Abstract
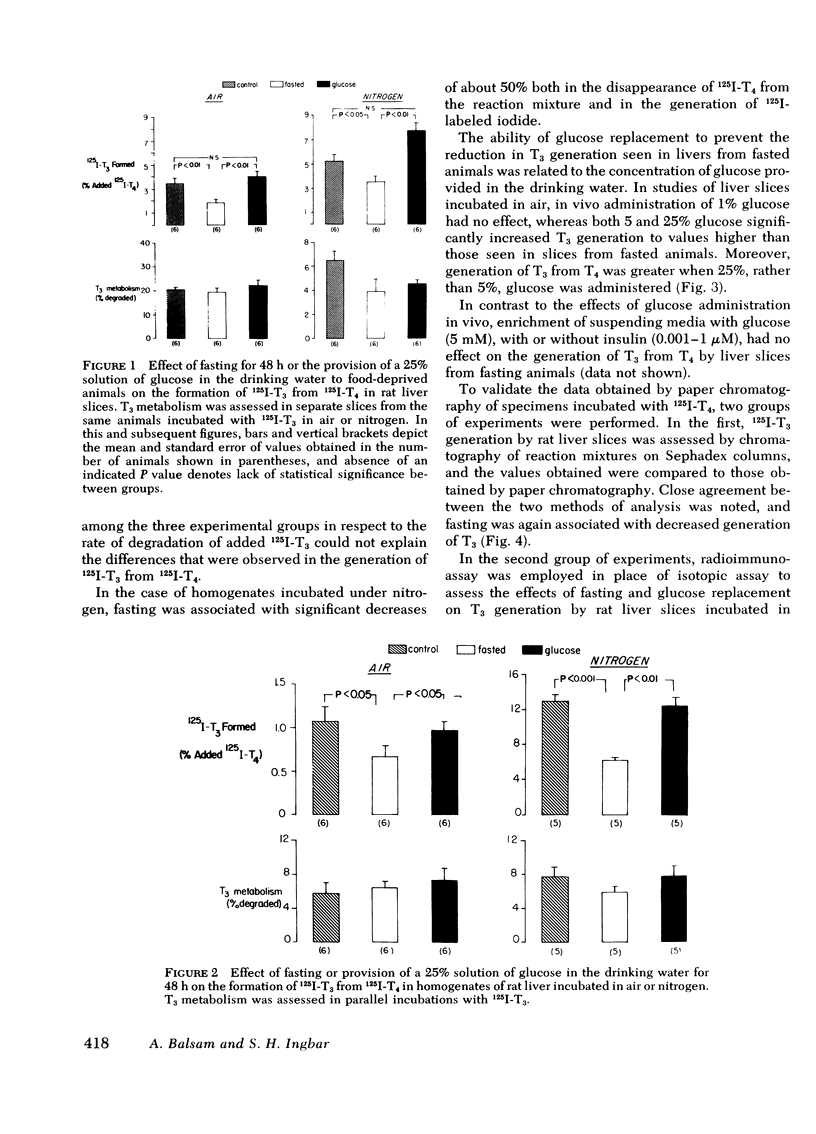
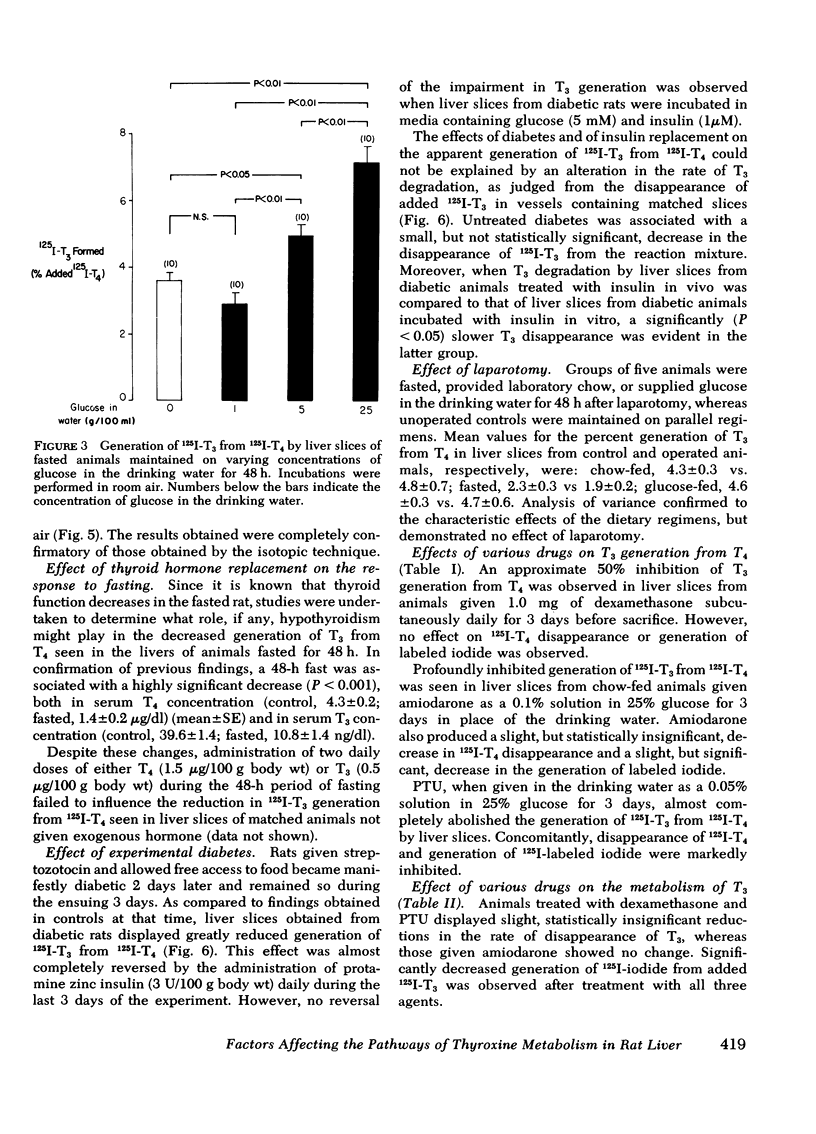
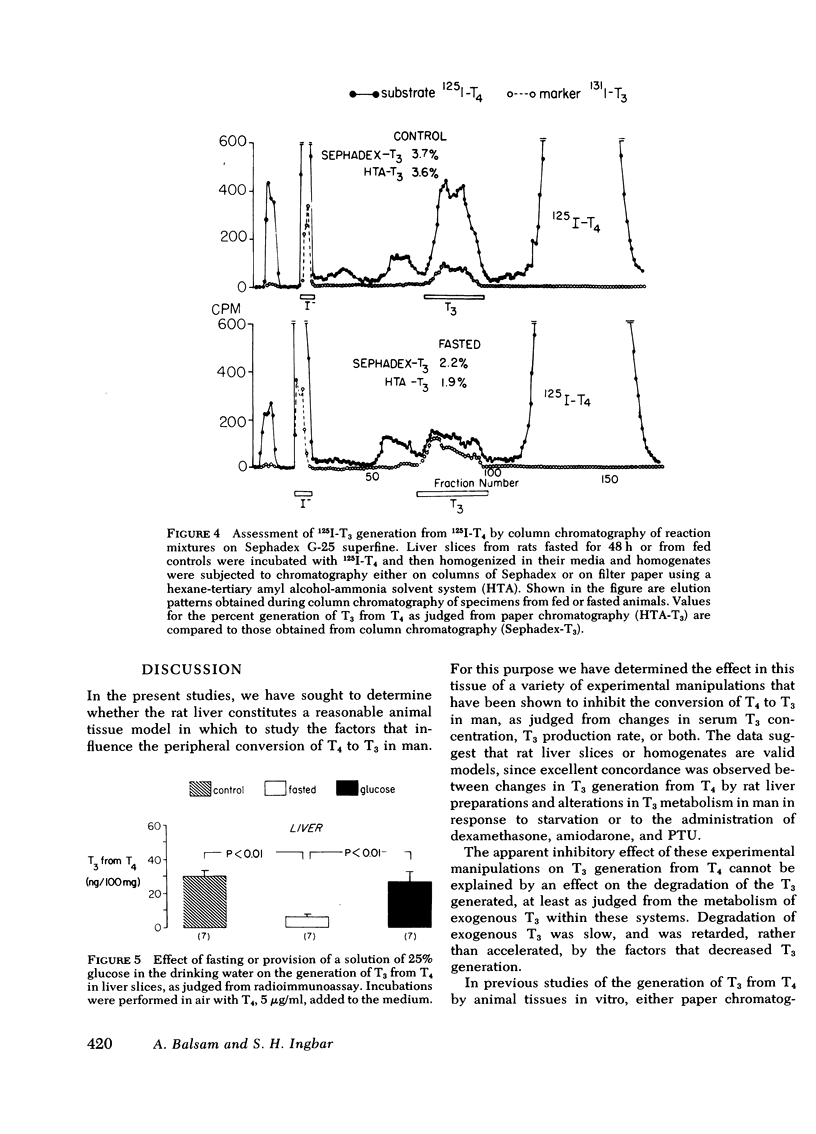
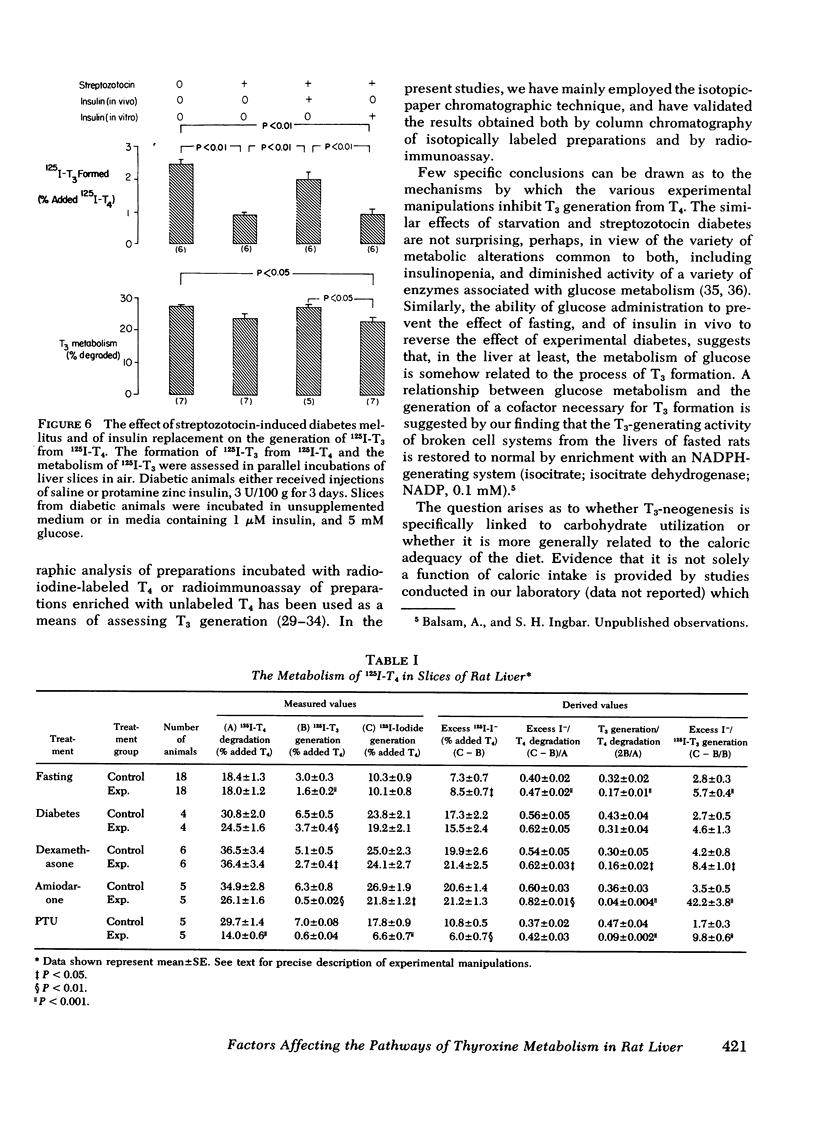
As judged from both paper and column chromatography, slices or homogenates of liver from rats fasted for 48 h displayed a lesser rate of generation of 125I-labeled 3,5,3′-triiodothyronine (T3) from 125I-labeled thyroxine (T4) added to incubation media than did preparations from normal chow-fed animals. A similar defect in the conversion of T4 to T3 in the livers of fasted animals was observed when preparations were incubated with substrate concentrations of T4 so that T3 generation could be assessed by radioimmunoassay. The effect of fasting could be prevented, wholly or in part, by administration of glucose in the drinking water to otherwise fasted animals, and the degree of prevention appeared to be proportional to the concentration of glucose employed. Diminished generation of T3 from T4 was similarly evident in the livers of animals with streptozotocin-induced diabetes mellitus, and this defect was overcome by the provision of insulin in vivo, but not in vitro. Decreased formation of T3 from T4 was also observed in preparations of liver from animals given dexamethasone, amiodarone, and propylthiouracil. In no case could these effects on the net formation of T3 from T4 be explained by effects of the experimental conditions on the degradation of the T3 generated, as judged from the rate of degradation of exogenous 125I-T3 measured in parallel incubates.
