Summary
Age-related neurodegenerative and neurochemical changes are thought to underlie decline in motor and cognitive functions, but compensatory processes in cortical and subcortical function may allow maintenance of performance level in some people. Our objective was to investigate age-related changes in the motor system of the human brain using functional MRI. Twenty six right handed volunteers were scanned whilst performing an isometric, dynamic, visually paced hand grip task, using dominant (right) and non-dominant (left) hands in separate sessions. Hand grip with visual feedback activated a network of cortical and subcortical regions known to be involved in the generation of simple motor acts. In addition, activation was seen in a putative human ‘grasping circuit’, involving rostral ventral premotor cortex (Brodmann area 44) and intraparietal sulcus. Within this network, a number of regions were more likely to be activated the older the subject. In particular, age-related changes in task-specific activations were demonstrated in left deep anterior central sulcus when using the dominant or non-dominant hand. Additional age-related increases were seen in caudal dorsal premotor cortex, caudal cingulate sulcus, intraparietal sulcus, insula, frontal operculum and cerebellar vermis. We have demonstrated a clear age-related effect in the neural correlates of motor performance, and furthermore suggest that these changes are non-linear. These results support the notion that an adaptable and plastic motor network is able to respond to age-related degenerative changes in order to maintain performance levels.
Keywords: ageing, functional MRI, hand grip, motor system
Introduction
Impairments in a number of cognitive tasks are seen as part of the normal ageing process in humans. Functional imaging studies have examined for differences in recruitment of brain regions during both motor (Calautti et al., 2001; Mattay et al., 2002) and cognitive tasks (D’Esposito et al., 1999; Esposito et al., 1999; Grady, 2000). Many of these studies have found greater activations in older subjects in a number of regions compared with younger subjects. However, this may only be the case for those older subjects in whom the level of performance is comparable to that in younger subjects (Reuter-Lorenz et al., 1999; Mattay et al., 2002). It has been suggested that interruption of the normal neural networks subserving cognitive performance by age-related neurodegenerative and neurochemical changes underlies decline in function (Wenk et al., 1989; Volkow et al., 1998), but that compensatory processes in cortical and subcortical function allow maintenance of performance level in some people.
Linear decreases in performance as a function of increasing age have been demonstrated with motor tasks such as repetitive finger tapping (Shimoyama et al., 1990), but more complex, non-linear effects are seen in more demanding timed tasks and visually guided hand movements (Houx and Jolles, 1993; Kauranen and Vanharanta, 1996; Smith et al., 1999). Functional imaging studies of changes in the motor system have used repetitive or cued finger tapping (Calautti et al., 2001; Mattay et al., 2002) and have shown greater age-associated activation in a number of brain regions within the motor system. We were interested to see whether such age-related changes would be seen during a phylogenetically older task (Napier, 1956) such as hand grip. Hand grip is a ubiquitous motor task performed in the real world, but has been studied infrequently with functional imaging. We hypothesized that grasping would result in activations in a widespread fronto-parietal network (Binkofski et al., 1998; Ehrsson et al., 2001), within which we would demonstrate age-related differential activations, and furthermore that any age effects would be non-linear in view of the degree of ‘on-line’ monitoring and precision required during our task (Smith et al., 1999). Furthermore, we explored the possibility that there is an interaction between age and task performance.
Methods
Subjects
Twenty six healthy volunteers, aged 21–80 years, comprising 17 male subjects (range 27–80 years, mean age 50.2 years) and nine female subjects (range 26–66 years, mean age 44.7 years), participated in the study. One subject performed tasks only with the dominant hand, and one subject only with the non-dominant hand. All subjects were right handed according to the Edinburgh handedness scale (Oldfield, 1971). They reported no history of neurological illness or psychiatric history and were not taking regular medication. Full written consent was obtained from all subjects in accordance to the Declaration of Helsinki. The study was approved by the Joint Ethics Committee of the Institute of Neurology and National Hospital for Neurology and Neurosurgery, London.
Motor paradigm
Subjects performed a dynamic isometric hand grip task using dominant (right) and non-dominant (left) hands in separate sessions, and in a randomized counterbalanced order, using a magnetic resonance imaging compatible manipulandum consisting of two force transducers (Honeywell FSG15N1A, Honeywell, NJ, USA) situated between two moulded plastic bars (width 6 cm). Compression of the two bars by isometric handgrip resulted in the generation of a differential voltage signal, linearly proportional to force exerted, which was fed into a signal conditioner (CED 1902, Cambridge Electronic Design, Cambridge, UK). This signal was digitized (CED 1401, Cambridge Electronic Design, Cambridge, UK) and fed into a computer running Cogent 2000 (Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/Cogent2000.html). The dynamic change in recorded signal was projected in real time onto a screen as a column whose height varied linearly with change in voltage and hence force. Prior to scanning, but whilst lying in the scanner, subjects were asked to grip the manipulandum with maximum force to generate a maximum voluntary contraction (MVC). During scanning, subjects performed paced isometric dynamic handgrips in blocks of 20 s, alternating with 20 s rest. A total of 24 blocks of handgrip and 24 rest blocks were performed per session. Target forces and rates of handgrip were constant within each 20 s block, but were varied between blocks in a randomized counterbalanced order. Target forces during scanning were set at 10, 20, 40 and 60% of MVC for each subject, and were indicated by a horizontal bar on the screen. The required rate of hand grip was indicated visually by a cross displayed at the bottom of the screen for 0.3 s at a rate of either 0.33 or 0.67 Hz. Both rates of grip were comfortable for all subjects. Variation in rate was included only to help maintain subject attention throughout the session. The appearance of the cross indicated that the subject was to perform a single brief handgrip, to be continued until the column representing force applied came into contact with the horizontal bar on the screen, at which point the grip could be released. Prior to scanning, subjects were pre-trained on at least eight handgrip-rest blocks, or until comfortable with the task, utilizing each of the eight force-rate combinations.
Data acquisition
A Siemens VISION system (Siemens, Erlangen, Germany), operating at 2 T, was used to acquire both T1-weighted anatomical images (1 × 1 × 1.5 mm voxels) and T2*-weighted MRI transverse echo-planar images (EPI) [64 × 64 3 × 3 mm2 pixels, echo time (TE) = 40 ms] with blood oxygen level dependent (BOLD) contrast. Each echoplanar image comprised 48 1.8-mm thick axial slices taken every 3 mm, positioned to cover the whole cerebrum. A total of 270 volumes were acquired continuously during each session, with an effective repetition time (TR) of 3.649 s per volume. The first six volumes were discarded to allow for T1 equilibration effects.
Image analysis
Imaging data were analysed using Statistical Parametric Mapping (SPM99; Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spm/) (Friston et al., 1995a; Worsley and Friston, 1995) implemented in Matlab5 (The Mathworks Inc., USA). All volumes were realigned spatially to the first volume in order to correct for interscan movement. No subject moved >2 mm in any direction, but some of this movement was task related. In order to remove some of this unwanted movement-related variance without removing variance attributable to the motor task, realigned images were processed using the ‘unwarp’ toolbox in SPM99 (Andersson et al., 2001), which is predicated on the assumption that susceptibility-by-movement interaction is responsible for a sizeable part of residual movement-related variance. Given the observed variance (after realignment) and the realignment parameters, estimates of how deformations changed with subject movement were made, which were subsequently used to minimize movement-related variance.
To correct for their different acquisition times, the signal measured in each slice was shifted relative to the acquisition of the middle slice using sinc interpolation in time. Resulting volumes were then normalized to a standard EPI template based on the Montreal Neurological Institute (MNI) reference brain in Talairach space (Talairach and Tournoux, 1988), and resampled to 3 × 3 × 3 mm3 voxels. In order to directly compare images obtained using either hand, images obtained using the non-dominant hand were flipped about the midsagital plane. Normalization was then performed using a symmetrical EPI template to create a separate set of normalized images. The symmetrical EPI template was created by averaging the standard EPI template and its mirror image about the midsagital plane. All normalized images were then smoothed with an isotropic, 8 mm, full-width, half-maximum Gaussian kernel to account for residual inter-subject differences and to allow valid statistical inference according to Gaussian random field theory (Friston et al., 1995b). The time series in each voxel were high pass filtered at 1/100 Hz to remove low frequency confounds, and scaled to a grand mean of 100 over voxels and scans within each session.
Statistical analysis was performed in two stages. In the first stage, using a single subject fixed effects model, all handgrips were defined as a single event type and modelled as delta functions (Friston et al., 1998). The data were modelled using a set of orthogonalized polynomial expansions up to the third order (by forward model selection). Each term is represented by the interaction between a delta function and the peak force exerted (expressed as a percentage of MVC) during each handgrip. In general, the nth order term is given by forcen·delta (Büchel et al., 1998) (Fig. 1). The resulting covariates were convolved with a canonical synthetic haemodynamic response function, and were used in a general linear model (Friston et al., 1995a), together with a single covariate representing the mean (constant) term over scans. The parameter estimates for each covariate resulting from the least mean squares fit of the model to the data were calculated, and statistical parametric maps of the t statistic (SPM{t}) resulting from linear contrasts of each covariate (Friston et al., 1995a) were generated and stored as separate images for each subject.
