Introduction
In 2007, readers of the British Medical Journal voted that the introduction of clean water and sewerage—the ‘sanitation revolution’ of the Victorian era—was the most important medical milestone since the 1840s,1 over anaesthesia, antibiotics, or vaccines. These improvements led to a dramatic reduction in morbidity and mortality associated with faecal-oral infections, such as typhoid fever and cholera. Today, water, sanitation and hygiene (WSH) measures remain critically important to global public health, especially among children in lower income countries, who are at greatest risk from enteric infections and their associated symptoms, complications and sequelae.
In this article, we review the evidence linking WSH measures to faecal-oral diseases in children. Although continued research is needed, existing evidence from the last 150 years supports extending life-saving WSH measures to at-risk populations worldwide.2 One recent estimate3 held that 95% of diarrhoeal deaths in children under 5 years of age could be prevented by 2025, at a cost of US$6.715 billion, through targeted scale-up of proven, cost-effective, life-saving interventions. These include access to safe and accessible excreta disposal, support for basic hygiene practices such as hand washing with soap, and provision of a safe and reliable water supply. We present estimates of the burden of WSH-related disease followed by brief overviews of water, sanitation and hygiene-related transmission routes and control measures.i We conclude with a summary of current international targets and progress.
Transmission routes and health impact
Human excreta can contain over 50 known bacterial, viral, protozoan and helminthic pathogens. The majority of excreta-related infections are obtained through ingestion, less often through inhalation. Excreta-related infections travel through a variety of routes from one host to the next, either as a result of direct transmission through contaminated hands, or indirect transmission via contamination of drinking water, soil, utensils, food and flies (figure 1). The importance of each transmission route varies between pathogens and settings, and different pathogens are more prevalent in some populations.
Figure 1.
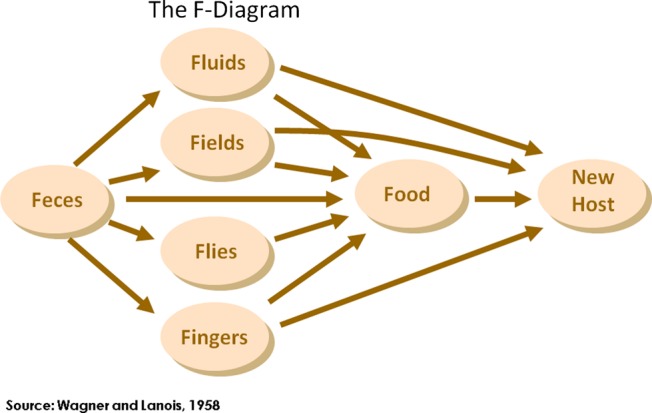
The F-diagram, showing the different faecal-oral transmission routes, and possible barriers to prevent excreta-related pathogens from finding a new host.
Diarrhoea, malnutrition and environmental enteropathy
Although great strides have been made in reducing diarrhoea mortality, especially as a result of the increased use of oral rehydration therapy (ORT), diarrhoea remains the second leading cause of death in children under 5 years of age, after pneumonia.4 It is responsible for an estimated 1.7 billion cases of diarrhoea, or on average 2.9 episodes/child/year, and an estimated 1.87 million deaths among children under 5 years of age.5 The highest burden of disease is in children in the age range of 6–11 months: 4.5 episodes/child/year.6 It has been estimated that 50% of diarrhoea deaths can be attributed to persistent diarrhoea,7 and while ORT can prevent many deaths from acute diarrhoeal diseases,8 access to appropriate treatment is often limited in resource-poor settings.
