Abstract
It has been hypothesized that a greater decline in circulating branched-chain amino acids (BCAAs) after weight loss induced by Roux-en-Y gastric bypass (RYGB) surgery than after calorie restriction alone has independent effects on glucose homeostasis, possibly by decreased signaling through the mammalian target of rapamycin (mTOR). We evaluated plasma BCAAs and their C3 and C5 acylcarnitine metabolites, muscle mTOR phosphorylation, and insulin sensitivity (insulin-stimulated glucose Rd) in obese subjects before and after ∼20% weight loss induced by RYGB (n = 10, BMI 45.6 ± 6.7 kg/m2) or laparoscopic adjustable gastric banding (LAGB) (n = 10, BMI 46.5 ± 8.8 kg/m2). Weight loss increased insulin-stimulated glucose Rd by ∼55%, decreased total plasma BCAA and C3 and C5 acylcarnitine concentrations by 20–35%, and did not alter mTOR phosphorylation; no differences were detected between surgical groups (all P values for interaction >0.05). Insulin-stimulated glucose Rd correlated negatively with plasma BCAAs and with C3 and C5 acylcarnitine concentrations (r values −0.56 to −0.75, P < 0.05). These data demonstrate that weight loss induced by either LAGB or RYGB causes the same decline in circulating BCAAs and their C3 and C5 acylcarnitine metabolites. Plasma BCAA concentration is negatively associated with skeletal muscle insulin sensitivity, but the mechanism(s) responsible for this relationship is not known.
Insulin resistance is a common metabolic complication of obesity (1,2) and an important risk factor for developing type 2 diabetes and the metabolic syndrome (3). Several mechanisms are purported to be responsible for obesity-induced insulin resistance, including abnormalities in fatty acid metabolism (4), systemic and adipose tissue inflammation (5), alterations in intracellular lipid metabolites (6), mitochondrial dysfunction (7), and endoplasmic reticulum stress (8). Recently, it has been proposed that an increase in circulating branched-chain amino acids (BCAAs), valine, leucine, and isoleucine, and their acylcarnitine metabolites, C3 and C5, is also involved in the pathogenesis of insulin resistance (9–11). Moreover, the plasma concentrations of these metabolites are prognostic for the development of type 2 diabetes (12) and for the improvement in insulin sensitivity in response to therapeutic interventions (13). The potential mechanism(s) responsible for the relationship between BCAA metabolism and insulin action is not clear. Studies conducted in rodent models and cultured rat myocytes found that increased exposure of skeletal muscle cells to BCAA impairs insulin action in concert with activation of the mammalian target of rapamycin (mTOR), which causes serine phosphorylation of insulin receptor substrate-1 and disrupts insulin signaling (9,14–16). In addition, amino acid infusion in human subjects activates the mTOR pathway and impairs skeletal muscle insulin action (17–19), whereas treatment with rapamycin, an mTOR inhibitor, increases skeletal muscle insulin sensitivity (17). Weight loss induced by bariatric surgery improves insulin sensitivity (20). However, the importance of BCAA metabolism in mediating the weight loss–induced improvement in insulin sensitivity after bariatric surgery is not clear. Data obtained from two centers found that both ∼10 and ∼22% weight loss induced by Roux-en-Y gastric bypass (RYGB) surgery caused a greater decrease in BCAA concentrations than matched weight loss induced by calorie restriction alone, which led to the hypothesis that alterations in BCAA metabolism contribute to a weight loss–independent improvement in insulin sensitivity after altering gastrointestinal tract anatomy by RYGB (21).
The purpose of the current study was to evaluate the hypothesis that weight loss induced by RYGB surgery causes greater alterations in BCAA metabolism and a greater reduction in skeletal muscle mTOR activation than matched weight loss induced by laparoscopic adjustable gastric banding (LAGB). Accordingly, plasma BCAA and C3 and C5 acylcarnitine concentrations, skeletal muscle mTOR phosphorylation, and skeletal muscle insulin sensitivity were assessed in obese subjects before and after ∼20% weight loss induced by RYGB or LAGB.