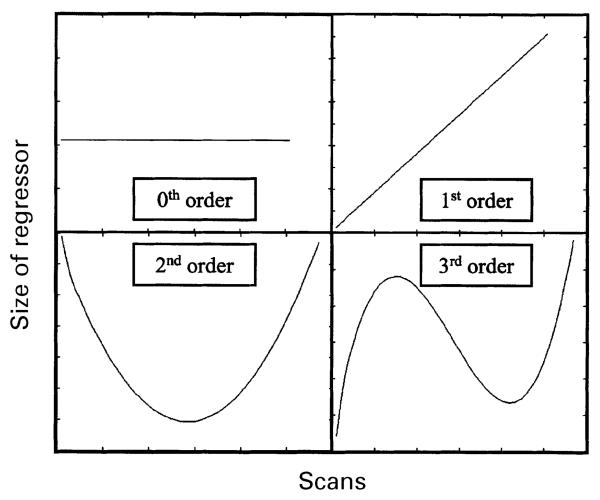
Fig. 1.
The concept of orthogonalized polynomial regressors is illustrated using these simple examples. The 0th order term represents the height of the delta functions used to model each event. The 1st, 2nd, and 3rd order terms represent the element-wise product of a linear (or non-linear) term and the 0th order term (Büchel et al., 1998). The orthogonalized 1st, 2nd, and 3rd order terms are shown.
In order to create activation maps representing the main effects of hand grip (0th order), as well as linear (1st order) and non-linear (2nd and 3rd order) changes of signal in relation to peak hand grip force, random-effects analyses were performed (Friston et al., 1999). The data for the second stage of analysis comprised the pooled parameter estimates for each covariate across all subjects. Contrast images for each subject were entered into a one sample t-tests for each covariate of interest. The SPM{t}s were thresholded at P < 0.05, corrected for multiple comparisons across whole brain.
We were interested to make direct comparisons between task-related brain activations using the dominant and non-dominant hands. Contrast images for the main effects of hand grip for the dominant and non-dominant hands were entered into a two sample t-test. By entering two contrast images per subject into the model, a potential source of non-sphericity was introduced. Non-sphericity correction within SPM was therefore applied. The comparison between hands was performed in three stages: (i) a direct comparison of the contrast images for dominant and non-dominant hand grip; (ii) a comparison of contrast images flipped and unflipped about the sagital midline for each hand; and (iii) a comparison of dominant hand unflipped contrast images, and flipped non-dominant hand contrast images. SPM{t}s were thresholded at P < 0.05, corrected for multiple comparisons across whole brain.
Our second experimental question concerns the possible effect of age on both the main affects of hand grip, and on the linear and non-linear effects of increasing force. Thus, we performed linear regression analyses within SPM99, in which the two orthogonal covariates were: (i) contrast images for each subject for the effect of interest (0th, 1st, 2nd or 3rd order effects); and (ii) a single value representing age2 for each subject (mean corrected across the group). We hypothesized a priori that we would find non-linear changes in activation in keeping with previous behavioural data (Smith et al., 1999), and so chose to use age2 rather than age as the second covariate. In order to identify differential age-related effects only within the motor network activated by hand grip, we performed a conjunction analysis between two orthogonal contrasts: effects of hand grip and effects of age2. Conjunction analysis relies on the conjoint testing of multiple, in this case two hypotheses. Thus for voxels that are significant in the conjunction analysis, we are able to reject the null hypotheses that there is no effect of age2 and no effect of hand grip, i.e. significant voxels exhibit a response to hand grip and this response varies as a function of age2 (Price and Friston, 1997). We are able to perform such a conjunction analysis at the random effects level because only one contrast image per subject is entered into the model, so that our covariates are independent and orthogonal, thus avoiding assumptions about non-sphericity. For significant voxels, the correlation coefficient for the plot of parameter estimate against age2 for each subject, together with the corresponding P-value, was calculated.
All SPM{t}s were transformed to the unit normal Z-distribution to create a statistical parametric map (SPM{Z}). All t-tests carried out within SPM were one tailed.
Anatomical identification was carefully performed by superimposing the maxima of activation foci both on the MNI brain and on the normalized structural images of each subject, and labelling with the aid of the atlas of Duvernoy (1999).
Results
Behavioural results
Mean MVC was 7.38 ± 1.05 kg using the dominant hand, and 7.12 ± 1.07 kg using the non-dominant hand. There was no correlation between age or age2 and peak MVC [dominant hand: r = 0.24, P = not significant (NS); non-dominant hand: r = 0.11, P = NS].
Main effects of handgrip
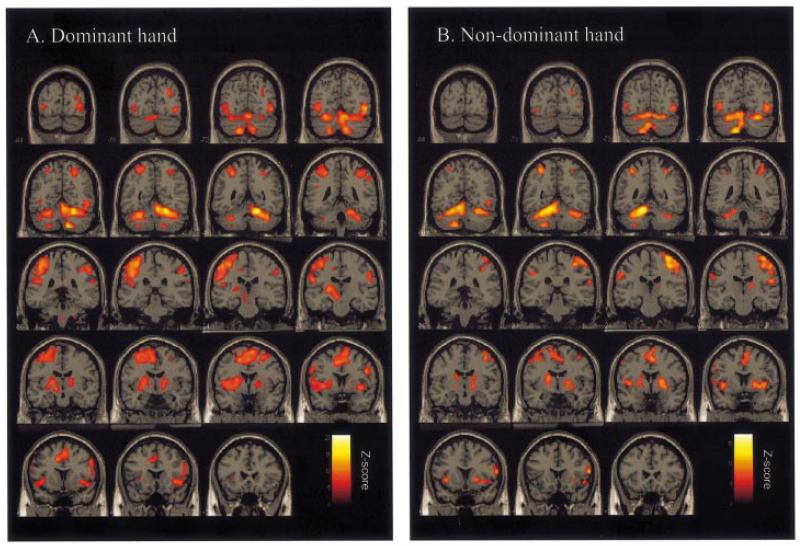
Regions activated by the main effects comparison of isometric dynamic hand grip compared with rest, irrespective of force applied, are shown in Table 1 and Fig. 2. Activations were seen in a network of regions, which was similar for dominant and non-dominant hands. The most lateralized activations were in contralateral sensorimotor cortex and ipsilateral superior cerebellum. Other activations were bilaterally distributed, including dorsal lateral premotor cortex (PMd) and ventral lateral premotor cortex (PMv), supplementary motor area (SMA), cingulate motor areas, inferior parietal cortex and intraparietal sulcus, insula cortex, cerebellar vermis, and both inferior and superior cerebellar hemispheres.
Table 1.
Main effects of hand grip
| Region | Dominant hand |
Non-dominant hand |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Side | Talairach coordinates in MNI space |
Z-value | Side | Talairach coordinates in MNI space |
Z-value | |||||
| x | y | z | x | y | z | |||||
| Central sulcus | CL | −34 | −30 | 56 | 7.38 | CL | 38 | −26 | 52 | 7.1 |
| CL | −30 | −30 | 66 | 6.4 | ||||||
| Postcentral gyrus | CL | −52 | −24 | 32 | 6.32 | CL | 54 | −18 | 50 | 6.46 |
| CL | −54 | −18 | 26 | 6.37 | CL | 48 | −26 | 58 | 6.31 | |
| Postcentral sulcus | CL | −50 | −22 | 50 | 6.61 | CL | 54 | −20 | 38 | 5.7 |
| Precentral gyrus (BA 4/6) | CL | 36 | −22 | 64 | 7.31 | |||||
| PMd | CL | −34 | −6 | 58 | 6.33 | CL | 42 | −10 | 54 | 5.87 |
| CL | −24 | −12 | 66 | 6.11 | IL | −38 | −4 | 52 | 5.76 | |
| IL | 28 | 0 | 54 | 5.94 | ||||||
| IL | 40 | 2 | 60 | 5.15 | ||||||
| Caudal PMv | CL | −54 | 6 | 6 | 5.85 | IL | −54 | 6 | 6 | 5.05 |
| IL | 50 | 6 | 28 | 6.6 | IL | −58 | 10 | 26 | 5.34 | |
| Rostral PMv (BA 44) | CL | −56 | 6 | 14 | 5.2 | CL | 62 | 16 | 12 | 6.95 |
| IL | 58 | 16 | 12 | 5.74 | IL | −58 | 6 | 4 | 5.05 | |
| Rostral cingulate sulcus | CL | −10 | 10 | 40 | 6.72 | |||||
| IL | 2 | 2 | 50 | 6.48 | ||||||
| Caudal cingulate sulcus | CL | −6 | −4 | 54 | 6.39 | IL | −10 | −6 | 62 | 5.67 |
| SMA | CL | −8 | −4 | 64 | 6.16 | CL | 8 | −2 | 68 | 6.31 |
| Pre-SMA | IL | 10 | 2 | 66 | 6.61 | CL | 8 | 4 | 58 | 6.28 |
| Insula cortex | CL | −38 | 2 | −2 | 6.3 | CL | 40 | 4 | 2 | 6.25 |
| CL | −40 | 14 | −4 | 5.53 | CL | 50 | 14 | −8 | 5.85 | |
| IL | 40 | 8 | −4 | 6.54 | IL | −36 | 12 | −2 | 6.07 | |
| IL | 44 | 2 | 10 | 5.55 | IL | −38 | 4 | 0 | 6.06 | |
| Supramarginal gyrus | CL | −44 | −36 | 24 | 6.12 | IL | −52 | −26 | 36 | 5.65 |
| IL | 52 | −36 | 44 | 5.98 | ||||||
| Intraparietal sulcus | IL | 48 | −38 | 52 | 6.15 | CL | 30 | −78 | 26 | 5.47 |
| IL | 28 | −76 | 26 | 5.96 | IL | −32 | −52 | 54 | 6.35 | |
| Superior parietal cortex | CL | −30 | −54 | 58 | 6.05 | |||||