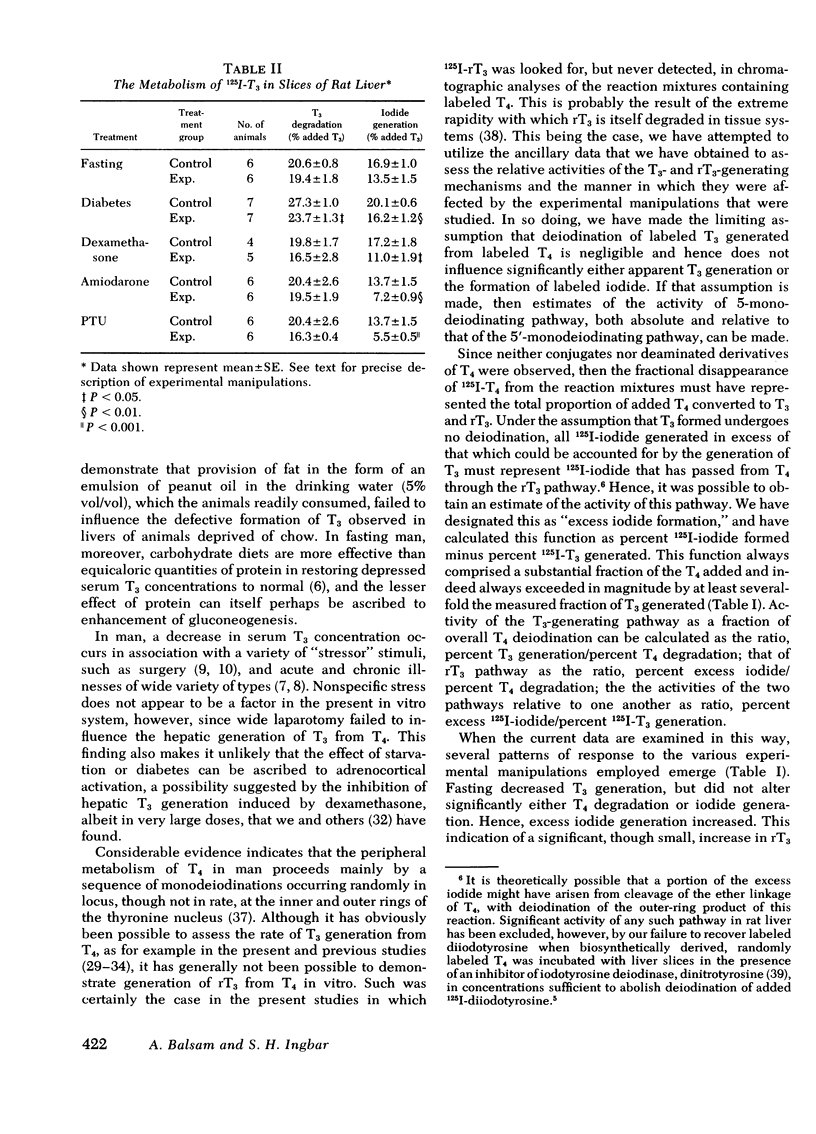
An analysis of the rate of disappearance of 125I-T4 from reaction mixtures in relation to the rate of appearance of 125I-T3 and 125I-iodide was employed to estimate the activity of the 5-monodeiodinating pathway of T4 metabolism that leads to the formation of 3,3′,5′-triiodothyronine (reverse T3). Such estimates indicated that reverse T3 formation was actively proceeding in the preparations studied, was slightly enhanced by fasting, was unaffected by dexamethasone and amiodarone, and was markedly inhibited by propylthiouracil.
In view of the similarities between the effect of these experimental manipulations on the generation of T3 from T4 by rat liver in vitro to their effects on the production rates and serum concentrations of T3 in man, it is concluded that the rat liver system provides a suitable model for the study of factors that influence the conversion of T4 to T3 in man. In addition, the findings strongly indicate that this process, at least in the liver, is closely linked to the utilization of carbohydrate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermudez F., Surks M. I., Oppenheimer J. H. High incidence of decreased serum triiodothyronine concentration in patients with nonthyroidal disease. J Clin Endocrinol Metab. 1975 Jul;41(1):27–40. doi: 10.1210/jcem-41-1-27. [DOI] [PubMed] [Google Scholar]
- Brandt M., Kahlet H., Hansen J. M., Skovsted L. Letter: Serum triiodothyronine and surgery. Lancet. 1976 Feb 28;1(7957):491–491. doi: 10.1016/s0140-6736(76)91521-x. [DOI] [PubMed] [Google Scholar]