The relationship between diarrhoeal disease and malnutrition is complex, though it is well accepted that malnourished children suffer more frequent episodes of diarrhoeal disease, while a child's nutritional status is affected following a diarrhoeal episode. A multiple country study found that 25% of stunting in children aged 24 months could be attributable to five or more diarrhoeal episodes experienced in the first 2 years of life.9 Malnutrition and stunting can lead to poorer school performance, early school drop-out and, as a result, lower economic well-being in later life.10 Over 440 million school days are missed annually due to WSH-related illnesses.11 Extended exposure to faecal pathogens may, in part, cause environmental enteropathy, a postulated condition (Humphrey, Lancet) characterised by malabsorption, villus atrophy, crypt hyperplasia, T-cell infiltration and general inflammation of the jejunum.12 This chronic infection of the small intestine could explain why sanitation may have a stronger association with gains in growth than with reductions in diarrhoea incidence.13 For example, a study in Peru found that diarrhoea could explain 16% of stunting, while access to sanitation and water services could explain 40%.14 Environmental enteropathy may reduce vaccine efficacy, either though induction of regulatory T cells which dampen the vaccine-specific immune response, or through the destruction of the live attenuated vaccine by an over-vigorous local immune response in the gut.15 This could explain why oral vaccines for the control of rotavirus have shown a lower efficacy in sub-Saharan Africa (39.3%) and Asia (48.3%), in contrast with efficacy in Europe/USA (85–98%).16–19
An estimated 88% of all child deaths as a result of diarrhoeal disease may be prevented through improvements in WSH.20 While there are promising emerging vaccines, including for rotavirus and cholera, there are several dozen pathogens transmitted in faeces, and WSH will remain critical to prevention of diarrhoeal diseases.
Helminths
Soil-transmitted helminths (STH); Ascaris lumbricoides, Trichuris trichiura and the human hookworms (Necator americanus and Ancylostoma duodenale) are our most common parasites and are estimated to infect up to 807 million, 604 million and 576 million people worldwide, respectively.21 Roughly a third of all Ascaris and Trichuris infections and 20% of hookworm infections are in children under the age of 15 years.22 STH ova leave the human body in excreta, and must mature in soil before they become infective. STH infections have shown a strong association with open defecation,23 and the use of fresh excreta or wastewater in agriculture.24 25 Consumption of dirt or soil (pica) by children, pregnant women, and others has also been suggested as a risk factor for STH.26 27 Schistosomiasis is estimated to infect 207 million people worldwide,21 and is caused by open defecation or anal cleansing28 close to or in water bodies where the miracidia can hatch and find a snail host to complete their lifecycle. The use of surface water sources like lakes, rivers and irrigation canals for domestic water needs, like washing, bathing and water collection have been associated with an increased risk of infection.29 Children are especially at risk as a result of their lower immunity, and because they may have more contact with contaminated water through play or water collection.30 Although direct mortality as a result of a helminth infection is low, morbidity is high. The global burden of disease has been variously estimated (as few as 3 million and as many as 50 million Disability Adjusted Life Year (DALY)31 32), and is uncertain but high.33 The burden of disease is attributable to the long-term impacts related to helminth infections, including anaemia, low birth weight, preterm birth, retarded growth, poorer cognitive performance and early school drop-out. Hookworm, in particular, is associated with undernutrition and possibly greater risk of malaria34–36 and HIV.37 38 The delivery of anthelminthics through schools has lately been the intervention of choice for reducing STH infections in children. The effectiveness of these programmes, especially with respect to improving cognitive performance, has recently been questioned, however, a large meta-analysis did not find a significant improvement,39 underscoring the need to interrupt transmission via effective sanitation in home and school environments.40
Prevention
Sanitation
Sanitation aims to prevent contamination of the environment by excreta and, therefore, to prevent transmission of pathogens that originate in faeces of an infected person. A wide range of technologies and methods exists to achieve this, which include sophisticated and high-cost methods like waterborne sewage systems and simple low-cost methods like the cat method, which involves the digging of a hole and covering faeces with soil after defecation. The WHO/UNICEF Joint Monitoring Programme (JMP) classifies the following as ‘improved’ sanitation that is more likely to be hygienic: a connection to a sewerage system, septic tanks, pour-flush toilets, ventilated improved pit latrines and pit latrines with a concrete slab. According to the 2010 JMP estimate, 11 countries make up 76% of the 2.5 billion people lacking improved sanitation, with one-third in India. Over one billion people practise open defecation, mostly in rural areas.41 Systematic reviews of the impact of sanitation on health have estimated a mean reduction of 32–36% in diarrhoea,42–44 though a Cochrane systematic review did not calculate a pooled effect due to heterogeneity of studies.45 The number of rigorous studies that have investigated the impact of sanitation on soil-transmitted helminth infections is very limited, as most of the sanitation interventions were conducted in conjunction with improvements in water supply. In Salvador, Brazil, neighbourhoods with storm water drains to prevent seasonal flooding with sewage, or full waterborne sewerage, were compared with neighbourhoods having neither. The study found that, when the level of community sanitation was better, the prevalence of ascariasis declined by up to 40%, and that domestic domain risk factors were more numerous and more significant in areas with better community sanitation.46
Safe child stool disposal
Many cultures consider the stools of infants fed on breast milk harmless, or at least less harmful than those of adults, because they are smaller, their faeces smell less, and contain less visual food residues.47–49 Additionally, most latrines are not designed for use of, and may not be used by, small children. They might be afraid to use them for the risk of falling in, bad smells, or the fear of dark spaces. Because nappies, child-sized potties and washing machines are not available in many poor settings, defecation on the floor is common and potentially seen as the most practical option until the child is potty trained. As a result, latrine use by children is low, as was shown by a study in Lima, Peru, where less than 25% of under five-year-olds used a toilet.49 Because of a much higher prevalence of diarrhoea and higher egg counts for STH in children, child stool often poses a greater health risk than those of adults.50 To date, safe disposal of children's stools has received relatively little attention in sanitation programmes.