| Parietal operculum | CL | −46 | −26 | 16 | 5.88 | CL | 56 | −18 | 22 | 6.16 |
| Frontal operculum | CL | −46 | 0 | 6 | 6 | |||||
| Superior temporal gyrus | CL | −48 | 8 | −10 | 5.72 | CL | 64 | −30 | 20 | 5.48 |
| Putamen | CL | −22 | −4 | 2 | 6.3 | CL | 28 | 4 | −2 | 6.14 |
| IL | −22 | 0 | 6 | 5.49 | ||||||
| Ventrolateral thalamus | CL | −14 | −16 | 2 | 6.05 | |||||
| IL | 16 | −10 | 12 | 5.87 | ||||||
| Posterior lateral thalamus | CL | 18 | −18 | 14 | 5.48 | |||||
| Red nucleus | CL | −12 | −14 | −6 | 6.06 | |||||
| Cerebellum (V) | IL | 20 | −50 | −20 | >8.0 | IL | −14 | −50 | −22 | 7.83 |
| Cerebellum (VI) | IL | 38 | −50 | −32 | 6.57 | IL | −12 | −70 | −22 | 6.09 |
| Cerebellum (VI) | CL | −30 | −54 | −30 | 7.05 | CL | 28 | −66 | −24 | 6.88 |
| Cerebellum (VI) | CL | −28 | −66 | −20 | 6.68 | CL | 34 | −58 | −26 | 6.31 |
| Cerebellum (CrI) | CL | −36 | −44 | −40 | 5.42 | IL | −46 | −58 | −34 | 6 |
| CL | 46 | −56 | −30 | 5.66 | ||||||
| Cerebellum (VIIIA) | CL | −20 | −68 | −46 | 6.95 | CL | 12 | −72 | −42 | 5.36 |
| Cerebellum (IX) | IL | 12 | −60 | −48 | 7.65 | IL | −14 | −56 | −48 | 7.2 |
| Cerebellar vermis (V) | M | 6 | −54 | −18 | 7.51 | |||||
| Cerebellar vermis (VI) | M | 6 | −66 | −20 | 7.05 | M | −4 | −68 | −24 | 6.88 |
| Cerebellar vermis (VIIIB) | M | −4 | −70 | −36 | 6.42 | |||||
Location of brain regions demonstrating significant activations for single handgrip compared with rest are given as MNI coordinates (x, y, z) in Talairach space. All voxels are significant at a threshold of P < 0.05, corrected for multiple comparisons across whole brain. MNI = Montreal Neurological Institute; SMA = supplementary motor area; PMd = dorsal lateral premotor cortex; PMv = ventral lateral premotor cortex; BA = Brodmann area; CL = contralateral; IL = ipsilateral; M = midline.
Fig. 2.
SPM{Z} representing the main effects of hand grip for (A) dominant hand and (B) non-dominant hand compared with rest, obtained in random effects two sample t-test analysis. Results are displayed on a canonical T1-weighted MRI in coronal sections 6 mm apart. All voxels are significant at P < 0.05, corrected for multiple comparisons across whole brain.
We were also interested to make comparisons between task-related brain activations using the dominant and non-dominant hands, by comparing flipped and unflipped, dominant and non-dominant hand contrast images (Table 2). Categorical comparisons of dominant and non-dominant hand grip demonstrated that the activation pattern generated by each hand differed from the other only by the activation of contralateral sensorimotor cortex and ipsilateral superior cerebellum (Fig. 3A and B). Secondly, comparison of flipped and unflipped contrast images for dominant hand suggested that the only lateralized task-related activations were in contralateral sensorimotor cortex, frontal operculum and ipsilateral cerebellum (Fig. 3C). The same comparison for non-dominant hand demonstrated that lateralized task-related activations were seen only in contralateral sensorimotor cortex, angular gyrus and ipsilateral cerebellum (Fig. 3D). Finally, the comparison of unflipped dominant with flipped non-dominant hand contrast images demonstrated increased activation for dominant hand in contralateral parietal operculum and insula cortex, and ipsilateral intraparietal sulcus and posterior inferior frontal gyrus [Brodmann area (BA) 44/45] (Fig. 3E).
Table 2.
Comparison of dominant and non-dominant hands
| Region | Side | Talairach coordinates MNI space |
Z-value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Dominant hand versus non-dominant hand | |||||
| Sensorimotor cortex | L | −34 | −30 | 58 | >8.0 |
| Cerebellum (V) | R | 18 | −50 | −22 | >8.0 |
| Cerebellum (XI) | R | 12 | −62 | −48 | 5.86 |
| Non-dominant hand versus dominant hand | |||||
| Sensorimotor cortex | R | 38 | −24 | 50 | >8.0 |
| Cerebellum (V) | L | −14 | −50 | −22 | >8.0 |
| Dominant hand versus flipped dominant hand | |||||
| Sensorimotor cortex | L | −34 | −28 | 56 | >8.0 |
| Cerebellum (V) | R | 16 | −48 | −22 | >8.0 |
| Cerebellum (XI) | R | 12 | −60 | −50 | 6.12 |
| Frontal operculum | L | −42 | −4 | 6 | 5.53 |
| Non-dominant hand versus flipped non-dominant hand | |||||
| Sensorimotor cortex | R | 38 | −26 | 54 | >8.0 |
| Cerebellum | L | −14 | −50 | −22 | >8.0 |
| Angular gyrus | R | 32 | −76 | 28 | 5.08 |
| Dominant handgrip versus flipped non-dominant handgrip | |||||
| Parietal operculum | L | −42 | −38 | 24 | 6 |
| Insula cortex | L | −42 | −4 | 6 | 5.58 |
| Intraparietal sulcus | R | 32 | −76 | 28 | 5.49 |
| Posterior inferior frontal gyrus | R | 56 | 20 | 26 | 5.46 |
Location of brain regions representing differences between the main effects of dominant and non-dominant hand grip are given as MNI coordinates (x, y, z) in Talairach space. Flipped images are those rotated about the midsagital plane. All voxels are significant at a threshold of P > 0.05, corrected for multiple comparisons across whole brain. MNI = Montreal Neurological Institute; R = right; L = left.
Fig. 3.
SPM{Z} representing the categorical comparison of the main effects of dominant and non-dominant hand grip, obtained in random effects, two sample, t-test analysis. Where images are ‘flipped’, this refers to flipping about the midsagital plane (see Methods for details). (A) Dominant versus non-dominant hand grip; (B) non-dominant versus dominant hand grip; (C) dominant hand grip versus flipped dominant hand grip; (D) non-dominant hand grip versus flipped non-dominant hand grip; (E) dominant hand grip versus flipped non-dominant hand grip; (F) flipped non-dominant hand grip versus dominant hand grip. The SPM{Z}s are shown as maximum intensity projections. The brain is shown from the right, top and back. All voxels are significant at P < 0.05, corrected for multiple comparisons across whole brain.
There were no voxels more active during rest than dominant hand grip, but regions within anterior cingulate cortex were more active during rest than non-dominant hand grip (peak voxel at x = 0, y = 34, z = −16; Z-score = 5.76). Non-significant decreases in BOLD signal were seen in the ipsilateral primary motor cortex (M1) during use of either hand.
Linear correlations between BOLD signal and force of handgrip
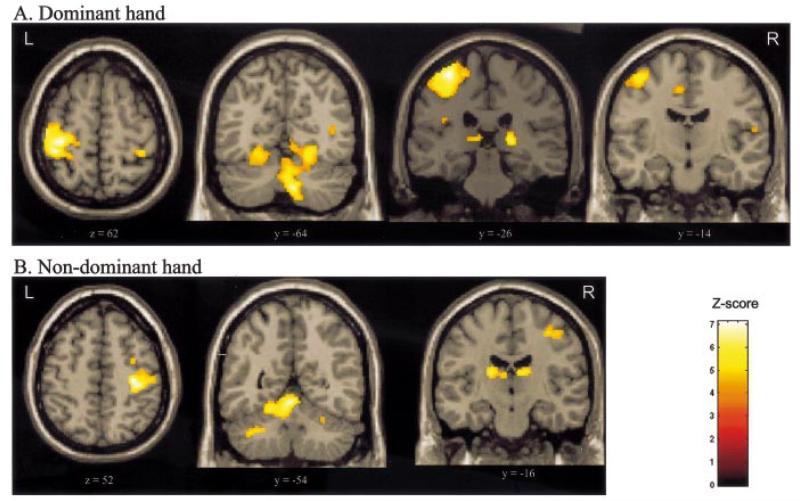
The statistical parametric map derived from the parameter estimates for the first order polynomial expansion of the handgrip force represents voxels in which the BOLD signal is linearly related to force of hand grip. These regions are shown in Table 3 and Fig. 4. Linear increases with either hand were seen in contralateral sensorimotor cortex, cerebellar vermis, ipsilateral cerebellar hemisphere (inferior cerebellum during dominant hand grip, superior cerebellum for non-dominant hand grip) and ipsilateral ventroposterior lateral thalamus. In addition, linear correlations between BOLD signal and force of handgrip were seen in caudal cingulate sulcus with dominant hand grip, and contralateral ventral posterolateral thalamus with non-dominant hand grip.