- Burger A., Dinichert D., Nicod P., Jenny M., Lemarchand-Béraud T., Vallotton M. B. Effect of amiodarone on serum triiodothyronine, reverse triiodothyronine, thyroxin, and thyrotropin. A drug influencing peripheral metabolism of thyroid hormones. J Clin Invest. 1976 Aug;58(2):255–259. doi: 10.1172/JCI108466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr W. A., Black E. G., Griffiths R. S., Hoffenberg R. Serum triiodothyronine and reverse triiodothyronine concentrations after surgical operation. Lancet. 1975 Dec 27;2(7948):1277–1279. doi: 10.1016/s0140-6736(75)90612-1. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. A study of extrathyroidal conversion of thyroxine (T4) to 3,3',5-triiodothyronine (T3) in vitro. Endocrinology. 1977 Aug;101(2):453–463. doi: 10.1210/endo-101-2-453. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. An assessment of daily production and significance of thyroidal secretion of 3, 3', 5'-triiodothyronine (reverse T3) in man. J Clin Invest. 1976 Jul;58(1):32–40. doi: 10.1172/JCI108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. J., Chopra U., Smith S. R., Reza M., Solomon D. H. Reciprocal changes in serum concentrations of 3,3',5-triiodothyronine (T3) in systemic illnesses. J Clin Endocrinol Metab. 1975 Dec;41(06):1043–1049. doi: 10.1210/jcem-41-6-1043. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Smith S. R. Circulating thyroid hormones and thyrotropin in adult patients with protein-calorie malnutrition. J Clin Endocrinol Metab. 