RESEARCH DESIGN AND METHODS
Twenty obese men and women scheduled to undergo either RYGB or LAGB procedures at Barnes-Jewish Hospital in St. Louis, Missouri, participated in this study. No subject had a history or evidence of serious disease, used tobacco products, took medications that could affect metabolism, or had diabetes. Blood and muscle tissue samples analyzed for the current study were obtained from the subjects during their participation in another study that evaluated insulin sensitivity by using the hyperinsulinemic-euglycemic clamp procedure (22). Body composition and metabolic characteristics of the study subjects before and after weight loss are shown in Supplementary Table 1 (22). Subjects gave their written informed consent before participating in this study, which was approved by the Human Research Protection Office of Washington University School of Medicine in St. Louis.
Experimental procedures.
Subjects were admitted to the Washington University School of Medicine Clinical Research Unit and consumed a standardized evening meal. The next morning, after subjects fasted for 12 h overnight, a catheter was inserted into a forearm vein for infusion and a second catheter was inserted into a radial artery for blood sampling. A primed-continuous infusion of [6,6-2H2]glucose was started and maintained for 7.5 h. After 3.5 h, insulin was infused at a rate of 50 mU/m2 body surface area ⋅ min for 4 h. During insulin infusion, euglycemia (plasma glucose concentration ∼100 mg/dL) was maintained by infusing 20% dextrose enriched to 2.5% with [6,6-2H2]glucose. Blood samples were obtained immediately before starting the tracer infusion and during the final 30 min of the basal period and the insulin clamp to determine plasma BCAA and C3 and C5 acylcarnitine concentrations and glucose kinetics. Skeletal muscle tissue samples from the vastus lateralis were obtained by percutaneous biopsy during basal conditions and during insulin infusion from most subjects (7 RYGB and 9 LAGB) before and after weight loss to determine mTOR phosphorylation. Tissue samples were immediately rinsed with ice-cold saline and frozen in liquid nitrogen and then stored at −80°C.
Analyses of blood samples.
Plasma glucose concentration was determined on an automated glucose analyzer (YSI 2300 STAT plus; Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin concentration was measured by ELISA (Millipore, Billerica, MA). Plasma amino acid and acylcarnitine concentrations were determined by tandem mass spectrometry (9). Plasma glucose tracer-to-tracee ratios were determined by using gas chromatography–mass spectrometry (23). Insulin-stimulated glucose Rd from plasma during insulin infusion was used as an index of insulin sensitivity.
Analysis of skeletal muscle samples.
Phosphorylated and total mTOR protein was measured by immunoblotting. Homogenized muscle lysates were subjected to SDS-PAGE and transferred to polyvinylidene fluoride membranes. Blots were then rinsed with Tris-buffered saline plus Tween (TBST) (0.14 mol/L NaCl; 0.02 mol/L Tris base, pH 7.6; and 0.1% Tween), blocked with 5% BSA in TBST for 1 h at room temperature, washed 3 × 10 min at room temperature, and incubated with primary antibodies for phosphorylated Ser2448 mTOR or total mTOR (cat no. 2972 and 2971, respectively; Cell Signaling Technology, Danvers, MA) (1:1,000 in 5% BSA overnight at 4°C). Blots were then washed 3 × 5 min with TBST, incubated with goat anti-rabbit secondary antibody with IgG horseradish peroxidase conjugate (cat no. NA934V; GE Healthcare, Buckinghamshire, U.K.) for 1 h at room temperature, washed again 3 × 10 min with TBST, washed 2 × 10 min with Tris-buffered saline, and developed with SuperSignal reagent. Protein bands were quantified by digital densitometry (LiCor Biosciences, Lincoln, NE), and results were expressed as the ratio of phosphorylated to total mTOR. The mean value before weight loss was normalized to 1, and values after weight loss were expressed relative to the normalized value.
Statistical analyses.