Water supply
While all communities have access to water, the quantity and quality available may be insufficient to meet basic needs, and access may not be near to the household. The physically demanding job of water collection is usually allocated to women and children. A study in 25 countries in sub-Saharan Africa estimated that children spent a collective four million hours every day fetching water, and keeping them away from school.41 Carrying water can also lead to injuries and growth stunting.51 When water is available within 1 km, or a 30 min return round trip from the household, water use does not change significantly until water is provided on the plot or very nearby (figure 2). When a tap is available within the household, or shared with a close neighbour, per capita water use can go up from 10–30 L/person/day to 30–100 L/person/day. Greater volumes of water available to a household tend to result in better hygiene, including increased hand washing.
Figure 2.
Distance to water source and use.
Figure 3.

An improved form of sanitation: a household pit latrine in Tanzania.
Over 780 million people now lack access to an ‘improved’ water source, and one study has estimated the number of people who rely on microbiologically or chemically unsafe water to be 1.8 billion, or about 28% of the global population.52 According to WHO/UNICEF JMP, ‘improved’ water sources include piped water, rainwater, protected springs and protected wells, which are thought to be less likely to be contaminated with pathogenic microbes. Recent research has shown, however, that even such improved water supplies may be subject to faecal contamination (ibid.) and that even occasional exposure to unsafe water—for example, from intermittent service or inadequate treatment—can undermine health benefits.53 Providing safe, reliable, piped-in water to every household is an essential goal, yielding optimal health gains, while contributing to the Millennium Development Goal (MDG) targets for poverty reduction, nutrition, childhood survival, school attendance, gender equity and environmental sustainability.
Food hygiene
Weaning food hygiene may be among the most important determinants of diarrhoeal disease risk in young children,54 55 although the current evidence is insufficient to arrive at conclusions about its relative importance in control of infections. It has been shown to be important in some contexts, with estimates of up to 70% of all diarrhoea caused by contaminated foods,54 and microbial counts that may exceed those found in drinking water.54–60 Food hygiene interventions, such as the promotion of reheating foods, preventing contact with flies and hand washing before feeding are the subject of current research, but effects on diarrhoeal disease risk have not been estimated from multiple trials. The sustained effects of behaviour change from food hygiene interventions have not been assessed.
Hand hygiene
Hand washing with soap before feeding children and after cleaning them can interrupt the transmission of faecal-oral microbes in the domestic environment. A review of the literature on hand hygiene suggests that hand washing with soap (HWWS) can reduce micro-organism levels close to zero,61 62 mainly through the mechanical action of rubbing and rinsing.62 63 Good hand washing practice should include water, a washing agent such as soap and a drying phase.50 64–66 Estimates of the impact of HWWS on health from systematic reviews suggest large effects (up to 48% reduction in diarrhoea).42 44 67–70 Hand washing with soap can also cut the risk of acute respiratory infections by 23%.71–73 Critically, persistent changes in behaviours may be possible following interventions,74 although more research is needed on the longer-term effects of behaviour change campaigns.
