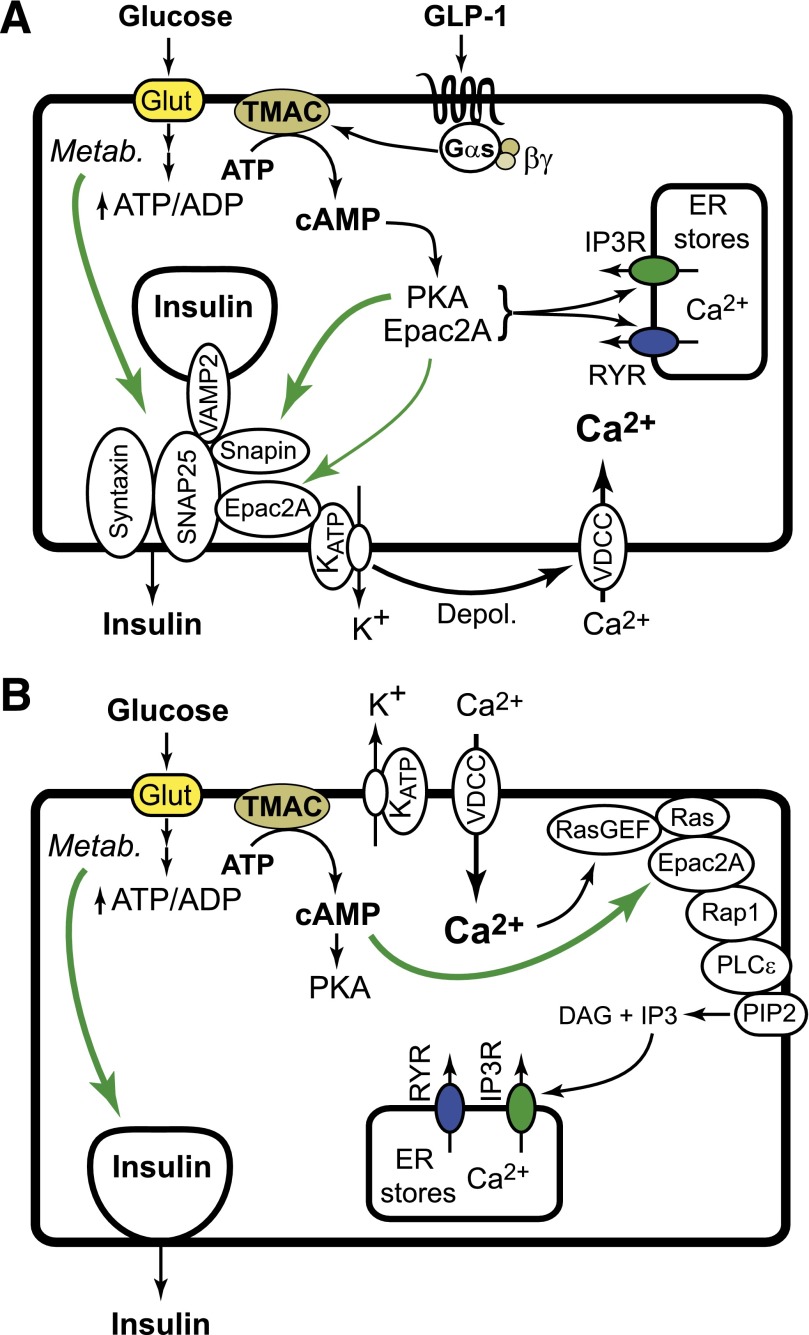
The high-fat diet (HFD)-fed mouse model of obesity-related type 2 diabetes mellitus (T2DM) continues to provide new insights concerning the molecular basis for pancreatic β-cell compensation under conditions of diet-induced insulin resistance. In the new study by Song et al. (1), β-cell compensation to preserve glucose-stimulated insulin secretion (GSIS) under conditions of the HFD is shown to be lost in mice in which there is a knockout (KO) of Rapgef4, the gene coding for a cAMP-regulated guanine nucleotide exchange factor designated as Epac2A. These findings obtained with mice fed the HFD suggest that Epac2A activators might be of use for the treatment of T2DM, or that aberrant Epac2A signaling in the β-cell might predispose to T2DM.