The effect of RYGB and LAGB surgery on metabolic outcomes was evaluated by two-way repeated-measures ANOVA, with time (before versus after weight loss) as within-subjects factor and surgical group (RYGB versus LAGB) as between-subjects factor. Results are presented as mean ± SD unless otherwise noted. A P value <0.05 was considered statistically significant.
RESULTS
Characteristics of the study subjects.
There were no significant differences in the effect of weight loss on percent body fat, intrahepatic triglyceride content, and fasting plasma glucose and insulin concentrations between the LAGB and RYGB groups (Supplementary Table 1). Insulin-stimulated glucose Rd increased from 38 ± 12 to 61 ± 9 and from 37 ± 15 to 57 ± 19 μmol/kg fat-free mass/min after ∼20% weight loss in the LAGB and RYGB groups, respectively, without any differences between the two surgical groups (P for interaction = 0.465).
Plasma BCAA and C3 and C5 acylcarnitine concentrations.
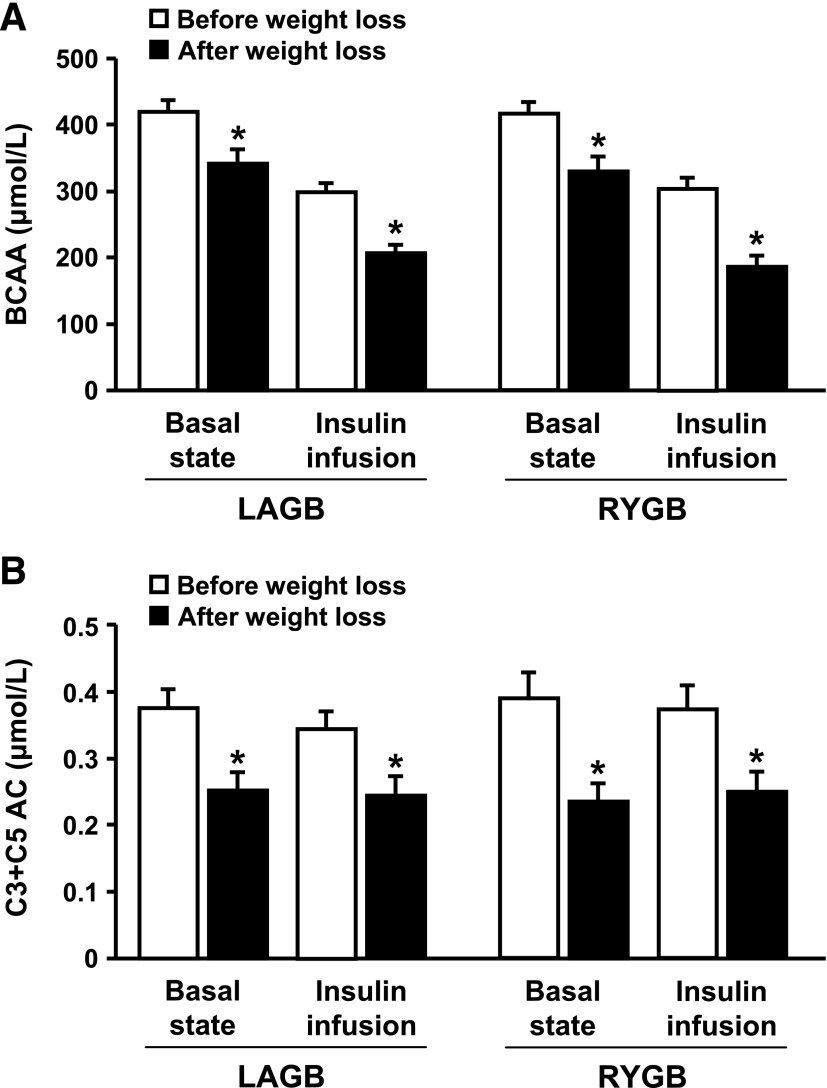
The molar sum of total BCAAs (valine and leucine/isoleucine) measured in fasting plasma decreased by ∼20% after weight loss in both LAGB and RYGB groups (P < 0.001), but the decrease was not different between groups (P for interaction = 0.732) (Fig. 1A). Total BCAA concentration decreased during insulin infusion and was ∼35% lower after than before weight loss in both groups. The concentrations of individual BCAAs and other plasma amino acids during basal conditions and insulin infusion, before and after weight loss, are shown in Supplementary Table 2.
