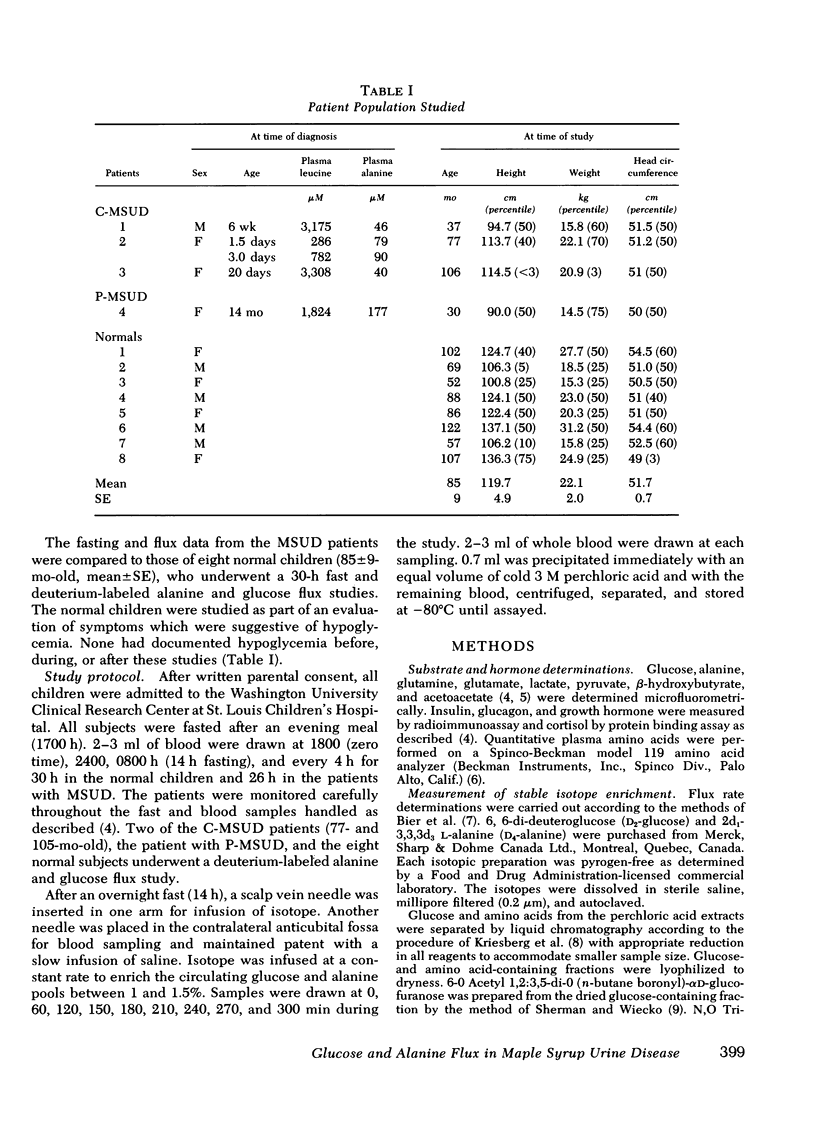
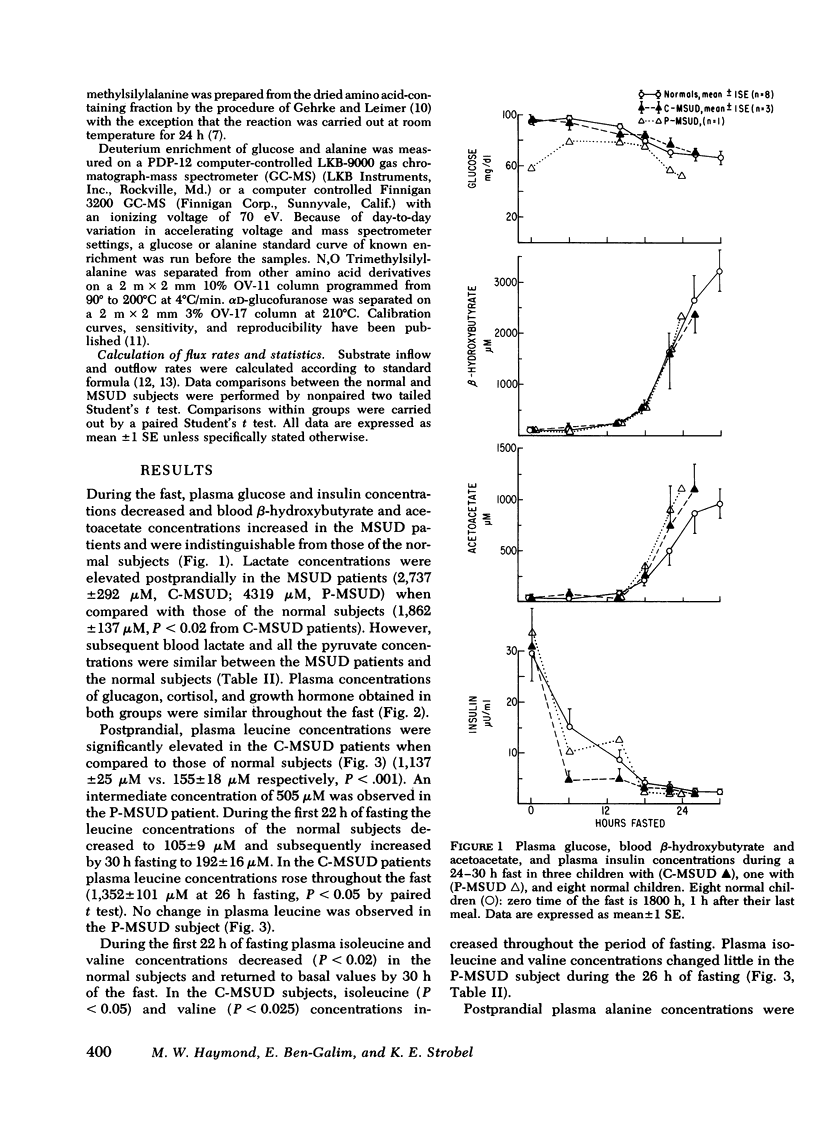
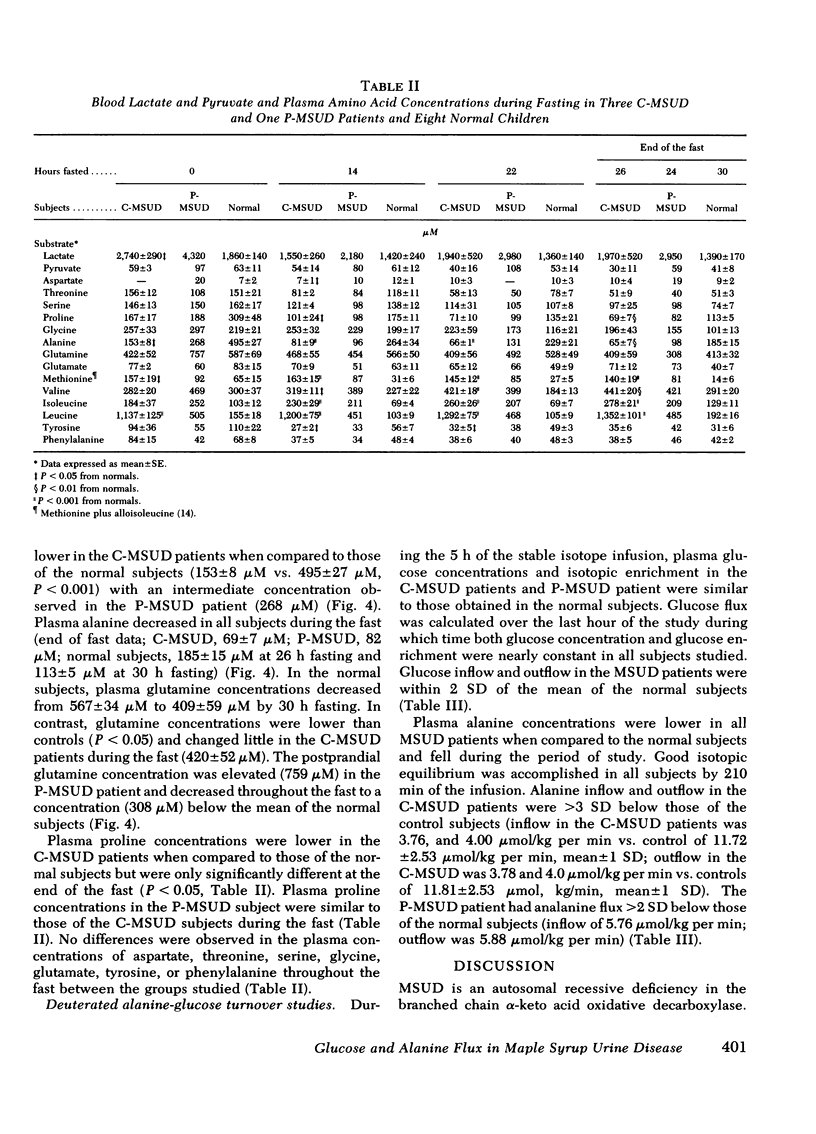
Abstract
In vitro studies have suggested that catabolism of branched chain amino acids is linked with alanine and glutamine formed in, and released from, muscle. To explore this possibility in vivo, static and kinetic studies were performed in three patients with classical, and one patient with partial, branched chain α-ketoacid decarboxylase deficiency (maple syrup urine disease, MSUD) and compared to similar studies in eight age-matched controls. The subjects underwent a 24-30-h fast, and a glucose-alanine flux study using stable isotopes. Basal plasma leucine concentrations were elevated (P <0.001) in patients with MSUD (1,140±125 μM vs. 155±18 μM in controls); and in contrast to the controls, branched chain amino acid concentrations in plasma increased during the fast in the MSUD patients. Basal plasma alanine concentrations were lower (P <0.01) in patients with classical MSUD (153±8 μM vs. 495±27 μM in controls). This discrepancy was maintained throughout the fast despite a decrease in alanine concentrations in both groups. Plasma alanine and leucine concentrations in the patient with partial MSUD were intermediate between those of the controls and the subjects with the classical form of the disease. Circulating ketone bodies and glucoregulatory hormones concentrations were similar in the MSUD and normal subjects during the fast.
