Abstract
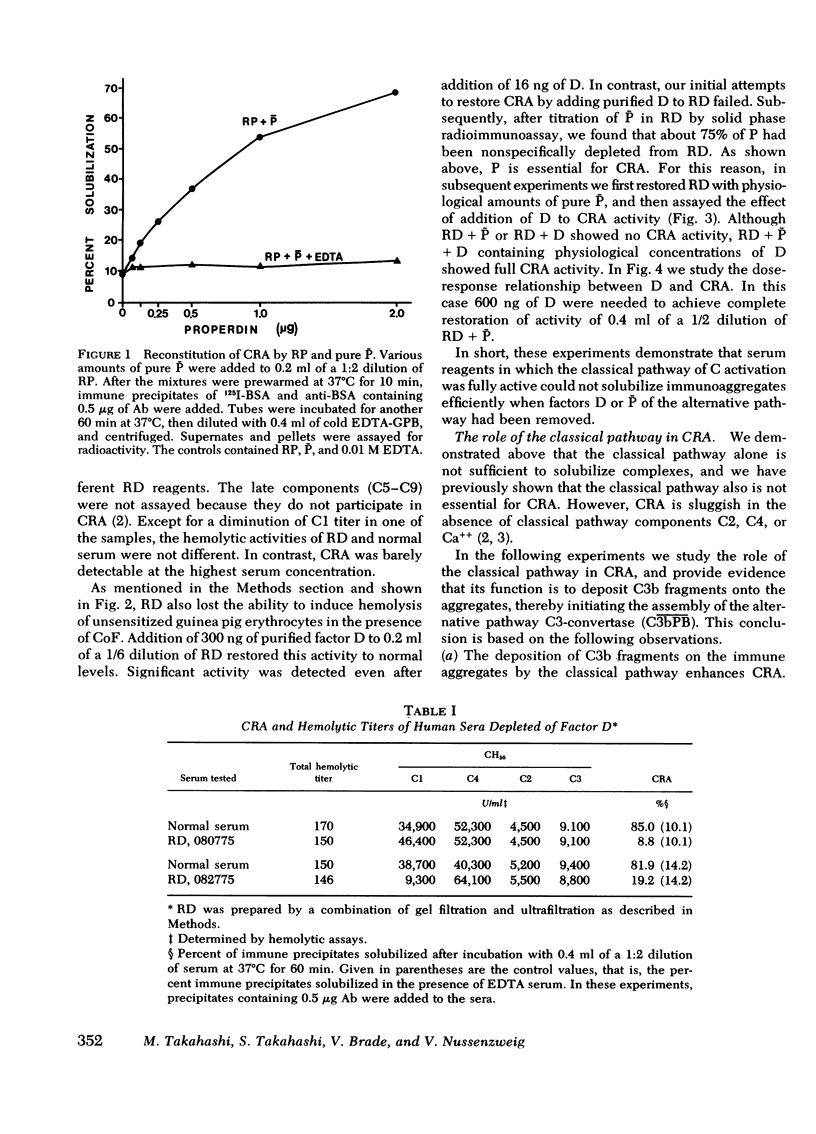
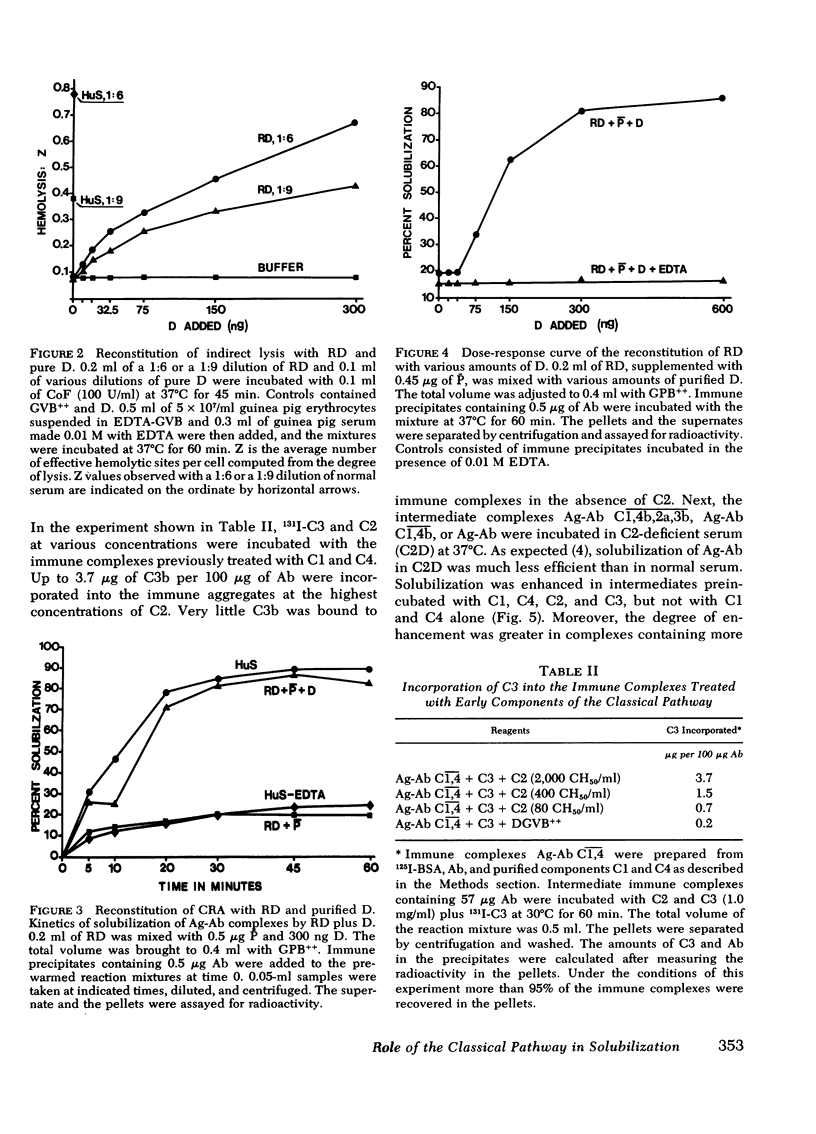
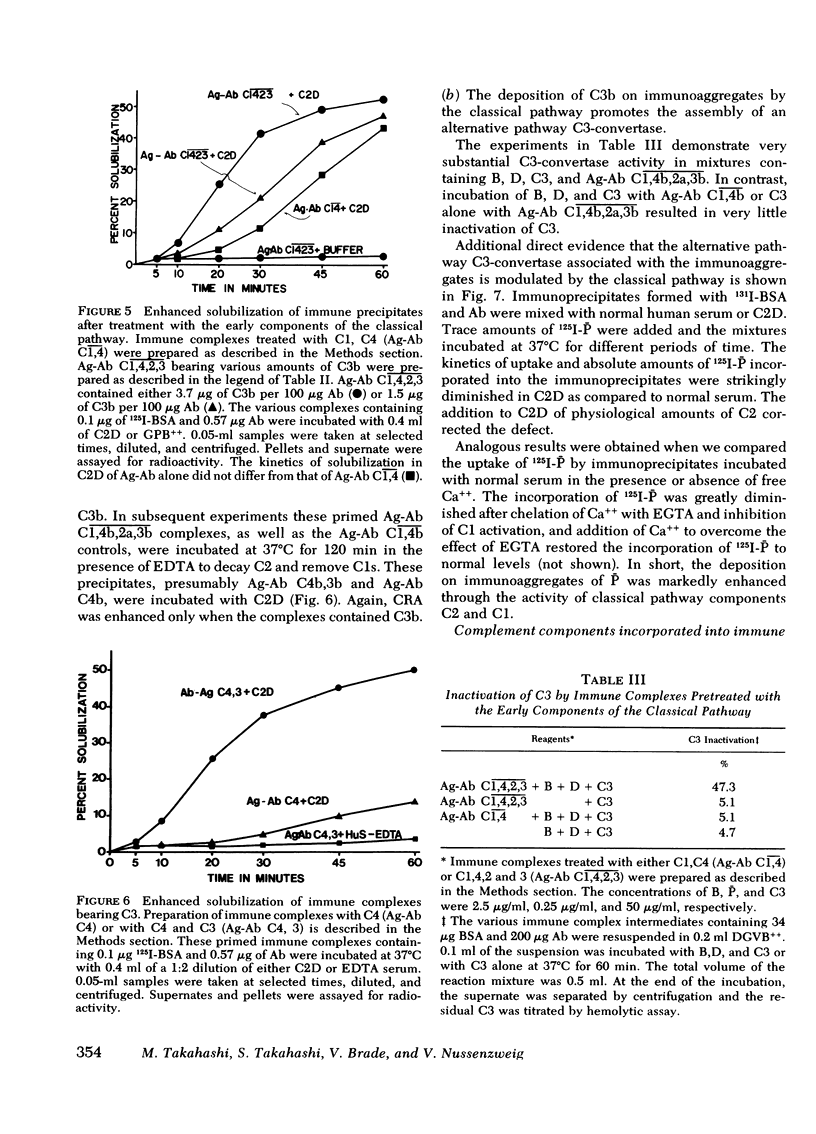
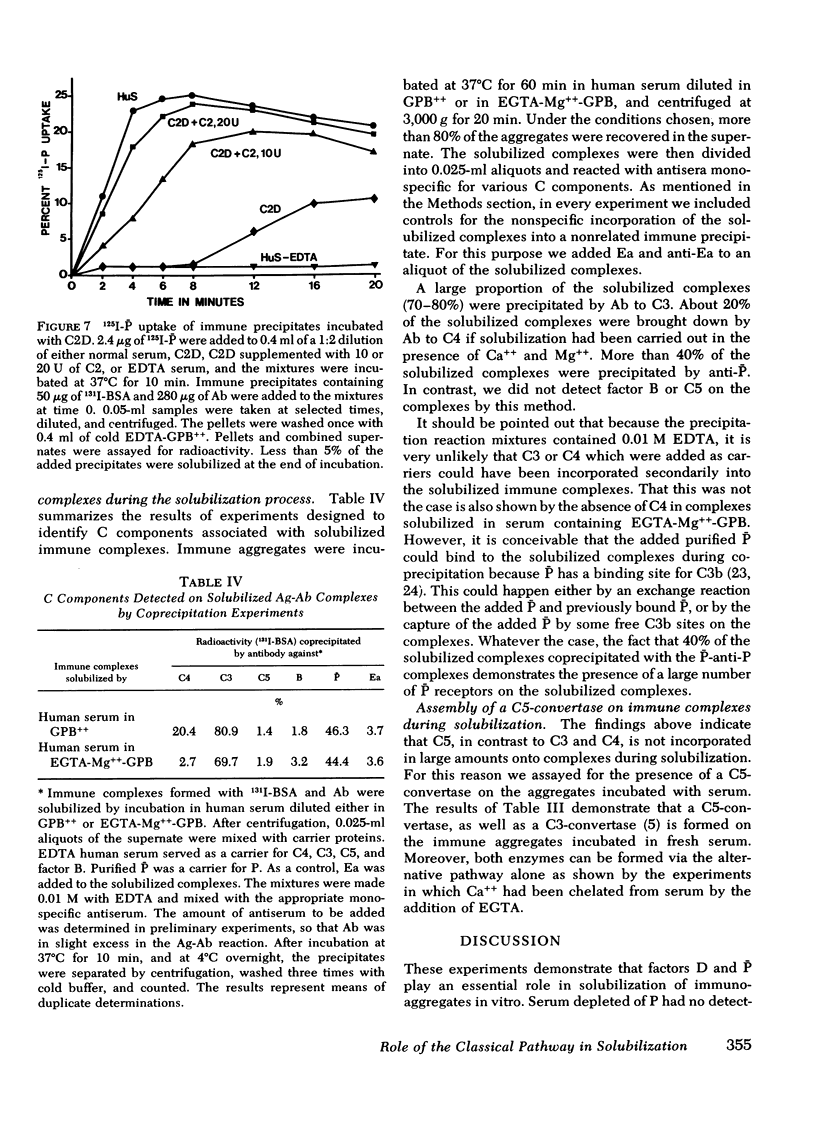
In this paper we examine the role of the classical pathway in the complement-mediated solubilization of immune precipitates (CRA). Serum reagents were depleted of the alternative pathway components properdin and factor D. Both depleted reagents lack CRA although they have almost intact hemolytic activity. Also, immune complexes were not solubilized when incubated with high concentrations of the classical pathway components (C1, C4, C2, and C3. We conclude that CRA is not mediated by the classical pathway alone. Activation of the classical pathway by the immune aggregates greatly enhances CRA. The effect of the classical pathway is to deposit C3b on the antigen-antibody lattice and promote the assembly of a lattice-associated, properdin-dependent C3-convertase. Although C3, C4, and properdin were detected on complexes solubilized by serum in the presence of Ca++ and Mg++, only C3 and properdin were found on the complexes when Ca++ had been chelated by ethylene glycol-bis-(beta-aminoethyl ether), N,N'-tetraacetic acid. In both situations the aggregates were capable of converting C5 in the fluid phase. However, no C5 was found on the solubilized complexes. These findings suggest that in contrast to nascent C3b and C4b, nascent C5-9 lacks binding affinity for immune aggregates.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolotin C., Morris S., Tack B., Prahl J. Purification and structural analysis of the fourth component of human complement. Biochemistry. 1977 May 3;16(9):2008–2015. doi: 10.1021/bi00628a039. [DOI] [PubMed] [Google Scholar]