1975 Feb;40(2):221–227. doi: 10.1210/jcem-40-2-221. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Solomon D. H., Chopra U., Young R. T., Chua Teco G. N. Alterations in circulating thyroid hormones and thyrotropin in hepatic cirrhosis: evidence for euthyroidism despite subnormal serum triiodothyronine. J Clin Endocrinol Metab. 1974 Sep;39(3):501–511. doi: 10.1210/jcem-39-3-501. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Williams D. E., Orgiazzi J., Solomon D. H. Opposite effects of dexamethasone on serum concentrations of 3,3',5'-triiodothyronine (reverse T3) and 3,3'5-triiodothyronine (T3). J Clin Endocrinol Metab. 1975 Nov;41(5):911–920. doi: 10.1210/jcem-41-5-911. [DOI] [PubMed] [Google Scholar]
- Croxson M. S., Ibbertson H. K. Low serum triiodothyronine (T3) and hypothyroidism in anorexia nervosa. J Clin Endocrinol Metab. 1977 Jan;44(1):167–174. doi: 10.1210/jcem-44-1-167. [DOI] [PubMed] [Google Scholar]
- Degroot L. J., Hoye K. Dexamethasone suppression of serum T3 and T4. J Clin Endocrinol Metab. 1976 May;42(5):976–978. doi: 10.1210/jcem-42-5-976. [DOI] [PubMed] [Google Scholar]
- Duick D. S., Warren D. W., Nicoloff J. T., Otis C. L., Croxson M. S. Effect of single dose dexamethasone on the concentration of serum triiodothyronine in man. J Clin Endocrinol Metab. 1974 Dec;39(6):1151–1154. doi: 10.1210/jcem-39-6-1151. [DOI] [PubMed] [Google Scholar]