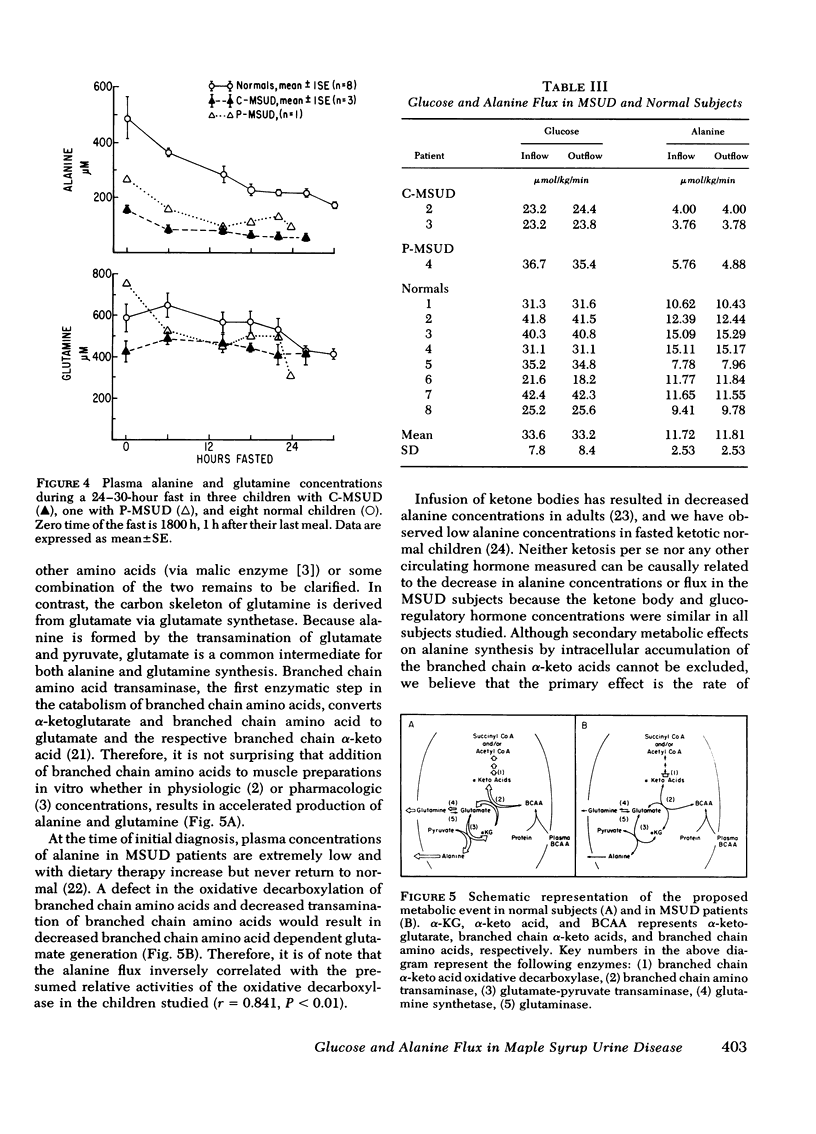
Alanine flux rates in two patients with classical MSUD (3.76 and 4.00 μmol/Kg per min) and the patient with partial MSUD (5.76 μmol/Kg per min) were clearly lower than those of the controls (11.72±2.53 [SD] μmol/Kg per min). After short-term starvation, glucose flux and fasting concentrations were similar in the MSUD patients and normal subjects.