Table 3.
Linear force-related regions
| Region | x | y | z | Z-value |
|---|---|---|---|---|
| Right (dominant) hand grip | ||||
| Left sensorimotor cortex | −36 | −36 | 60 | 5.15* |
| −34 | −28 | 64 | 5.12* | |
| Right inferior cerebellum | 8 | −66 | −42 | 5.17* |
| Cerebellar vermis | 4 | −42 | 0 | 4.05† |
| Right thalamus (VPL) | 20 | −26 | 6 | 4.41† |
| Left caudal cingulate sulcus | −6 | −12 | 48 | 3.63† |
| Left (non-dominant) hand grip | ||||
| Right sensorimotor cortex | 34 | −22 | 52 | 4.96* |
| Cerebellar vermis | −4 | −52 | −12 | 4.78† |
| Left superior cerebellum | −10 | −50 | −20 | 4.44** |
| Left thalamus (VPL) | −18 | −16 | 16 | 4.44† |
| Right thalamus (VPL) | 12 | −16 | 16 | 4.24** |
Location of brain regions in which increasing BOLD signal was linearly related to peak force of hand grip are given as Montreal Neurological Institute coordinates (x, y, z) in Talairach space. VPL = ventral posterolateral.
Voxel significant at P > 0.05 after correction for multiple comparisons across whole brain.
Peak voxel within a cluster which is significant at P > 0.05 after correction for multiple comparisons across whole brain.
Voxel significant at P > 0.05 after correction for multiple comparisons across predefined volume of interest (VOI). For right hand grip, VOI defined by spheres of 10 mm radius centred on the following coordinates, derived from Dettmers et al. (1995): left sensorimotor area (x = −20, y = −30, z = 64), left cingulate motor area (x = −2, y = −14, z = 48), right thalamus (x = 18, y = −18, z = 8) and cerebellar vermis (x = 4, y = −44, z = −8). For left hand grip, corresponding coordinates in the opposite hemisphere were used.
Fig. 4.
Regions in which there exists a significant linear (1st order) relationship between BOLD signal and increasing hand grip force for (A) dominant hand and (B) non-dominant hand. Results are displayed on a canonical T1-weighted MRI. All clusters are significant at P < 0.05, corrected for multiple comparisons across whole brain.
Non-linear correlations between BOLD signal and force of handgrip
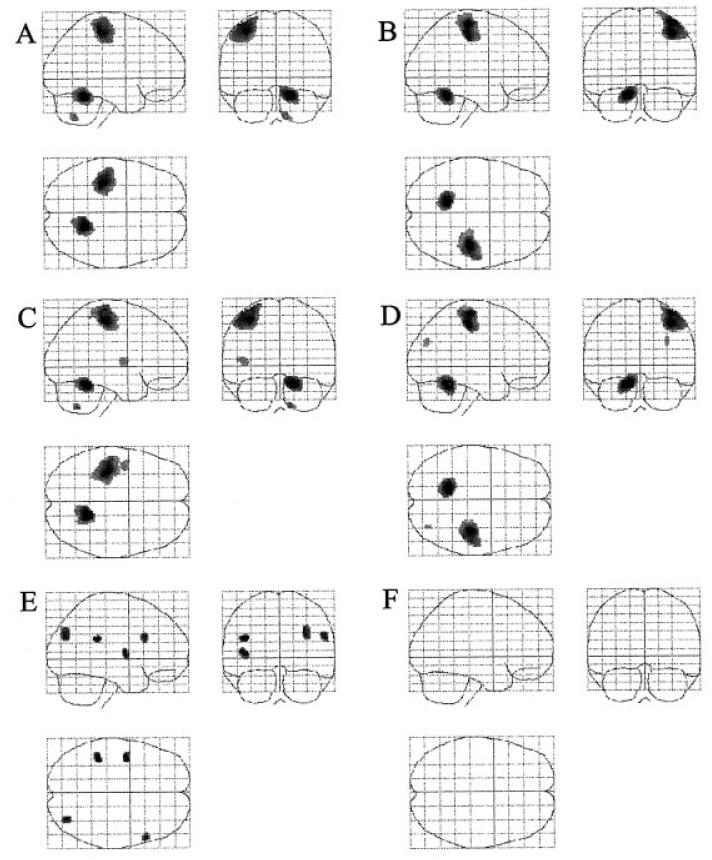
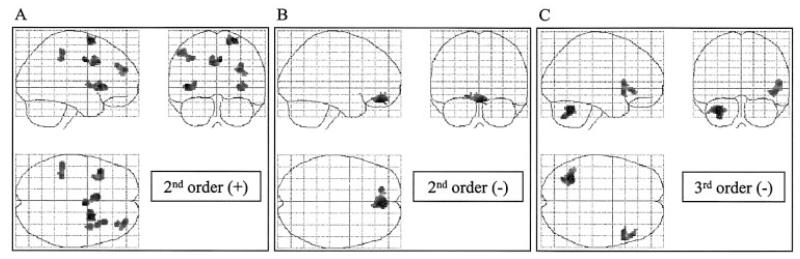
The statistical parametric maps derived from the parameter estimates for the second or third order polynomial expansion of the handgrip force represent voxels in which the BOLD signal exhibits a non-linear relationship with force of hand grip (Fig. 1). No such relationship was consistently demonstrated with use of the non-dominant hand. Both positive and negative second order effects were seen with dominant hand use (Table 4). Positive second order effects were seen in contralateral rostral cingulate sulcus and intraparietal sulcus, ipsilateral superior frontal sulcus, caudal cingulate sulcus and dorsolateral prefrontal cortex, and bilaterally in insula cortex (Fig. 5A). Ipsilateral intraparietal sulcus exhibited a non-significant trend (P = 0.065, corrected for multiple comparisons across whole brain, for cluster with the peak voxel at x = 44 y = −42 z = 52). A negative second order (Fig. 1) effect was seen in a cluster with peak voxel in right medial orbitofrontal cortex (Fig. 5B). The cluster of activated voxels extended from x = −20 to x = +10. No areas demonstrating significant positive third order effects were seen, but negative third order effects were seen in contralateral superior cerebellum, ipsilateral insula cortex and ipsilateral frontal operculum (Fig. 5C).
Table 4.
Non-linear, force-related regions for dominant hand grip
| Region of activation | x | y | z | Z-score |
|---|---|---|---|---|
| Second order (+) | ||||
| Right superior frontal sulcus | 24 | 6 | 68 | 4.61 |
| Left insula cortex | −42 | 18 | 2 | 4.49 |
| Right caudal cingulate sulcus | 4 | −4 | 40 | 4.23 |
| Left rostral cingulated sulcus | −6 | 14 | 36 | 4.04 |
| Right DLPFC | 32 | 52 | 26 | 3.95 |
| Left intraparietal sulcus | −36 | −36 | 42 | 3.81 |
| Right insula cortex | 34 | 14 | −2 | 3.74 |
| Second order (−) | ||||
| Right medial orbitofrontal cortex | 4 | 46 | −20 | 4.44 |
| Third order (+) No significant voxels or clusters at group level Third order (−) | ||||
| Left superior cerebellum | −32 | −60 | −30 | 4.45 |
| Right insula cortex | 46 | 16 | −6 | 3.71 |
| Right frontal operculum | 50 | 28 | −4 | 3.76 |
Location of brain regions in which there is a non-linear relationship between BOLD signal and force applied during a single dominant hand grip are given as Montreal Neurological Institute coordinates (x, y, z) in Talairach space. Clusters are significant at P < 0.05 after correction for multiple comparisons across whole brain, and the peak voxel within each cluster is reported. DLPFC = dorsolateral prefrontal cortex.
Fig. 5.
SPM{Z}s representing regions in which there exists significant non-linear relationships between BOLD signal and increasing hand grip force using the dominant hand. The SPM{Z}s are shown as maximum intensity projections. The brain is shown from the right, top and back. All clusters are significant at P < 0.05, corrected for multiple comparisons across whole brain.