Water quality
Because universal safe, reliable, on-plot water supply remains an elusive goal for the majority of the world's population, household-level water treatment (HWT) has been proposed as an interim solution to provide safer drinking water at the point of use.75 76 In many settings, both rural and urban, populations may have access to sufficient quantities of water, but that water may be unsafe for consumption as a result of microbial or chemical contamination. Effective HWT, such as boiling, filtration or chlorination, has been shown to improve microbial water quality significantly.77–79 Safe storage of treated water is necessary to prevent recontamination through unsafe water handling.80
Improving water quality at the point of consumption can protect children from waterborne disease. The findings of meta-analyses show a much stronger protective effect for water quality interventions at the household level (rather than at source level) on diarrhoeal disease outcomes (up to 40%42 44 81: but no blinded trials of household water treatment have shown protective effects. HWT is unique among WSH interventions in that it may, in some forms, be possible to blind with a placebo device, chemical, or method. A review by Cairncross et al67 estimated diarrhoea risk reductions of 17% associated with improved water quality, which is consistent with earlier reviews by Esrey et al,43 68 and issues of bias potentially affecting the evidence base for water quality interventions have been articulated.81 82
One of the challenges that promoters of HWT have reported is low adherence—consistent, correct and sustained use—which can limit expected health gains.83 84 Unlike centrally treated, piped-in water supplies, HWT is normally a batch process that must be undertaken by the end users on a daily basis in order to provide consistent protection against waterborne pathogens. A number of studies of HWT have reported reduced use of interventions over time, suggesting that low adherence may limit the usefulness of HWT as a strategy.77 85–88
Progress toward and beyond the MDGs
Improvements in water supply and sanitation, if implemented sustainably, will have an important impact on a wide variety of different infectious diseases, and could improve the quality of life of millions of children worldwide, and provide them a proper start in life. In 2010, the UN General Assembly affirmed that clean drinking water and sanitation are human rights, with the UN Human Rights Council later recognising these rights to be derived from the rights to an adequate standard of living, health, life and dignity.41 These pronouncements cannot by themselves drive progress in expanding services, but do add moral weight to the pursuit of universal coverage. Although great strides have been made in the provision of sanitation and water, it is estimated that if current trends continue, 605 million people will still lack access to an improved water source in 2015, and 2.4 billion people will lack access to basic sanitation.41 Implementing better measures of what constitutes ‘access’ to water and sanitation—currently, monitoring is based primarily on technology types, without regard to key quality or accessibility factors—would paint an even bleaker picture of the global shortfall in coverage.
Households with a so-called ‘improved’ water supply connection that is intermittent and provides microbiologically unsafe water are counted among those with access to water meeting the MDG target for ‘sustainable access to safe drinking water’.89 Similarly, sanitation progress made in some areas have accounted only for construction of latrines, when there are no good options for treatment or disposal of waste after they fill up (figure 3).
In order to consolidate the gains of the last decade, and contribute to lowering the burden of childhood morbidity and mortality, post-MDG goals for water and sanitation should focus on strengthening key WSH institutions, creating demand and ownership, and fostering sustainable behaviour change. This requires an acknowledgment that the motivations for improving access are different for policy makers and intended recipients. The MDGs include language on reducing poverty and disease mortality, but for most of the beneficiaries the advantages and motives for the adoption of WSH measures are not directly health-related, but improvements in quality of life, including factors related to privacy, comfort, status, dignity, protection from harassment and savings in cost and time. Harnessing the power of these motivations to expand access to WSH, by leveraging investment by households—not only by governments and donor agencies—may help increase coverage of these life-saving measures among those who would most benefit from them.
Contributors: All authors wrote and reviewed the paper, JHJE acts as guarantor of the content.
Funding: SC's and JHJE's time was funded by the DFID research programme consortium on Sanitation and Hygiene (SHARE: grant no. P04990).
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.
Although we do cite pooled estimates of effect for WSH interventions on child health from a number of systematic reviews, readers should note that there is a rich emerging literature that attempts comparisons of impact between water supply, water quality, hygiene and sanitation; Waddington et al 2009 provides a good summary that is still current. WSH interventions are not very amenable to randomisation (in the case of infrastructure) and are almost never blinded in trials, with the exception of a minority of water quality intervention trials. Therefore, randomised, controlled trials (RCTs) may be subject to significant bias, and RCTs constitute the majority of studies included in systematic reviews of WSH interventions. We cite these reviews where appropriate as important but potentially flawed estimates that may be considered suggestive only. A broader perspective on the evidence base may be more helpful.
