red blood cells. Proc Soc Exp Biol Med. 1966 Feb;121(2):327–329. doi: 10.3181/00379727-121-30770. [DOI] [PubMed] [Google Scholar]