Protein kinase A (PKA)-independent signaling properties of cAMP are mediated by Epac2A in β-cells, and Epac2A acts as a cofactor with PKA in order to mediate the potentiation of GSIS by cAMP-elevating hormone glucagon-like peptide 1 (GLP-1) (2–5). Since GLP-1 is the prototype of a new class of insulin secretagogues for use in the treatment of T2DM (6), speculation exists concerning what additional roles Epac2A might play in β-cell biology. Song et al. (1) now report that when mice are fed an HFD (7), there exists β-cell compensation in which Epac2A enables GSIS to occur in the absence of administered GLP-1. Thus, Epac2A expression in islets is of importance to the cAMP-dependent potentiation of GSIS by GLP-1 (Fig. 1A), while also being of importance to the maintenance of GSIS under conditions of an HFD (Fig. 1B). These new findings concerning Epac2A extend on the prior study of Song et al. (8) in which it was demonstrated that cAMP-dependent PKA–mediated phosphorylation of soluble N-ethylmaleimide–sensitive attachment protein receptor (SNARE) complex-associated protein Snapin leads to a potentiation of GSIS from islets of mice fed a normal diet.
FIG. 1.
A: The normal diet. Under the conditions of a normal diet, the KO of Epac2A does not disrupt GSIS. However, the action of GLP-1 to potentiate GSIS and to increase [Ca2+]i is reduced in islets of Epac2A KO mice. Epac2A mediates the action of GLP-1 to facilitate glucose-dependent closure of KATP channels, thereby stimulating Ca2+ influx while also mobilizing Ca2+. These actions of GLP-1 may explain how it restores first-phase GSIS in T2DM. B: The high-fat diet. Under conditions of the HFD, β-cell compensation occurs so that Epac2A enables GSIS in the absence of GLP-1. Thus, a KO of Epac2A may uncouple glucose metabolism from cAMP production, Epac2A activation, and Rap1/PLCε activation. Depol., depolarizaton; ER, endoplasmic reticulum; Glut, glucose transporter; IP3R, inositol trisphosphate; RasGEF, Ras guanine nucleotide exchange factor; RYR, ryanodine receptors; Metab., metabolism; TMAC, transmembrane adenylyl cyclase; VDCC, voltage-dependent Ca2+ channel.
In the new study by Song et al. (1), a KO of Epac2A disrupts the action of GLP-1 receptor agonist exendin-4 (Ex-4) to potentiate a glucose-stimulated increase of [Ca2+]i in islets of mice fed a normal diet (1). Furthermore, glucose alone has a reduced ability to stimulate an increase of [Ca2+]i in islets of Epac2A KO mice fed the HFD. These defects of Ca2+ handling correlate with a reduction of first-phase GSIS from islets of Epac2A KO mice (1). Thus, for the normal diet, Epac2A activation by Ex-4 reinforces the action of glucose to generate a Ca2+ signal that triggers first-phase GSIS (Fig. 1A). However, under conditions of the HFD, Epac2A is instead activated in response to glucose alone, thereby generating a Ca2+ signal that triggers first-phase GSIS (Fig. 1B). These findings are noteworthy in view of a recent report that glucose has little ability to increase levels of [Ca2+]i in human islets of T2DM donors (9). Evidently, reduced coupling of glucose metabolism to ATP-sensitive K+ channel (KATP) closure may occur in T2DM, thereby diminishing Ca2+ influx that triggers first-phase GSIS. Since Ex-4 restores first-phase GSIS in patients with T2DM (10), this action of Ex-4 might be mediated, at least in part, by Epac2A.
Interplay of PKA and Epac2A is also indicated by the new findings of Song et al. (1). For mice in which upregulated PKA activity exists because of a KO of PKA regulatory subunit 1α (Δprkar1a), GSIS is enhanced under conditions of a normal diet or an HFD. When Δprkar1a mice are crossed with Epac2A KO mice, the resultant Δprkar1a/EPAC2A KO mice exhibit reduced GSIS under normal and high-fat dietary conditions. Thus, Epac2A expression is permissive for PKA-stimulated GSIS in these double KO mice (1). Similarly, Δprkar1a mouse islets show an exaggerated increase of [Ca2+]i in response to glucose, and this is reduced in Δprkar1a/EPAC2A KO mice (1). Collectively, these data indicate that interplay of PKA and Epac2A is important to cAMP-dependent stimulation of Ca2+ influx and/or mobilization in β-cells. Interestingly, Song et al. also demonstrate that Epac2A mediates the action of cAMP to promote assembly of SNARE proteins VAMP and SNAP-25 (1). Since these SNARE proteins mediate Ca2+-dependent fusion of secretory granules with the plasma membrane, Epac2A also controls insulin exocytosis directly.
How does one explain how Epac2A enables GSIS under conditions of the HFD? An explanation is provided by one new study demonstrating that glucose metabolism is coupled to cAMP production with consequent Epac2A-mediated activation of Rap1 GTPase in order to stimulate insulin secretion (11). Since Rap1 mediates cAMP-dependent potentiation of “restless newcomer” exocytosis in order to potentiate first-phase GSIS in mouse β-cells (12), glucose-dependent activation of Epac2A and Rap1 might serve to maintain GSIS in mice fed the HFD (Fig. 1B).