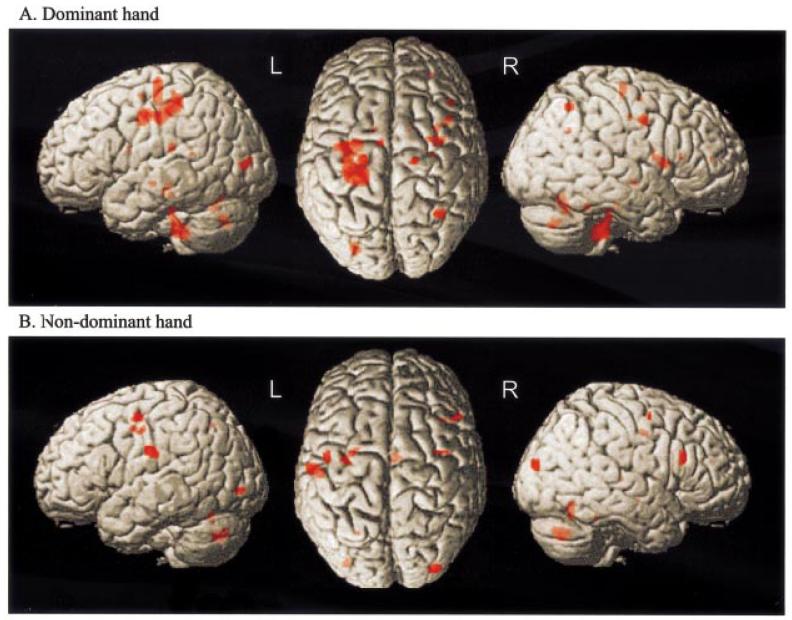
Effects of age
Regions significant for the conjunction of the effects of age2 and effects of handgrip are shown in Table 5 and Fig. 6. When using the dominant hand, positive correlations with age2 were observed in a number of voxels, particularly in a large cluster in the contralateral (left) hemisphere ranging from y = −36 to y = −4. Peaks within this cluster were situated in postcentral sulcus, inferior central sulcus and precentral gyrus. Further correlations were observed in contralateral caudal cingulate sulcus, and ipsilateral superior frontal sulcus, frontal operculum, insula cortex and intraparietal sulcus, as well as ipsilateral superior cerebellum, cerebellar vermis, ipsilateral ventral posterolateral thalamus and bilateral caudate nuclei. When using the non-dominant hand, positive correlations were seen in ipsilateral (left) inferior postcentral gyrus, inferior central sulcus and superior frontal sulcus. Further correlations were observed in contralateral (right) caudal cingulate sulcus, PMd, inferior frontal sulcus (BA 45), cerebellar vermis and thalamus (mediodorsal nuclei).
Table 5.
Effects of increasing age
| Region of activation | x | y | z | Z-score | Correlation coefficient |
|---|---|---|---|---|---|
| Conjunction between effects of age2 and effects of dominant (right) handgrip versus rest | |||||
| Left inferior central sulcus | −34 | −8 | 42 | 6.23 | +0.73 |
| Left postcentral gyrus | −24 | −34 | 50 | 7.13 | +0.80 |
| Left precentral gyrus (BA 4/6) | −22 | −18 | 58 | 5.50 | +0.67 |
| Left caudal cingulate sulcus −8 | −6 | 58 | 5.32 | +0.65 | |
| Cerebellar vermis | 6 | −62 | −26 | 5.41 | +0.66 |
| 8 | −64 | −18 | 5.30 | +0.65 | |
| −6 | −68 | −34 | 5.35 | +0.65 | |
| Right superior cerebellum | 22 | −46 | −24 | 5.27 | +0.64 |
| Right superior frontal sulcus | 34 | −4 | 40 | 5.75 | +0.69 |
| Right frontal operculum | 42 | 12 | 8 | 5.67 | +0.68 |
| Right intraparietal sulcus | 34 | −58 | 52 | 5.62 | +0.68 |
| Right insula cortex | 34 | 6 | 12 | 5.43 | +0.66 |
| Right thalamus (vpl) | 12 | −24 | 10 | 4.97 | +0.65 |
| Right caudate | 12 | 4 | 18 | 5.15 | +0.63 |
| Left caudate | −12 | 4 | 16 | 4.96 | +0.64 |
| Conjunction between effects of age2 and effects of rest versus dominant (right) handgrip | |||||
| Right M1 | 46 | −22 | 56 | 5.39 | +0.66 |
| Conjunction between effects of age2 and effects of non-dominant (left) handgrip versus rest | |||||
| Left inferior central sulcus | −32 | −10 | 42 | 5.46 | +0.66 |
| Left inferior postcentral gyrus | −58 | −16 | 28 | 5.77 | +0.69 |
| Left superior frontal sulcus | −26 | −4 | 44 | 5.16 | +0.64 |
| Cerebellar vermis | 8 | −72 | −36 | 6.53 | +0.75 |
| 10 | −64 | −14 | 5.41 | +0.65 | |
| 0 | −64 | −24 | 5.03 | +0.62 | |
| Right inferior frontal gyrus (BA 45) | 50 | 22 | 24 | 5.42 | +0.66 |
| Right dorsal premotor cortex | 38 | −4 | 56 | 5.20 | +0.63 |
| Right caudal cingulate sulcus | 6 | −8 | 42 | 5.22 | +0.64 |
| Right mediodorsal thalamus | 8 | −22 | 8 | 5.22 | +0.64 |
| Conjunction between effects of age2 and effects of rest versus non-dominant (left) handgrip | |||||
| Left M1 | −28 | −30 | 60 | 5.44 | +0.64 |
Regions significant for the conjunction of effects of handgrip and effects of age2. Locations given as Montreal Neurological Institute coordinates (x, y, z) in Talairach space. All voxels significant at P < 0.05 after correction for multiple comparisons across whole brain. BA = Brodman area; M1 = primary motor cortex; vpl = ventral posterolateral.
Fig. 6.
SPM{Z}s representing the conjunction of main effects of handgrip and effects of age2 for (A) dominant hand grip and (B) non-dominant hand grip. Results are surface rendered onto a canonical brain. The brain is shown (from left to right) from the left side, from above (left hemisphere on the left) and from the right. All voxels are significant at P < 0.05, corrected for multiple comparisons across whole brain.
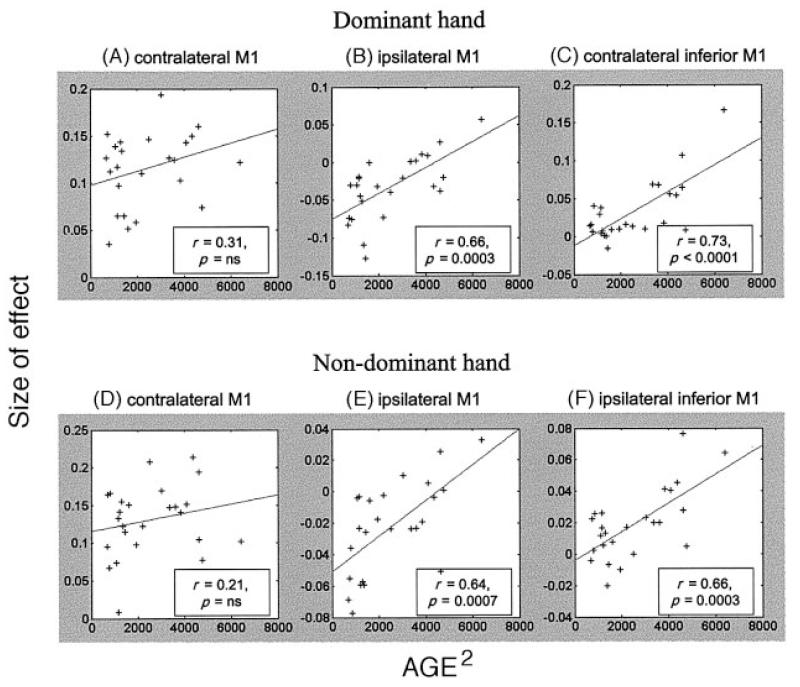
Conjunction analysis was also performed between the effects of age2 and the effects of rest compared with hand grip. For both dominant and non-dominant hands, significant correlations were seen in ipsilateral M1, such that in younger subjects there was more likely to be a deactivation of ipsilateral M1 during hand grip compared with rest (Fig. 7).
Fig. 7.
Parameter estimates for the main effect of hand grip plotted against age2 for (A) contralateral M1 (dominant hand: x = −34, y = −30, z = 56), (B) ipsilateral M1 (dominant hand: x = 46, y = −22, z = 56), (C) contralateral (left) inferior M1 (dominant hand: x = −34, y = −8, z = 42), (D) contralateral M1 (non-dominant hand: x = 38, y = −26, z = 52), (E) ipsilateral M1 (non-dominant hand: x = −28, y = −30, z = 60), and (F) ipsilateral (left) inferior M1 (non-dominant hand: x = −32, y = −10, z = 42). The correlation coefficient r and the corresponding P-value are given for each plot. The parameter estimates are calculated for single peak voxels within each region.
There was no significant negative correlation between age2 and BOLD signal (i.e. increasing signal in younger subjects), and nor were there any regions demonstrating significant correlations between age2 and linear or non-linear effects of hand grip.
Post hoc regression analysis using age rather than age2 as the independent variable revealed correlations in the same regions, but was less significant.
Effects of MVC
There was no correlation between MVC and main effects of handgrip for either hand.
Effects of gender
There were no differences in activation patterns between male and female subjects.
Discussion
We have identified age-related changes in the neural correlates of a hand grip task seen previously only by categorical comparison of young and old subjects (Calautti et al., 2001; Mattay et al., 2002). By studying a large cohort of subjects spanning a wide range of ages, we have been able to perform a correlation analysis to determine that older subjects increasingly activate regions within the motor network involved in hand grip in a non-linear fashion. Furthermore, some of this age-related network is lateralized independent of the hand used. These changes are likely to represent adaptive plasticity within the motor network in order to maintain performance in the face of age-related changes in the brain. The network of regions activated by our hand grip task and the effect of altering the peak force exerted will now be discussed in order to put the age-related changes into context.