FIG. 1.
Total plasma BCAA (A) and C3 and C5 acylcarnitine (AC) (B) concentrations in the basal state and during insulin infusion in obese subjects before and after 20% weight loss induced by LAGB or RYGB. Values are means ± SEM (n = 10 for each surgery group). *Value after weight loss is significantly different from value before weight loss, P < 0.05.
Plasma C3 acylcarnitine (propionyl carnitine) is derived from the catabolism of isoleucine and valine, whereas C5 acylcarnitines (α-methylbutyryl and isovaleryl carnitines) are derived from metabolism of isoleucine and leucine. Plasma C3 and C5 acylcarnitine concentrations decreased by ∼35% after weight loss in both LAGB and RYGB groups (all P values <0.001), during both basal conditions and insulin infusion, but the decrease was not different between groups (all P values for interaction >0.35) (Fig. 1B). Insulin infusion did not affect plasma C3 and C5 acylcarnitine concentrations before or after weight loss. The concentrations of other acylcarnitines during basal conditions and insulin infusion, before and after weight loss, are shown in Supplementary Table 3.
Skeletal muscle mTOR phosphorylation.
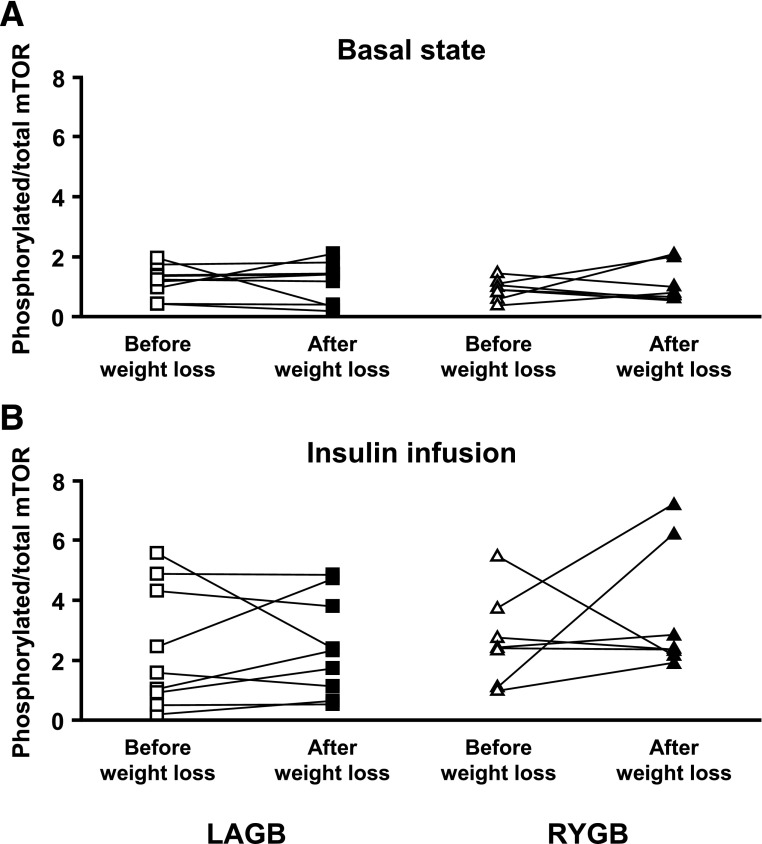
No significant change in basal skeletal muscle mTOR phosphorylation (ratio of phosphorylated to total mTOR) was detected after subjects lost ∼20% of their body weight induced by either LAGB or RYGB surgery, and there was no difference between groups (P for interaction = 0.424) (Fig. 2A). As expected (24), insulin infusion increased skeletal muscle mTOR phosphorylation (P < 0.001), but there was no effect of weight loss and no differences between surgical groups in insulin-stimulated mTOR phosphorylation (P for interaction = 0.306) (Fig. 2B).