- Geffner D. L., Azukizawa M., Hershman J. M. Propylthiouracil blocks extrathyroidal conversion of thyroxine to triiodothyronine and augments thyrotropin secretion in man. J Clin Invest. 1975 Feb;55(2):224–229. doi: 10.1172/JCI107925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green W. L. Inhibition of thyroidal iodotyrosine deiodination by tyrosine analogues. Endocrinology. 1968 Aug;83(2):336–347. doi: 10.1210/endo-83-2-336. [DOI] [PubMed] [Google Scholar]
- Green W. L. Separation of iodo compounds in serum by chromatography on Sephadex columns. J Chromatogr. 1972 Oct 5;72(1):83–91. doi: 10.1016/0021-9673(72)80010-4. [DOI] [PubMed] [Google Scholar]
- Hüfner M., Knöpfle M. Pharmacological influences on T4 to T3 conversion in rat liver. Clin Chim Acta. 1976 Nov 1;72(3):337–341. doi: 10.1016/0009-8981(76)90196-0. [DOI] [PubMed] [Google Scholar]
- Ingbar S. H., Braverman L. E. Active form of the thyroid hormone. Annu Rev Med. 1975;26:443–449. doi: 10.1146/annurev.me.26.020175.002303. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Schimmel M., Utiger R. D. Changes in serum 3,3',5'-triiodothyronine (reverse T3) concentrations with altered thyroid hormone secretion and metabolism. J Clin Endocrinol Metab. 1977 Sep;45(3):447–456. doi: 10.1210/jcem-45-3-447. [DOI] [PubMed] [Google Scholar]
- Miyai K., Yamamoto T., Azukizawa M., Ishibashi K., Kumahara Y. Serum thyroid hormones and thyrotropin in anorexia nervosa. J Clin Endocrinol Metab. 1975 Feb;40(2):334–338. doi: 10.1210/jcem-40-2-334. [DOI] [PubMed] [Google Scholar]