Motor paradigm
We set out to examine the effects of age on recruitment of cortical and subcortical brain regions during a motor task. However, the network subserving motor performance will be differentially engaged depending on the task used and the level of performance. We chose to use hand grip, a ubiquitous motor task, and to modulate one aspect of the performance level of this task (i.e. peak force exerted during hand grip) by visual feedback. We hypothesized that this task would activate a large number of regions so that we would be able to examine for differential effects of age in a wide network, as well as for any interaction between age and grip force. Performance levels, as measured by peak forces exerted, were maintained across age ranges by asking subjects to perform at a fixed percentage of their own MVC. Mattay et al. (2002) reported that reaction times in a cued finger tap task varied amongst older subjects, and that the activation patterns in those with delayed reaction times (i.e. impaired performance) did not differ from young subjects, whereas older subjects in whom reaction times were not delayed demonstrated increases in activation in a number of motor regions compared with younger subjects. It has been suggested that one way older subjects maintain their level of performance is by increased recruitment of brain regions during the task. Thus, if the level of performance is unchanged across subjects, the sensitivity for detecting age-related differences in neuronal activation will be greater.
Main effects of handgrip
Previous functional imaging studies involving arm and hand movements have demonstrated activation in contralateral primary sensorimotor cortex, ipsilateral anterior cerebellum, superior parietal cortex and ventrolateral thalamus (Colebatch et al., 1991; Grafton et al., 1991, 1992). We have shown, however, that isometric dynamic handgrip with visual feedback activates a more extensive network of cortical and subcortical regions known to be part of the motor system (Fink et al., 1997). Of particular interest is the finding of bilateral activation in the rostral part of PMv (BA 44) and intraparietal sulcus when either hand is used. There is strong evidence to support the notion that BA 44 in humans is the functional homologue of area F5 in the macaque (von Bonin and Bailey, 1947), both from comparative architectonic analysis (Petrides and Pandya, 1994) and functional imaging studies (Rizzolatti et al., 1996; Binkofski et al., 2000). In addition, intraparietal sulcus in humans is likely to be the functional homologue of anterior intraparietal area (AIP) in the macaque (Faillenot et al., 1997; Binkofski et al., 1998). Area F5 is directly connected to primary motor cortex, and receives inputs from the secondary somatosensory area (SII) and posterior parietal cortex, particularly AIP (Muakkassa and Strick, 1979; Ghosh and Gattera, 1995; Luppino et al., 1999). On the basis of intracortical microstimulation studies, both F5 and AIP have been implicated in a network of cortical regions associated with grasping (Rizzolatti et al., 1981, 1988; Kurata and Tanji 1986; Taira et al., 1990; Hepp-Reymond et al., 1994). The notion of a ‘grasping circuit’ involving these structures was described by Jeannerod et al. (1995), and the human equivalent is thought to involve BA 44 together with intraparietal sulcus. However, until recently such a network had not been demonstrated in humans (Grafton et al., 1996; Rizzolatti et al., 1996). Studies placing the emphasis on the exploratory (Binkofski et al., 1999) or precision (Ehrsson et al., 2000, 2001) nature of grip succeeded in demonstrating activation of BA 44 and intraparietal sulcus, and our hand grip task activated BA 44 and intraparietal sulcus/supramarginal gyrus bilaterally with either hand, lending further support to the notion of a human grasping network involving these regions. Given the diversity of tasks that activate this network (blinded object manipulation, finger thumb precision grip with tactile feedback, and now hand grip with visual feedback), we would suggest that it is the continued monitoring of hand performance that is important, rather than the type of hand task. Lesion studies in humans have suggested that with regard to the role played by either left or right BA 44 in the performance of motor tasks, left BA 44 is involved in ‘on-line’ control of visually guided movements (Jackson and Husain, 1996), whereas right BA 44 may have a role in directing attention as it has been associated with unilateral visual neglect in humans (Husain and Kennard, 1996). We are not able to define this differential functionality any further on the basis of our results.
Correlation between BOLD signal and handgrip force
Using single cell recording in macaque monkeys (Ashe 1997), the correlation between firing rates of cortical neurones and force of both isometric and dynamic grip tasks has been demonstrated to be mainly monotonic or linear. Linear relationships have been observed primarily in primary motor cortex (Evarts, 1968; Smith et al., 1975; Hepp-Reymond et al., 1978; Evarts et al., 1983; Wannier et al., 1991; Georgopoulos et al., 1992), and also for neurones in somatosensory cortex (Wannier et al., 1991), premotor cortex (Hepp-Reymond et al., 1994), supplementary motor area (Smith et al., 1975) and thalamus (Anner-Baratti et al., 1986). Functional imaging techniques allow the activity in larger populations of neurones to be studied simultaneously across all brain regions, which allows inference about activity relationships between one population of neurones and another. However, local differences in behaviour of neurones will be masked. Despite this limitation, Dettmers et al. (1995) found linear increases in regional cerebral blood flow (rCBF) with increasing force of finger key presses in a number of regions, particularly contralateral M1, primary sensory cortex, caudal cingulate sulcus, posterior SMA (SMA proper), cerebellar vermis and ipsilateral thalamus. We were able to replicate these findings, with the exception of SMA. Using a dynamic hand grip task, Thickbroom et al. (1999) demonstrated an increase in extent but not size of BOLD signal in contralateral motor cortex in relation to increasing force, and were unable to demonstrate any relationship with force using a static grip task (Thickbroom et al., 1998). Compared with the dynamic grip task of Thickbroom et al. (1999), our task was performed across a much wider range of forces (10–60% MVC), making it more sensitive to detection of such changes.
We found significant non-linear correlations of brain activity with increasing force in multiple regions across the brain in individual subjects. There was, however, significant individual variability. The absence of any consistent non-linear correlates across the group when using the non-dominant hand suggests that the variability is more marked using this hand. Furthermore, we report dominant hand group effects at the cluster level, with no significant single voxels at the chosen threshold (P < 0.05, corrected for multiple comparisons across whole brain). Non-linear changes in firing rates of single cortical cells in precentral gyrus of the macaque monkey have been reported (Cheney and Fetz, 1980; Evarts et al., 1983), particularly in higher or lower force ranges. These non-linearities have been interpreted as either recruitment or saturation effects of different populations of neurones within functionally similar cortical regions. This hypothesis is supported by the observation that the BOLD signal in voxels within the same cortical region behaved in a very different fashion in relation to increasing force, e.g. voxels within right insula cortex exhibited both positive second order effects and negative third order effects (group analysis; Table 3). Recent evidence has suggested an alternative explanation. Hepp-Reymond et al. (1999) investigated context-specific force encoding in a number of premotor regions and primary motor cortex of the macaque monkey. A number of neurones were found in all sites, in which the rate of change of firing was dependent on context, i.e. context may change the gain control of the motor system. For example, it is possible that increased attention to accuracy is required at both low and high forces. This might explain the positive second order effect seen in regions such as intraparietal sulcus, dorsolateral prefrontal cortex (DLPFC), insula cortex and cingulate sulcus. In our experimental design, we did not include explicit contextual or strategic changes, but clearly further work is required to establish whether this phenomenon occurs in the human motor system.
Effects of age
Two previous studies made categorical comparisons between an older and a younger group using the dominant hand to demonstrate relative overactivity in a number of motor-related regions, and one study failed to show a difference using the non-dominant hand (Calautti et al., 2001; Mattay et al., 2002). Such categorical comparisons may miss more subtle age-related changes (Calautti et al., 2001), so we chose to perform a linear regression analysis. Linear age-related changes have been demonstrated using motor tasks such as repetitive finger tapping (Shimoyama et al., 1990), but non-linear effects are seen in more demanding tasks involving timed tasks, choice reaction times, and visually guided hand movements (Houx et al., 1993; Kauranen et al., 1996; Smith et al., 1999). We asked our subjects to be accurate in exerting the target force during each hand grip, increasing the demands of the task. Our a priori hypothesis, therefore, was that we would see non-linear effects. The notion that this task represents a precise and relatively demanding motor task compared with finger tapping, for example, is supported by the similarities seen between our regions of activation for hand grip, and those seen in other studies involving precision (Ehrsson et al., 2000, 2001). The subsequent discussion refers only to the correlation analysis performed using age2.
Activity in the part of contralateral primary motor cortex activated by hand grip across group did not show a correlation with age2. However, activity in ipsilateral hand area of primary motor cortex was reduced during hand grip compared with rest in younger subjects, but the degree of ‘deactivation’ was lesser in older subjects (Fig. 7). These findings account for why significant deactivations reported with ipsilateral hand movement in other studies (Allison et al., 2000) were not seen when averaging across the group in our study. The mechanism of ipsilateral deactivation of primary motor cortex is thought to be via transcallosal inhibition, and this process may be reduced in older subjects. In support of this idea, paired pulse transcranial magnetic stimulation (TMS) has demonstrated reduced intracortical inhibition in motor cortex of older subjects (Peinemann et al., 2001), and it is possible that ageing also leads to impaired transcallosal inhibition of ipsilateral primary motor cortex in elderly subjects.