FIG. 2.
Skeletal muscle mTOR phosphorylation (ratio of phosphorylated to total mTOR) in the basal state (A) and during insulin infusion (B) before and after 20% weight loss induced by LAGB or RYGB. Individual data are shown (n = 9 for LAGB and 7 for RYGB).
Relationships among BCAA, acylcarnitines, and insulin sensitivity.
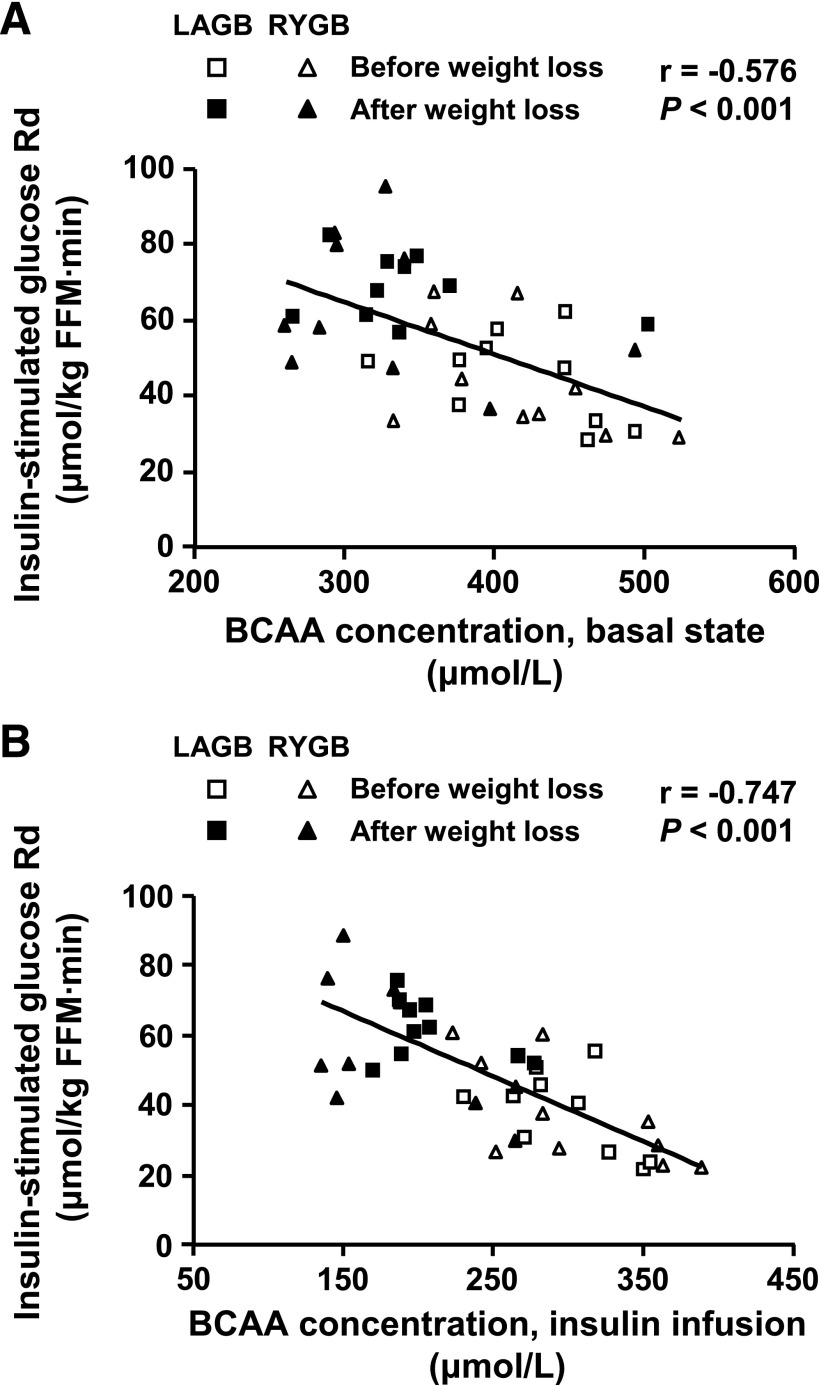
Insulin-stimulated glucose Rd correlated negatively with total plasma BCAA concentration in the basal state (Fig. 3A) and during insulin infusion (Fig. 3B). Plasma C3 and C5 acylcarnitine concentrations were also negatively correlated with insulin sensitivity in the basal state (r = −0.556, P < 0.001) and during insulin infusion (r = −0.622, P < 0.001).