References
- 1.Ferriman A. BMJ readers choose sanitation as greatest medical advance since 1840. Br Med J 2007;334:11117235067 [Google Scholar]
- 2.Chopra M, Mason E, Borrazzo J. et al. Ending of preventable deaths from pneumonia and diarrhoea: an achievable goal. Lancet 2013 [DOI] [PubMed] [Google Scholar]
- 3.Bhutta ZA, Das JK, Walker N, et al. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost? Lancet 2013;381:1417–29 [DOI] [PubMed] [Google Scholar]
- 4.Walker CL, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013;381:1405–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boschi-Pinto C, Velebit L, Shibuya K. Estimating child mortality due to diarrhoea in developing countries. Bull World Health Organ 2008;86:710–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer Walker CL, Perin J, Aryee MJ, et al. Diarrhea incidence in low- and middle-income countries in 1990 and 2010: a systematic review. BMC Public Health 2012;12:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathers CD, et al. Global Burden of Disease in 2002: data sources, methods and results. In: Global Programme on Evidence for Health Policy Discussion Paper No. 54 (Revised 2004). World Health Organization, 2002 [Google Scholar]
- 8.Sur D, Bhattacharya SK. Acute diarrhoeal diseases–an approach to management. J Indian Med Assoc 2006;104:220–3 [PubMed] [Google Scholar]
- 9.Checkley W, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol 2008;37:816–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008;371:340–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moszynski P. Worldwide water crisis is a “silent emergency”, UN agency says. BMJ 2006;333:986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 2009;374:1032–5 [DOI] [PubMed] [Google Scholar]
- 13.Esrey SA. Water, waste, and well-being: a multicountry study. Am J Epidemiol 1996;143:608–23 [DOI] [PubMed] [Google Scholar]
- 14.Checkley W, Gilman R, Black R. et al. Effect of water and sanitation on childhood health in a poor Peruvian peri-urban community. Lancet 2004;363:112–18 [DOI] [PubMed] [Google Scholar]
- 15.Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol 2010;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010;376:606–14 [DOI] [PubMed] [Google Scholar]
- 17.Zaman K, Anh DD, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010;376:615–23 [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Palacios GM, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006;354:11–22 [DOI] [PubMed] [Google Scholar]
- 19.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006;354:23–33 [DOI] [PubMed] [Google Scholar]
- 20.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet 2003;361:2226–34 [DOI] [PubMed] [Google Scholar]
- 21.Hotez PJ, Brindley PJ, Bethony JM, et al. Helminth infections: the great neglected tropical diseases. J Clin Invest 2008;118:1311–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Silva NR, Brooker S, Hotez PJ, et al. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol 2003;19:547–51 [DOI] [PubMed] [Google Scholar]
- 23.Ziegelbauer K, Speich B, Mausezahl D, et al. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med 2012;9:e1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ensink JHJ, Blumenthal UJ, Brooker S. Wastewater quality and the risk of intestinal nematode infection in sewage farming families in hyderabad, India. Am J Trop Med Hyg 2008;79:561–7 [PMC free article] [PubMed] [Google Scholar]
- 25.Humphries DL, Stephenson LS, Pearce EJ, et al. The use of human faeces for fertilizer is associated with increased intensity of hookworm infection in Vietnamese women. Trans R Soc Trop Med Hyg 1997;91:518–20 [DOI] [PubMed] [Google Scholar]
- 26.Geissler PW, Mwaniki D, Thiong'o F, et al. Geophagy as a risk factor for geohelminth infections: a longitudinal study of Kenyan primary schoolchildren. Trans R Soc Trop Med Hyg 1998;92:7–11 [DOI] [PubMed] [Google Scholar]
- 27.Saathoff E, Olsen A, Kvalsvig JD, et al. Geophagy and its association with geohelminth infection in rural schoolchildren from northern KwaZulu-Natal, South Africa. Trans R Soc Trop Med Hyg 2002;96:485–90 [DOI] [PubMed] [Google Scholar]