- Brade V., Lee G. D., Nicholson A., Shin H. S., Mayer M. M. The reaction of zymosan with the properdin system in normal and C4-deficienct guinea pig serum. Demonstration of C3- and C5-cleaving multi-unit enzymes, both containing factor B, and acceleration of their formation by the classical complement pathway. J Immunol. 1973 Nov;111(5):1389–1400. [PubMed] [Google Scholar]
- Brai M., Osler A. G. Studies of the C3 shunt activation in cobra venom induced lysis of unsensitized erythrocytes. Proc Soc Exp Biol Med. 1972 Jul;140(3):1116–1121. doi: 10.3181/00379727-140-36623. [DOI] [PubMed] [Google Scholar]
- Chapitis J., Lepow I. H. Multiple sedimenting species of properdin in human serum and interaction of purified properdin with the third component of complement. J Exp Med. 1976 Feb 1;143(2):241–257. doi: 10.1084/jem.143.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czop J., Nussenzweig V. Studies on the mechanism of solubilization of immune precipitates by serum. J Exp Med. 1976 Mar 1;143(3):615–630. doi: 10.1084/jem.143.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensky J., Hinz C. F., Jr, Todd E. W., Wedgwood R. J., Boyer J. T., Lepow I. H. Properties of highly purified human properdin. J Immunol. 