- Moshang T., Jr, Parks J. S., Baker L., Vaidya V., Utiger R. D., Bongiovanni A. M., Snyder P. J. Low serum triiodothyronine in patients with anorexia nervosa. J Clin Endocrinol Metab. 1975 Mar;40(3):470–473. doi: 10.1210/jcem-40-3-470. [DOI] [PubMed] [Google Scholar]
- Nomura S., Pittman C. S., Chambers J. B., Jr, Buck M. W., Shimizu T. Reduced peripheral conversion of thyroxine to triiodothyronine in patients with hepatic cirrhosis. J Clin Invest. 1975 Sep;56(3):643–652. doi: 10.1172/JCI108134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Propylthiouracil inhibits the conversion of L-thyroxine to L-triiodothyronine. An explanation of the antithyroxine effect of propylthiouracil and evidence supporting the concept that triiodothyronine is the active thyroid hormone. J Clin Invest. 1972 Sep;51(9):2493–2497. doi: 10.1172/JCI107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnay G. I., O'Brian J. T., Bush J., Vagenakis A. G., Azizi F., Arky R. A., Ingbar S. H., Braverman L. E. The effect of starvation on the concentration and binding of thyroxine and triiodothyronine in serum and on the response to TRH. J Clin Endocrinol Metab. 1974 Jul;39(1):191–194. doi: 10.1210/jcem-39-1-191. [DOI] [PubMed] [Google Scholar]
- Rudolph M., Sakurada T., Fang S. L., Vagenakis A. G., Braverman L. E., Ingbar S. H. Appearance of labeled metabolites in the serum of man after the administration of labeled thyroxine, triiodothyronine (T3), and reverse triiodothyronine (rT3). J Clin Endocrinol Metab. 1978 Jun;46(6):923–928. doi: 10.1210/jcem-46-6-923. [DOI] [PubMed] [Google Scholar]