- 28.Sow S, Polman K, Vereecken K, et al. The role of hygienic bathing after defecation in the transmission of Schistosoma mansoni. Trans R Soc Trop Med Hyg 2008;102:542–7 [DOI] [PubMed] [Google Scholar]
- 29.Dalton PR, Pole D. Water-contact patterns in relation to Schistosoma haematobium infection. Bull World Health Organ 1978;56:417–26 [PMC free article] [PubMed] [Google Scholar]
- 30.Mafiana CF, Ekpo UF, Ojo DA. Urinary schistosomiasis in preschool children in settlements around Oyan Reservoir in Ogun State, Nigeria: implications for control. Trop Med Int Health 2003;8:78–82 [DOI] [PubMed] [Google Scholar]
- 31.Chan MS. The global burden of intestinal nematode infections–fifty years on. Parasitol Today 1997;13:438–43 [DOI] [PubMed] [Google Scholar]
- 32.WHO Reducing Risks, Promoting Healthy Life. The World Health Report 2002. Annex Table 3. Burden of Disease in DALYs by Cause, Sex and Mortality Stratus in WHO Regions, Estimates for 2001, 2002, WHO: Geneva, 230 [Google Scholar]
- 33.Brooker S. Estimating the global distribution and disease burden of intestinal nematode infections: adding up the numbers—a review. Int J Parasitol 2010;40:1137–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degarege A, Animut A, Legesse M, et al. Malaria severity status in patients with soil-transmitted helminth infections. Acta Trop 2009;112:8–11 [DOI] [PubMed] [Google Scholar]
- 35.Boel M, Carrara VI, Rijken M, et al. Complex Interactions between soil-transmitted helminths and malaria in pregnant women on the Thai-Burmese border. PLoS Negl Trop Dis 2010;4:e887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pullan RL, Kabatereine NB, Bukirwa H, et al. Heterogeneities and consequences of Plasmodium species and hookworm coinfection: a population based study in Uganda. J Infect Dis 2011;203:406–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb EL, Kyosiimire-Lugemwa J, Kizito D, et al. The effect of anthelmintic treatment during pregnancy on HIV plasma viral load: results from a randomized, double-blind, placebo-controlled trial in Uganda. J Acquir Immune Defic Syndr 2012;60:307–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanyaolu AO, Oyibo WA, Fagbenro-Beyioku AF, et al. Comparative study of entero-parasitic infections among HIV sero-positive and sero-negative patients in Lagos, Nigeria. Acta Trop 2011;120:268–72 [DOI] [PubMed] [Google Scholar]
- 39.Taylor-Robinson DC, et al. Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin and school performance. Cochrane Database Syst Rev 2012;7:CD000371. [DOI] [PubMed] [Google Scholar]
- 40.Remais JV, Eisenberg JN. Balance between clinical and environmental responses to infectious diseases. Lancet 2012;379:1457–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.UNICEF and WHO Progress on drinking water and sanitation. 2012 update, in Joint Monitoring Programme for Water Supply and Sanitation, 2012
- 42.Waddington H, Snilstveit, White H, et al. Water, sanitation and hygiene interventions to combat childhood diarrhoea in developing countries. Delhi: International Initiative for Impact Evaluation, 2009 [Google Scholar]
- 43.Esrey SA, Potash JB, Roberts L, et al. Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bull World Health Organ 1991;69:609–21 [PMC free article] [PubMed] [Google Scholar]
- 44.Fewtrell L, Kaufmann R, Kay D, et al. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis 2005;5:42–52 [DOI] [PubMed] [Google Scholar]
- 45.Clasen TF, Bostoen K, Schmidt WP, et al. Interventions to improve disposal of human excreta for preventing diarrhoea. Cochrane Database Syst Rev 2010;6:CD007180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moraes LR, Cancio JA, Cairncross S. Impact of drainage and sewerage on intestinal nematode infections in poor urban areas in Salvador, Brazil. Trans R Soc Trop Med Hyg 2004;98:197–204 [DOI] [PubMed] [Google Scholar]
- 47.Gil A, Lanata C, Kleinau E, et al. Children's feces disposal practices in developing countries and interventions to prevent diarrheal diseases; A literature review. Environmental Health Project 2004: Office of Health, Infectious Diseases and Nutrition, U.S. Agency for International Development