1968 Jan;100(1):142–158. [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med. 1975 Oct 1;142(4):856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F., Ruddy S. Formation of a hemolytically active cellular intermediate by the interaction between properdin factors B and D and the activated third component of complement. J Exp Med. 1973 Dec 1;138(6):1305–1313. doi: 10.1084/jem.138.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F., Ruddy S. Properdin factor D: characterization of its active site and isolation of the precursor form. J Exp Med. 1974 Feb 1;139(2):355–366. doi: 10.1084/jem.139.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., Porter R. R., Sim R. B. The unactivated form of the first component of human complement, C1. Biochem J. 1976 Sep 1;157(3):541–548. doi: 10.1042/bj1570541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The role of properdin in the alternate pathway of complement activation. J Exp Med. 1974 Jan 1;139(1):44–57. doi: 10.1084/jem.139.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu S., Fujita T., Doi S., Nagaki K., Inai S. Conversion of C5 precipitin line in the serum treated with activating substances of complement system. J Immunol Methods. 1977;15(4):373–381. doi: 10.1016/0022-1759(77)90098-9. [DOI] [PubMed] [Google Scholar]
- LEPOW I. H., PILLEMER L., SCHOENBERG M. D., TODD E. W., WEDGWOOD R. J. The properdin system and immunity. X. Characterization of partially purified human properdin. J Immunol. 1959 Oct;83:428–436. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MUELLER-EBERHARD H. J., LEPOW I. H. C'1 ESTERASE EFFECT ON ACTIVITY AND PHYSICOCHEMICAL PROPERTIES OF THE FOURTH COMPONENT OF COMPLEMENT. J Exp Med. 1965 May 1;121:819–833. doi: 10.1084/jem.121.