- Saberi M., Sterling F. H., Utiger R. D. Reduction in extrathyroidal triiodothyronine production by propylthiouracil in man. J Clin Invest. 1975 Feb;55(2):218–223. doi: 10.1172/JCI107924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurada T., Rudolph M., Fang S. L., Vagenakis A. G., Braverman L. E., Ingbar S. H. Evidence that triiodothyronine and reverse triiodothyronine are sequentially deiodinated in man. J Clin Endocrinol Metab. 1978 Jun;46(6):916–922. doi: 10.1210/jcem-46-6-916. [DOI] [PubMed] [Google Scholar]
- Spaulding S. W., Chopra I. J., Sherwin R. S., Lyall S. S. Effect of caloric restriction and dietary composition of serum T3 and reverse T3 in man. J Clin Endocrinol Metab. 1976 Jan;42(1):197–200. doi: 10.1210/jcem-42-1-197. [DOI] [PubMed] [Google Scholar]
- Utiger R. D. Serum triiodothyronine in man. Annu Rev Med. 1974;25:289–302. doi: 10.1146/annurev.me.25.020174.001445. [DOI] [PubMed] [Google Scholar]
- Vagenakis A. G., Burger A., Portnary G. I., Rudolph M., O'Brian J. R., Azizi F., Arky R. A., Nicod P., Ingbar S. H., Braverman L. E. Diversion of peripheral thyroxine metabolism from activating to inactivating pathways during complete fasting. J Clin Endocrinol Metab. 1975 Jul;41(1):191–194. doi: 10.1210/jcem-41-1-191. [DOI] [PubMed] [Google Scholar]
- Wiersinga W. M., Touber J. L. The influence of beta-adrenoceptor blocking agents on plasma thyroxine and triiodothyronine. J Clin Endocrinol Metab. 1977 Aug;45(2):293–298. doi: 10.1210/jcem-45-2-293. [DOI] [PubMed] [Google Scholar]