FIG. 3.
Relationship between skeletal muscle insulin sensitivity, assessed as the insulin-stimulated glucose Rd from plasma (μmol glucose/kg fat-free mass [FFM]/minute) during a hyperinsulinemic-euglycemic clamp procedure and plasma total BCAA concentration in the basal state (A) and during insulin infusion (B) in obese subjects before and after bariatric surgery–induced weight loss.
DISCUSSION
It has been hypothesized that a greater decline in circulating BCAAs after weight loss induced by RYGB than after equivalent weight loss induced by calorie restriction alone has independent effects on insulin sensitivity and glucose homeostasis (21). In this study, we found that marked weight loss (∼20% of initial body weight) induced by RYGB or LAGB surgery caused the same improvement in skeletal muscle insulin sensitivity and the same decrease in plasma BCAA and C3 and C5 acylcarnitine concentrations. These data do not support the notion that RYGB has weight loss–independent effects on BCAA metabolism after marked weight loss is achieved. The reasons for the same decline in circulating BCAAs and C3 and C5 acylcarnitines in response to our two surgical interventions, as opposed to the preferential decrease in these same metabolites in response to RYGB relative to dietary therapy alone in a previous study (21), are not clear. It is possible that differences in the time required to achieve the same weight loss or differences in diet composition between the RYGB and diet therapy groups influenced previous findings. In addition, we cannot exclude the possibility that weight loss induced by mechanical restriction or diet therapy alone has different effects on BCAA metabolism.
Our data also demonstrate that plasma total BCAA concentration is negatively correlated with skeletal muscle insulin sensitivity. Our study is unable to determine whether this correlation is a simple association or represents a true cause-and-effect relationship, related to an effect of BCAA on insulin action or an effect of insulin action on BCAA metabolism. Data from studies conducted in animal models (9,14–16) and human subjects (17–19) have shown that administration of amino acids, particularly BCAAs, induces skeletal muscle insulin resistance accompanied by increased signaling through the mTOR pathway. However, we found that weight loss decreased plasma BCAA concentrations and improved insulin sensitivity without altering skeletal muscle mTOR phosphorylation during either basal conditions or insulin infusion. Alterations in the activity of the mTOR pathway are therefore not likely responsible for the weight loss–induced improvement in skeletal muscle insulin sensitivity in people. Our results cannot exclude the possibility that decreased plasma BCAAs improve insulin sensitivity through other mechanisms, such as relief of “metabolic overload” of mitochondria in insulin-sensing tissues (11). This possibility is consistent with our finding of reduced C3 and C5 acylcarnitines (by-products of BCAA catabolism) in response to weight loss. It is also possible that weight loss–induced improvement in insulin sensitivity decreases plasma BCAA concentrations by reducing skeletal muscle protein breakdown and the release of BCAA into the circulation (25). This notion is supported by our observation that insulin infusion caused a marked decrease in total plasma BCAA concentrations. Moreover, the relative insulin-induced decrease in BCAA concentrations was greater after weight loss, when subjects were more insulin sensitive, than before weight loss.
In summary, we found that marked weight loss induced by RYGB or LAGB caused the same reduction in circulating BCAA and C3 and C5 acylcarnitine concentrations and the same increase in skeletal muscle insulin sensitivity. Additional studies are needed to determine the mechanism(s) responsible for the weight loss–induced decline in circulating BCAAs, and whether the change in BCAA metabolism contributes to the improvement in insulin sensitivity.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grants DK 37948 and DK 56341 (Nutrition Obesity Research Center), UL1 RR024992 (Clinical and Translational Science Award), RR-00954 (Biomedical Mass Spectrometry Resource), and PO1 DK58398.