- 48.Curtis V, Kanki B, Mertens T, et al. Potties, pits and pipes: explaining hygiene behaviour in Burkina Faso. Soc Sci Med 1995;41:383–93 [DOI] [PubMed] [Google Scholar]
- 49.Yeager BA, Huttly SRA, Bartolini R, et al. Defecation practices of young children in a Peruvian shanty town. Soc Sci Med 1999;49:531–41 [DOI] [PubMed] [Google Scholar]
- 50.Feachem R, Bradley D, Garelick H, et al. Sanitation and disease: health aspects of excreta and wastewater management. World Bank studies in water supply and sanitation 3. Chichester, UK: John Wiley & Sons, 1983:501 [Google Scholar]
- 51.Geere JA, Hunter PR, Jagals P. Domestic water carrying and its implications for health: a review and mixed methods pilot study in Limpopo Province, South Africa. Environ Health 2010;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onda K, LoBuglio J, Bartram J. Global access to safe water: accounting for water quality and the resulting impact on MDG progress. Int J Environ Res Public Health 2012;9:880–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunter PR, Pond K, Jagals P, et al. An assessment of the costs and benefits of interventions aimed at improving rural community water supplies in developed countries. Sci Total Environ 2009;407:3681–5 [DOI] [PubMed] [Google Scholar]
- 54.Motarjemi Y, Kaferstein F, Moy G, et al. Contaminated weaning food: a major risk factor for diarrhoea and associated malnutrition. Bull World Health Organ 1993;71:79–92 [PMC free article] [PubMed] [Google Scholar]
- 55.Toure O, Coulibaly S, Arby A, et al. Piloting an intervention to improve microbiological food safety in Peri-Urban Mali. Int J Hyg Environ Health 2012;216:138–45. [DOI] [PubMed] [Google Scholar]
- 56.Barrell RA, Rowland MG. Commercial milk products and indigenous weaning foods in a rural West African Environment: a bacteriological perspective. J Hyg (Lond) 1980;84:191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imong SM, Rungruengthanakit K, Ruangyuttikarn C, et al. The bacterial content of infant weaning foods and water in rural northern Thailand. J Trop Pediatr 1989;35:14–18 [DOI] [PubMed] [Google Scholar]
- 58.Henry FJ, Patwary Y, Huttly SRA, et al. Bacterial contamination of weaning foods and drinking water in rural Bangladesh. Epidemiol Infect 1990;104:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lanata CF. Studies of food hygiene and diarrhoeal disease. Int J Environ Health Res 2003;13(Suppl 1):S175–83 [DOI] [PubMed] [Google Scholar]
- 60.Kung'u JK, Boor KJ, Ame SM, et al. Bacterial populations in complementary foods and drinking-water in households with children aged 10–15 months in Zanzibar, Tanzania. J Health Popul Nutr 2009;27:41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kampf G, Kramer A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin Microbiol Rev 2004;17:863–93, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sprunt K, Redman W, Leidy G. Antibacterial effectiveness of routine hand washing. Pediatrics 1973;52:264–71 [PubMed] [Google Scholar]
- 63.Lowbury EJ, Lilly HA, Bull JP. Disinfection of hands: removal of transient organisms. Br Med J 1964;2:230–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jumaa PA. Hand hygiene: simple and complex. Int J Infect Dis 2005;9:3–14 [DOI] [PubMed] [Google Scholar]
- 65.Kaltenthaler E, Waterman R, Cross P. Faecal indicator bacteria on the hands and the effectiveness of hand-washing in Zimbabwe. J Trop Med Hyg 1991;94:358–63 [PubMed] [Google Scholar]
- 66.Hoque BA, Briend A. A comparison of local handwashing agents in Bangladesh. J Trop Med Hyg 1991;94:61–4 [PubMed] [Google Scholar]
- 67.Cairncross S, Hunt C, Boisson S, et al. Water, sanitation and hygiene for the prevention of diarrhoea. Int J Epidemiol 2010;39(Suppl 1):i193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Esrey SA, Feachem RG, Hughes JM. Interventions for the control of diarrhoeal diseases among young children: improving water supplies and excreta disposal facilities. Bull World Health Organ 1985;63:757–72 [PMC free article] [PubMed] [Google Scholar]
- 69.Huttly SR, Morris SS, Pisani V. Prevention of diarrhoea in young children in developing countries. Bull World Health Organ 1997;75:163–74 [PMC free article] [PubMed] [Google Scholar]