Plasticity in the β-cell cAMP signaling network is a consequence of the compensatory process in which the relative contributions of PKA and Epac2A to GSIS are dictated by nutritional status, metabolic demands, and pathophysiological processes that generate insulin resistance (13). Betatrophin is a β-cell trophic factor released from the liver under conditions of insulin resistance (14), and its existence provides a new paradigm for understanding how the HFD induces β-cell compensation. Potentially, circulating factors such as betatrophin induce coordinate expression of an Epac2A signal transduction “module” comprised of Epac2A, Rap1, and a Rap1-regulated phospholipase C-ε (PLCε) (Fig. 1B) (15). This signaling module is implicated in the control of β-cell excitability and Ca2+ handling by virtue of its ability to promote glucose-dependent closure of KATP channels, to stimulate Ca2+ influx, and to mobilize Ca2+ (15). In fact, a KO of PLCε uncouples Epac2A activation from the stimulation of insulin secretion (16).
Finally, it should be noted that insulin tolerance tests reveal that Epac2A KO mice have increased insulin sensitivity (1). Thus, glucose tolerance is relatively undisturbed in Epac2A KO mice fed a normal diet or an HFD. Since the Epac2A KO mice tested by Song et al. are whole-body KOs (12), there exists a clear rationale to repeat these studies of glucoregulation using β-cell–specific Epac2A KO mice in which a confounding increase of insulin sensitivity might not occur.
ADDENDUM
While this article was in proof, it was reported by Kai et al. that an alternative isoform of Epac designated as Epac1 also plays a role in the control of β-cell function.
Kai AK, Lam AK, Chen Y, et al. Exchange protein activated by cAMP 1 (Epac1)-deficient mice develop β-cell dysfunction and metabolic syndrome. FASEB J. 27 June 2013 [Epub ahead of print]
ACKNOWLEDGMENTS
This work was supported by American Diabetes Association Basic Science Awards to G.G.H. (7-12-BS-077) and C.A.L. (1-12-BS-109). O.G.C. acknowledges the support of SUNY Upstate Medical University.
No conflicts of interest relevant article were reported.
Footnotes
See accompanying original article, p. 2796.
REFERENCES
- 1.Song W-J, Mondal P, Li Y, Lee SE, Hussain MA. Pancreatic β-cell response to increased metabolic demand and to pharmacologic secretagogues requires exchange protein activated by cAMP islet /brain isoform 2A. Diabetes 2013;62:2796–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holz GG. Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor–mediated signal transduction in the pancreatic β-cell. Diabetes 2004;53:5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev 2005;85:1303–1342 [DOI] [PubMed] [Google Scholar]
- 4.Kashima Y, Miki T, Shibasaki T, et al. Critical role of cAMP-GEFII—Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem 2001;276:46046–46053 [DOI] [PubMed] [Google Scholar]
- 5.Eliasson L, Ma X, Renström E, et al. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol 2003;121:181–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 2009;5:262–269 [DOI] [PubMed] [Google Scholar]
- 7.Winzell MS, Ahrén B. The high-fat diet–fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004;53(Suppl. 3):S215–S219 [DOI] [PubMed] [Google Scholar]
- 8.Song W-J, Seshadri M, Ashraf U, et al. Snapin mediates incretin action and augments glucose-dependent insulin secretion. Cell Metab 2011;13:308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doliba NM, Qin W, Najafi H, et al. Glucokinase activation repairs defective bioenergetics of islets of Langerhans isolated from type 2 diabetics. Am J Physiol Endocrinol Metab 2012;302:E87–E102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2005;90:5991–5997 [DOI] [PubMed] [Google Scholar]
- 11.Idevall-Hagren O, Jakobsson I, Xu Y, Tengholm A. Spatial control of Epac2 by cAMP and Ca2+-mediated activation of Ras in pancreatic β cells. Sci Signal 2013;6:ra29 [DOI] [PubMed]
- 12.Shibasaki T, Takahashi H, Miki T, et al. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA 2007;104:19333–19338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinke SA, Hellemans K, Schuit FC. Plasticity of the β cell insulin secretory competence: preparing the pancreatic β cell for the next meal. J Physiol 2004;558:369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi P, Park JS, Melton DA. Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell 2013;153:747–758 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Leech CA, Dzhura I, Chepurny OG, et al. Molecular physiology of glucagon-like peptide-1 insulin secretagogue action in pancreatic β cells. Prog Biophys Mol Biol 2011;107:236–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dzhura I, Chepurny OG, Leech CA, et al. Phospholipase C-ε links Epac2 activation to the potentiation of glucose-stimulated insulin secretion from mouse islets of Langerhans. Islets 2011;3:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]