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Miller G. W., Nussenzweig V. A new complement function: solubilization of antigen-antibody aggregates. Proc Natl Acad Sci U S A. 1975 Feb;72(2):418–422. doi: 10.1073/pnas.72.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minta J. O., Goodkofsky I., Lepow I. H. Solid phase radioimmunoassay of properdin. Immunochemistry. 1973 May;10(5):341–350. doi: 10.1016/0019-2791(73)90082-7. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Götze O. C3 proactivator convertase and its mode of action. J Exp Med. 1972 Apr 1;135(4):1003–1008. doi: 10.1084/jem.135.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON R. A., Jr An alternative mechanism for the properdin system. J Exp Med. 1958 Oct 1;108(4):515–535. doi: 10.1084/jem.108.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILSSON U. R., MUELLER-EBERHARD H. J. ISOLATION OF BETA IF-GLOBULIN FROM HUMAN SERUM AND ITS CHARACTERIZATION AS THE FIFTH COMPONENT OF COMPLEMENT. J Exp Med. 1965 Aug 1;122:277–298. doi: 10.1084/jem.122.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Nicholson A., Brade V., Lee G. D., Shin H. S., Mayer M. M. Kinetic studies of the formation of the properdin system enzymes on zymosan: evidence that nascent C3b controls the rate of assembly. J Immunol. 1974 Mar;112(3):1115–1123. [PubMed] [Google Scholar]