- 70.Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infect Dis 2003;3:275–81 [DOI] [PubMed] [Google Scholar]
- 71.Rabie T, Curtis V. Handwashing and risk of respiratory infections: a quantitative systematic review. Trop Med Int Health 2006;11:258–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luby SP, Agboatwalla M, Feikin DR, et al. Effect of handwashing on child health: a randomised controlled trial. Lancet 2005;366:225–33 [DOI] [PubMed] [Google Scholar]
- 73.Sandora TJ, Taveras EM, Shih MC, et al. A randomized, controlled trial of a multifaceted intervention including alcohol-based hand sanitizer and hand-hygiene education to reduce illness transmission in the home. Pediatrics 2005;116:587–94 [DOI] [PubMed] [Google Scholar]
- 74.Cairncross S, Shordt K, Zacharia S, et al. What causes sustainable changes in hygiene behaviour? A cross-sectional study from Kerala, India. Soc Sci Med 2005;61:2212–20 [DOI] [PubMed] [Google Scholar]
- 75.Mintz ED, Reiff FM, Tauxe RV. Safe water treatment and storage in the home. A practical new strategy to prevent waterborne disease. JAMA 1995;273:948–53 [PubMed] [Google Scholar]
- 76.WHO Combating waterborne disease at the household level in International Network to Promote Household Water Treatment and Safe Storage. Geneva: World Health Organization, 2007 [Google Scholar]
- 77.Luby S, Agboatwalla M, Raza A, et al. A low-cost intervention for cleaner drinking water in Karachi, Pakistan. Int J Infect Dis 2001;5:144–50 [DOI] [PubMed] [Google Scholar]
- 78.Clasen TF, Thao DH, Boisson S, et al. Microbiological effectiveness and cost of boiling to disinfect drinking water in rural Vietnam. Environ Sci Technol 2008;42:4255–60 [DOI] [PubMed] [Google Scholar]
- 79.Brown J, Sobsey MD, Loomis D. Local drinking water filters reduce diarrheal disease in Cambodia: a randomized, controlled trial of the ceramic water purifier. Am J Trop Med Hyg 2008;79:394–400 [PubMed] [Google Scholar]
- 80.Wright J, Gundry S, Conroy R. Household drinking water in developing countries: a systematic review of microbiological contamination between source and point-of-use. Trop Med Int Health 2004;9:106–17 [DOI] [PubMed] [Google Scholar]
- 81.Clasen T, Schmidt W-P, Rabie T, et al. Interventions to improve water quality for preventing diarrhoea: systematic review and meta-analysis. Br Med J 2007;334:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmidt WP, Cairncross S. Household water treatment in poor populations: is there enough evidence for scaling up now? Environ Sci Technol 2009;43:986–92 [DOI] [PubMed] [Google Scholar]
- 83.Enger KS, Nelson KL, Clasen T, et al. Linking quantitative microbial risk assessment and epidemiological data: informing safe drinking water trials in developing countries. Environ Sci Technol 2012;46:5160–7 [DOI] [PubMed] [Google Scholar]
- 84.Brown J, Clasen T. High adherence is necessary to realize health gains from water quality interventions. PLoS One 2012;7:e36735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boisson S, Kiyombo M, Sthreshley L, et al. Field assessment of a novel household-based water filtration device: a randomised, placebo-controlled trial in the Democratic Republic of Congo. PLoS One 2010;5:e12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mausezahl D, Christen A, Pacheco GD, et al. Solar drinking water disinfection (SODIS) to reduce childhood diarrhoea in rural Bolivia: a cluster-randomized, controlled trial. PLoS Med 2009;6:e1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mong Y, Kaiser R, Ibrahim D, et al. Impact of the safe water system on water quality in cyclone-affected communities in Madagascar. Am J Public Health 2001;91:1577–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Colindres RE, Jain S, Bowen A, et al. After the flood: an evaluation of in-home drinking water treatment with combined flocculent-disinfectant following Tropical Storm Jeanne—Gonaives, Haiti, 2004. J Water Health, 2007;5:367–74 [DOI] [PubMed] [Google Scholar]
- 89.DANIDA Reaching the MDG target for sanitation in Africa; A call for realism, in Technical Advisory Services. Copenhagen, Denmark: Ministry of Foreign Affairs of Denmark, 2010 [Google Scholar]