Increased activation with age was seen in a number of left sided areas, whichever hand was used. In particular, increased activation was seen in older subjects deep within the left central sulcus (Fig. 7C, F), corresponding to the putative area 4p (Geyer et al., 1996). Area 4p has been demonstrated to be increasingly activated with increasing attention to motor performance (Binkofski et al., 2002), which is consistent with the notion of increased utilization of neural resources in older subjects in order to maintain performance levels. This finding is also interesting in view of studies that have suggested that the left hemisphere, in right handed subjects, is dominant for controlling most cognitive aspects of movement with either hand (Haaland and Harrington, 1996). It has previously been assumed that this is related to a dominance of left sided association areas, such as prefrontal and parietal cortex (Haaland and Harrington, 1996), but the absence of age-related changes in these association areas in our data would support the idea that the left sensorimotor cortex itself plays a role in this hemispheric difference.
Age-related increased activations were also seen in regions close to our putative ‘grasping circuit’. Older subjects were more likely to recruit right frontal operculum and intraparietal sulcus with dominant hand use, and right inferior frontal gyrus with non-dominant hand use. These frontal regions are both very close to BA 44, with a likelihood of being within BA 44 of 5–25% according to the probability map of Tomaiuolo et al. (1999). If this excess activation is in compensation for increasing cognitive demands of the task in older subjects, it again provides evidence to support a functional differentiation between the networks in dominant and non-dominant hemispheres.
Other age-related increases in activation were seen in caudal cingulate sulcus (contralateral when either hand was used) and dorsal premotor cortex/superior frontal sulcus (BA 6/8) regions (bilateral for non-dominant hand and ipsilateral for dominant hand). Caudal regions of the cingulate sulcus are activated in many simple motor tasks, and preferentially during sequential movement (Deiber et al., 1999). Movement complexity has not been shown to alter cingulate activation (Shibasaki et al., 1993). The PMd regions activated increasingly in our older subjects are situated rostrally within the premotor region. It has been suggested that rostral premotor cortex (or pre-PMd) is more sensitive to sensory cues involved during movement (Picard and Strick, 2001), which would be in keeping with the notion that older subjects might find the task more demanding of higher level cognitive processes.
Although we have demonstrated an increase in activations in older subjects, it is not clear whether this is due to a change in threshold for activation, or a change in gain setting. We were interested therefore to see if there were any age-related differences in the relationship between force exerted and BOLD signal across the group. We observed a number of brain regions in which older subjects were more likely to show a linear increase in BOLD signal in relationship to increasing force, including contralateral primary motor cortex, right BA 44/45, bilateral intraparietal sulcus/supramarginal gyrus, and bilateral secondary somatosensory area (SII) (P < 0.001, uncorrected for multiple comparisons). None of these regions was significant at our threshold, but it is interesting to speculate that the trend represents an increase in gain setting in older subjects. A task with greater cognitive demand may be better suited to demonstrate this difference.
Changes downstream from the cortical and subcortical motor structures also have an influence on performance. Decline in muscle mass and strength is seen in older human subjects (Buckwalter et al., 1993; Ranganathan et al., 2001), and loss of anterior horn cells has been reported in older animals (Machado-Salas et al., 1977). Both might impair the transformation of descending corticospinal impulses into the generation of force, and lead to an increased effort in maintaining task performance. However, we asked subjects to perform at a proportion of their own maximal MVC, and so effort is largely controlled for in our task, making these peripheral changes a less likely explanation for our results.
Conclusions
Isometric dynamic hand grip is a robust activator of a widespread network of cortical and subcortical regions in the human brain. By incorporating ‘on-line’ visual feedback, we have demonstrated activation in the ‘grasping circuit’ involving bilateral rostral PMv (BA 44) and intraparietal sulcus, previously seen only in tasks involving pinch grip and blinded object manipulation. Within this motor network, we have demonstrated significant changes in the way the motor system is activated with increasing age. One explanation is that neurodegenerative and neurochemical changes occurring as part of the normal ageing process result in less efficient integration of visuospatial and sensorimotor processing. Thus, for older subjects to perform the task to the same level as younger subjects requires greater computational effort, which is reflected at the systems level as increased activation in key regions. There are clear parallels with changes seen in studies in which the complexity of motor task is increased (Catalan et al., 1998), or in which naïve subjects learn a new motor task (Karni et al., 1995; Toni et al., 1998). In both cases, alterations in task difficulty are reflected in adaptive changes in a motor network. Taken together with our findings, these results reflect the adaptable and plastic nature of the networks that subserve motor function, in response to increasing demands relating to task complexity or neuronal loss. An extreme example of this is seen in patients suffering motor deficit as a result of stroke, who appear to rely on this adaptability as the substrate for some part of their functional recovery (Chollet et al., 1991; Weiller et al., 1993). However, the capacity for plastic change may be finite. After injury-induced reorganization of the brain, the capacity for subsequent adaptive change is reduced (Kolb et al., 1998). It is possible that the adaptive changes we have observed in older brains may in turn limit the capacity for further reorganization after injury. This clearly has implications for what we can expect from therapeutic techniques designed to promote cerebral reorganization after stroke in older subjects. A greater understanding of age-related changes in the functional reorganization of the brain will be crucial in unravelling the relationship between normal ageing and pathological process.
Acknowledgements
We wish to thank Peter Aston and Eric Featherstone (Wellcome Department of Imaging Neuroscience) for the design and programming involved in creating the hand grip manipulandum. N.S.W. and R.S.J.F. are supported by the Wellcome Trust.
Abbreviations
- AIP
anterior intraparietal area
- BA
Brodmann area
- BOLD
blood oxygen level dependent
- EPI
echoplanar image
- fMRI
functional MRI
- M1
primary motor cortex
- MNI
Montreal Neurological Institute
- MVC
maximum voluntary contraction
- PMd
dorsal lateral premotor cortex
- PMv
ventral lateral premotor cortex
- rCBF
regional cerebral blood flow
- SMA
supplementary motor area
- SPM
statistical parametric mapping
References
- Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC. Functional MRI cerebral activation and deactivation during finger movement. Neurology. 2000;54:135–42. doi: 10.1212/wnl.54.1.135. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–19. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Anner-Baratti R, Allum JH, Hepp-Reymond MC. Neural correlates of isometric force in the ‘motor’ thalamus. Exp Brain Res. 1986;63:567–80. doi: 10.1007/BF00237479. [DOI] [PubMed] [Google Scholar]
- Ashe J. Force and the motor cortex. Behav Brain Res. 1997;86:1–15. doi: 10.1016/s0166-4328(96)00145-3. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Dohle C, Posse S, Stephan KM, Hefter H, Seitz RJ, et al. Human anterior intraparietal area subserves prehension: a combined lesion and functional MRI activation study. Neurology. 1998;50:1253–9. doi: 10.1212/wnl.50.5.1253. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur J Neurosci. 1999;11:3276–86. doi: 10.1046/j.1460-9568.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Amunts K, Stephan KM, Posse S, Schormann T, Freund HJ, et al. Broca’s region subserves imagery of motion: a combined cytoarchitectonic and fMRI study. Hum Brain Mapp. 2000;11:273–85. doi: 10.1002/1097-0193(200012)11:4<273::AID-HBM40>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Fink GR, Geyer S, Buccino G, Gruber O, Shah NJ, et al. Neural activity in human primary motor cortex areas 4a and 4p is modulated differentially by attention to action. J Neurophysiol. 2002;88:514–9. doi: 10.1152/jn.2002.88.1.514. [DOI] [PubMed] [Google Scholar]
- Büchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage. 1998;8:140–8. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Woo SL, Goldberg VM, Hadley EC, Booth F, Oegema TR, et al. Soft-tissue aging and musculoskeletal function. J Bone Joint Surg Am. 1993;75:1533–48. doi: 10.2106/00004623-199310000-00015. [DOI] [PubMed] [Google Scholar]
- Calautti C, Serrati C, Baron JC. Effects of age on brain activation during auditory-cued thumb-to-index opposition: a positron emission tomography study. Stroke. 2001;32:139–46. doi: 10.1161/01.str.32.1.139. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M. The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain. 1998;121:253–64. doi: 10.1093/brain/121.2.253. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Functional classes of primate corticomotoneuronal cells and their relation to active force. J Neurophysiol. 1980;44:773–91. doi: 10.1152/jn.1980.44.4.773. [DOI] [PubMed] [Google Scholar]
- Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Deiber MP, Passingham RE, Friston KJ, Frackowiak RS. Regional cerebral blood flow during voluntary arm and hand movements in human subjects. J Neurophysiol. 1991;65:1392–401. doi: 10.1152/jn.1991.65.6.1392. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M. Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol. 1999;81:3065–77. doi: 10.1152/jn.1999.81.6.3065. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, et al. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol. 1995;74:802–15. doi: 10.1152/jn.1995.74.2.802. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy with MRI, and blood supply. 2nd ed Springer-Verlag; New York: 1999. [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision-versus power-grip tasks: an fMRI study. J Neurophysiol. 2000;83:528–36. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren E, Forssberg H. Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol. 2001;85:2613–23. doi: 10.1152/jn.2001.85.6.2613. [DOI] [PubMed] [Google Scholar]