- Nicol P. A., Lachmann P. J. The alternate pathway of complement activation. The role of C3 and its inactivator (KAF). Immunology. 1973 Feb;24(2):259–275. [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Medicus R. G., Gïtze O., Müller-Eberhard H. J. Properdin- and nephritic factor-dependent C3 convertases: requirement of native C3 for enzyme formation and the function of bound C3b as properdin receptor. J Exp Med. 1975 Sep 1;142(3):760–772. doi: 10.1084/jem.142.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODD E. W., PILLEMER L., LEPOW I. H. The properdin system and immunity. IX. Studies on the purification of human properdin. J Immunol. 1959 Oct;83:418–427. [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Czop J., Ferreira A., Nussenzweig V. Mechanism of solubilization of immune aggregates by complement. Implications for immunopathology. Transplant Rev. 1976;32:121–139. doi: 10.1111/j.1600-065x.1976.tb00231.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Tack B. F., Nussenzweig V. Requirements for the solubilization of immune aggregates by complement: assembly of a factor B-dependent C3-convertase on the immune complexes. J Exp Med. 1977 Jan 1;145(1):86–100. doi: 10.1084/jem.145.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verroust P. J., Wilson C. B., Cooper N. R., Edgington T. S., Dixon F. J. Glomerular complement components in human glomerulonephritis. J Clin Invest. 1974 Jan;53(1):77–84. doi: 10.1172/JCI107562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby W. F., Mayer M. M. Antibody-complement complexes. Science. 1965 Nov 12;150(3698):907–908. doi: 10.1126/science.150.3698.907. [DOI] [PubMed] [Google Scholar]