S.K. is a shareholder of Aspire Bariatrics. No other potential conflicts of interest relevant to this article were reported.
F.M. collated and analyzed data and wrote the manuscript. D.B. performed the metabolic studies, collated and analyzed data, and reviewed the manuscript. G.G.S. and B.N.F. performed the mTOR analysis and reviewed and edited the manuscript. J.C.E. performed the surgical interventions and reviewed and edited the manuscript. O.I. performed the amino acid and acylcarnitine analyses. C.B.N. contributed to the design and discussion of the study and reviewed and edited the manuscript. S.K. designed the study, obtained funding, reviewed the data, contributed to the discussion, and reviewed and edited the manuscript. S.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the following Washington University School of Medicine staff: Courtney Tiemann for help with subject recruitment and dietary counseling; Freida Custodio, Jennifer Shew, and Ioana Gruchevska for technical assistance; Janine Kampelman for performing skeletal muscle biopsies; and the staff of the Clinical Research Unit for help in performing the studies. The authors also thank the study subjects for their participation.
Footnotes
Clinical trial reg. no. NCT00981500, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0185/-/DC1.
REFERENCES
- 1.Kolterman OG, Insel J, Saekow M, Olefsky JM. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest 1980;65:1272–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olefsky JM. Insulin resistance in humans. Gastroenterology 1982;83:1313–1318 [PubMed] [Google Scholar]
- 3.Lebovitz HE. Insulin resistance—a common link between type 2 diabetes and cardiovascular disease. Diabetes Obes Metab 2006;8:237–249 [DOI] [PubMed] [Google Scholar]
- 4.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 2012;148:852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 2000;106:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002;51:2944–2950 [DOI] [PubMed] [Google Scholar]
- 8.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004;306:457–461 [DOI] [PubMed] [Google Scholar]
- 9.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adeva MM, Calviño J, Souto G, Donapetry C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids 2012;43:171–181 [DOI] [PubMed] [Google Scholar]
- 11.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 2012;15:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah SH, Crosslin DR, Haynes CS, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012;55:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saha AK, Xu XJ, Balon TW, Brandon A, Kraegen EW, Ruderman NB. Insulin resistance due to nutrient excess: is it a consequence of AMPK downregulation? Cell Cycle 2011;10:3447–3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saha AK, Xu XJ, Lawson E, et al. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes 2010;59:2426–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem 2001;276:38052–38060 [DOI] [PubMed] [Google Scholar]
- 17.Krebs M, Brunmair B, Brehm A, et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes 2007;56:1600–1607 [DOI] [PubMed] [Google Scholar]
- 18.Krebs M, Krssak M, Bernroider E, et al. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 2002;51:599–605 [DOI] [PubMed] [Google Scholar]
- 19.Tremblay F, Krebs M, Dombrowski L, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 2005;54:2674–2684 [DOI] [PubMed] [Google Scholar]
- 20.Bradley D, Magkos F, Klein S. Effects of bariatric surgery on glucose homeostasis and type 2 diabetes. Gastroenterology 2012;143:897–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laferrère B, Reilly D, Arias S, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med 2011;3:re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley D, Conte C, Mittendorfer B, et al. Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest 2012;122:4667–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magkos F, Fabbrini E, Korenblat K, Okunade AL, Patterson BW, Klein S. Reproducibility of glucose, fatty acid and VLDL kinetics and multi-organ insulin sensitivity in obese subjects with non-alcoholic fatty liver disease. Int J Obes (Lond) 2011;35:1233–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott PH, Brunn GJ, Kohn AD, Roth RA, Lawrence JC., Jr Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci USA 1998;95:7772–7777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forlani G, Vannini P, Marchesini G, Zoli M, Ciavarella A, Pisi E. Insulin-dependent metabolism of branched-chain amino acids in obesity. Metabolism 1984;33:147–150 [DOI] [PubMed] [Google Scholar]