- Esposito G, Kirkby BS, Van Horn JD, Ellmore TM, Berman KF. Context-dependent, neural system-specific neurophysiological concomitants of ageing: mapping PET correlates during cognitive activation. Brain. 1999;122:963–79. doi: 10.1093/brain/122.5.963. [DOI] [PubMed] [Google Scholar]
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 1968;31:14–27. doi: 10.1152/jn.1968.31.1.14. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Fromm C, Kroller J, Jennings VA. Motor cortex control of finely graded forces. J Neurophysiol. 1983;49:1199–215. doi: 10.1152/jn.1983.49.5.1199. [DOI] [PubMed] [Google Scholar]
- Faillenot I, Toni I, Decety J, Gregoire MC, Jeannerod M. Visual pathways for object-oriented action and object recognition: functional anatomy with PET. Cereb Cortex. 1997;7:77–85. doi: 10.1093/cercor/7.1.77. [DOI] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. J Neurophysiol. 1997;77:2164–74. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995a;2:189–210. [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995b;3:165–89. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Ashe J, Smyrnis N, Taira M. The motor cortex and the coding of force. Science. 1992;256:1692–5. doi: 10.1126/science.256.5064.1692. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, et al. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–7. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Gattera R. A comparison of the ipsilateral cortical projections to the dorsal and ventral subdivisions of the macaque premotor cortex. Somatosens Mot Res. 1995;12:359–78. doi: 10.3109/08990229509093668. [DOI] [PubMed] [Google Scholar]
- Grady CL. Functional brain imaging and age-related changes in cognition. Biol Psychol. 2000;54:259–81. doi: 10.1016/s0301-0511(00)00059-4. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Woods RP, Mazziotta JC, Phelps ME. Somatotopic mapping of the primary motor cortex in humans: activation studies with cerebral blood flow and positron emission tomography. J Neurophysiol. 1991;66:735–43. doi: 10.1152/jn.1991.66.3.735. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Woods RP, Phelps ME. Human functional anatomy of visually guided finger movements. Brain. 1992;115:565–87. doi: 10.1093/brain/115.2.565. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination. Exp Brain Res. 1996;112:103–11. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL. Hemispheric asymmetry of movement. Curr Opin Neurobiol. 1996;6:796–800. doi: 10.1016/s0959-4388(96)80030-4. [DOI] [PubMed] [Google Scholar]
- Hepp-Reymond MC, Wyss UR, Anner R. Neuronal coding of static force in the primate motor cortex. J Physiol (Paris) 1978;74:287–91. [PubMed] [Google Scholar]
- Hepp-Reymond MC, Husler EJ, Maier MA, Ql HX. Force-related neuronal activity in two regions of the primate ventral premotor cortex. Can J Physiol Pharmacol. 1994;72:571–9. doi: 10.1139/y94-081. [DOI] [PubMed] [Google Scholar]
- Hepp-Reymond MC, Kirkpatrick-Tanner M, Gabernet L, Qi HX, Weber B. Context-dependent force coding in motor and premotor cortical areas. Exp Brain Res. 1999;128:123–33. doi: 10.1007/s002210050827. [DOI] [PubMed] [Google Scholar]
- Houx PJ, Jolles J. Age-related decline of psychomotor speed: effects of age, brain health, sex, and education. Percept Mot Skills. 1993;76:195–211. doi: 10.2466/pms.1993.76.1.195. [DOI] [PubMed] [Google Scholar]
- Husain M, Kennard C. Visual neglect associated with frontal lobe infarction. J Neurol. 1996;243:652–7. doi: 10.1007/BF00878662. [DOI] [PubMed] [Google Scholar]
- Jackson SR, Husain M. Visuomotor functions of the lateral pre-motor cortex. Curr Opin Neurobiol. 1996;6:788–95. doi: 10.1016/s0959-4388(96)80029-8. [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci. 1995;18:314–20. [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–8. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Kauranen K, Vanharanta H. Influences of aging, gender, and handedness on motor performance of upper and lower extremities. Percept Mot Skills. 1996;82:515–25. doi: 10.2466/pms.1996.82.2.515. [DOI] [PubMed] [Google Scholar]
- Kolb B, Forgie M, Gibb R, Gorny G, Rowntree S. Age, experience and the changing brain. Neurosci Biobehav Rev. 1998;22:143–59. doi: 10.1016/s0149-7634(97)00008-0. [DOI] [PubMed] [Google Scholar]
- Kurata K, Tanji J. Premotor cortex neurons in macaques: activity before distal and proximal forelimb movements. J Neurosci. 1986;6:403–11. doi: 10.1523/JNEUROSCI.06-02-00403.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4) Exp Brain Res. 1999;128:181–7. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- Machado-Salas J, Scheibel ME, Scheibel AB. Neuronal changes in the aging mouse: spinal cord and lower brain stem. Exp Neurol. 1977;54:504–12. doi: 10.1016/0014-4886(77)90253-9. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hairi AR, Das S, Callicott JH, et al. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–5. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- Muakkassa KF, Strick PL. Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized ‘premotor’ areas. Brain Res. 1979;177:176–82. doi: 10.1016/0006-8993(79)90928-4. [DOI] [PubMed] [Google Scholar]
- Napier JRJ. The prehensile movements of the human hand. J Bone Joint Surg. 1956;38B:902–13. doi: 10.1302/0301-620X.38B4.902. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Conrad B, Siebner HR. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett. 2001;313:33–6. doi: 10.1016/s0304-3940(01)02239-x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative architectonic analysis of the human and the macaque frontal cortex. In: Grafman J, Boller F, editors. Handbook of neuropsychology. Vol. 9. Elsevier; Amsterdam: 1994. pp. 17–58. [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–72. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. Neuroimage. 1997;5:261–70. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- Ranganathan VK, Siemionow V, Sahgal V, Yue GH. Effects of aging on hand function. J Am Geriatr Soc. 2001;49:1478–84. doi: 10.1046/j.1532-5415.2001.4911240.x. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P, Stanczac L, Miller A. Neural recruitment and cognitive aging: two hemispheres are better than one, especially as you age. Psychol Sci. 1999;10:494–500. [Google Scholar]
- Rizzolatti G, Scandolara C, Gentilucci M, Camarda R. Response properties and behavioral modulation of ‘mouth’ neurons of the postarcuate cortex (area 6) in macaque monkeys. Brain Res. 1981;225:421–4. doi: 10.1016/0006-8993(81)90847-7. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M. Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp Brain Res. 1988;71:491–507. doi: 10.1007/BF00248742. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D, et al. Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp Brain Res. 1996;111:246–52. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Sadato N, Lyshkow H, Yonekura Y, Honda M, Nagamine T, et al. Both primary motor cortex and supplementary motor area play an important role in complex finger movement. Brain. 1993;116:1387–98. doi: 10.1093/brain/116.6.1387. [DOI] [PubMed] [Google Scholar]
- Shimoyama I, Ninchoji T, Uemura K. The finger-tapping test. A quantitative analysis. Arch Neurol. 1990;47:681–4. doi: 10.1001/archneur.1990.00530060095025. [DOI] [PubMed] [Google Scholar]
- Smith AM, Hepp-Reymond MC, Wyss UR. Relation of activity in precentral cortical neurons to force and rate of force change during isometric contractions of finger muscles. Exp Brain Res. 1975;23:315–32. doi: 10.1007/BF00239743. [DOI] [PubMed] [Google Scholar]
- Smith CD, Umberger GH, Manning EL, Slevin JT, Wekstein DR, Schmitt FA, et al. Critical decline in fine motor hand movements in human aging. Neurology. 1999;53:1458–61. doi: 10.1212/wnl.53.7.1458. [DOI] [PubMed] [Google Scholar]
- Taira M, Mine S, Georgopoulos AP, Murata A, Sakata H. Parietal cortex neurons of the monkey related to the visual guidance of hand movement. Exp Brain Res. 1990;83:29–36. doi: 10.1007/BF00232190. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; Stuttgart: 1988. [Google Scholar]
- Thickbroom GW, Phillips BA, Morris I, Byrnes ML, Mastaglia FL. Isometric force-related activity in sensorimotor cortex measured with functional MRI. Exp Brain Res. 1998;121:59–64. doi: 10.1007/s002210050437. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Phillips BA, Morris I, Byrnes ML, Sacco P, Mastaglia FL. Differences in functional magnetic resonance imaging of sensorimotor cortex during static and dynamic finger flexion. Exp Brain Res. 1999;126:431–8. doi: 10.1007/s002210050749. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, MacDonald JD, Caramanos Z, Posner G, Chiavaras M, Evans AC, et al. Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. Eur J Neurosci. 1999;11:3033–46. doi: 10.1046/j.1460-9568.1999.00718.x. [DOI] [PubMed] [Google Scholar]
- Toni I, Krams M, Turner R, Passingham RE. The time course of changes during motor sequence learning: a whole-brain fMRI study. Neuroimage. 1998;8:50–61. doi: 10.1006/nimg.1998.0349. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–9. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- von Bonin G, Bailey P. The neocortex of the Macaca mulatta. University of Illinois Press; Urbana (IL): 1947. [Google Scholar]
- Wannier TM, Maier MA, Hepp-Reymond MC. Contrasting properties of monkey somatosensory and motor cortex neurons activated during the control of force in precision grip. J Neurophysiol. 1991;65:572–89. doi: 10.1152/jn.1991.65.3.572. [DOI] [PubMed] [Google Scholar]
- Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol. 1993;33:181–9. doi: 10.1002/ana.410330208. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Pierce DJ, Struble RG, Price DL, Cork LC. Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobiol Aging. 1989;10:11–19. doi: 10.1016/s0197-4580(89)80005-3. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited—again. Neuroimage. 1995;2:173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]