These data indicate that branched chain amino acid catabolism is an important rate limiting event for alanine production in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adibi S. A. Metabolism of branched-chain amino acids in altered nutrition. Metabolism. 1976 Nov;25(11):1287–1302. doi: 10.1016/s0026-0495(76)80012-1. [DOI] [PubMed] [Google Scholar]
- Bier D. M., Arnold K. J., Sherman W. R., Holland W. H., Holmes W. F., Kipnis D. M. In-vivo measurement of glucose and alanine metabolism with stable isotopic tracers. Diabetes. 1977 Nov;26(11):1005–1015. doi: 10.2337/diab.26.11.1005. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Dancis J., Hutzler J., Rokkones T. Intermittent branched-chain ketonuria. Variant of maple-syrup-urine disease. N Engl J Med. 1967 Jan 12;276(2):84–89. doi: 10.1056/NEJM196701122760204. [DOI] [PubMed] [Google Scholar]
- Dickinson J. C., Rosenblum H., Hamilton P. B. Ion exchange chromatography of the free amino acids in the plasma of infants under 2,500 gm at birth. Pediatrics. 1970 Apr;45(4):606–613. [PubMed] [Google Scholar]
- Felig P., Pozefsky T., Marliss E., Cahill G. F., Jr Alanine: key role in gluconeogenesis. Science. 1970 Feb 13;167(3920):1003–1004. doi: 10.1126/science.167.3920.1003. [DOI] [PubMed] [Google Scholar]
- Felig P. The glucose-alanine cycle. Metabolism. 1973 Feb;22(2):179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Bier D. M., Cryer P. E., Pagliara A. S. Hypoglycemia in compensated chronic renal insufficiency. Substrate limitation of gluconeogenesis. Diabetes. 1974 Dec;23(12):982–986. doi: 10.2337/diab.23.12.982. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. II. The precursor role of amino acids in alanine and glutamine synthesis. J Biol Chem. 1976 Feb 10;251(3):836–843. [PubMed] [Google Scholar]
- Gehrke C. W., Leimer K. Trimethylsilylation of amino acids derivatization and chromatography. J Chromatogr. 1971 May 6;57(2):219–238. doi: 10.1016/0021-9673(71)80035-3. [DOI] [PubMed] [Google Scholar]
- Goedde H. W., Langenbeck U., Brackertz D., Keller W., Rokkones T., Halvorsen S., Kiil R., Merton B. Clinical and biochemical-genetic aspects of intermittent branched-chain ketoaciduria. Report of two Scandinavian families. Acta Paediatr Scand. 1970 Jan;59(1):83–87. doi: 10.1111/j.1651-2227.1970.tb15519.x. [DOI] [PubMed] [Google Scholar]
- Haymond M. W., Karl I. E., Feigin R. D., DeVivo D., Pagliara A. S. Hypoglycemia and maple syrup urine disease: defective gluconeogenesis. Pediatr Res. 1973 May;7(5):500–508. doi: 10.1203/00006450-197305000-00003. [DOI] [PubMed] [Google Scholar]
- Haymond M. W., Karl I. E., Pagliara A. S. Ketotic hypoglycemia: an amino acid substrate limited disorder. J Clin Endocrinol Metab. 1974 Apr;38(4):521–530. doi: 10.1210/jcem-38-4-521. [DOI] [PubMed] [Google Scholar]
- Haymond M. W., Karl I., Weldon V. V., Pagliara A. S. The role of growth hormone and cortisone on glucose and gluconeogenic substrate regulation in fasted hypopituitary children. J Clin Endocrinol Metab. 1976 May;42(5):846–856. doi: 10.1210/jcem-42-5-846. [DOI] [PubMed] [Google Scholar]
- Kreisberg R. A., Siegal A. M., Owen W. C. Alanine and gluconeogenesis in man: effect of ethanol. J Clin Endocrinol Metab. 1972 May;34(5):876–883. doi: 10.1210/jcem-34-5-876. [DOI] [PubMed] [Google Scholar]
- Odessey R., Khairallah E. A., Goldberg A. L. Origin and possible significance of alanine production by skeletal muscle. J Biol Chem. 1974 Dec 10;249(23):7623–7629. [PubMed] [Google Scholar]
- SNYDERMAN S. E., NORTON P. M., ROITMAN E., HOLT L. E., Jr MAPLE SYRUP URINE DISEASE, WITH PARTICULAR REFERENCE TO DIETOTHERAPY. Pediatrics. 1964 Oct;34:454–472. [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Schulman J. D., Lustberg T. J., Kennedy J. L., Museles M., Seegmiller J. E. A new variant of maple syrup urine disease (branched chain ketoaciduria). Clinical and biochemical evaluation. Am J Med. 1970 Jul;49(1):118–124. doi: 10.1016/s0002-9343(70)80121-8. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Hendler R. G., Felig P. Effect of ketone infusions on amino acid and nitrogen metabolism in man. J Clin Invest. 1975 Jun;55(6):1382–1390. doi: 10.1172/JCI108057. [DOI] [PMC free article] [PubMed] [Google Scholar]


