Abstract
The frequency of pancreatic β-cell replication declines dramatically with age, potentially contributing to the increased risk of type 2 diabetes in old age. Previous studies have shown the involvement of cell-autonomous factors in this phenomenon, particularly the decline of polycomb genes and accumulation of p16/INK4A. Here, we demonstrate that a systemic factor found in the circulation of young mice is able to increase the proliferation rate of old pancreatic β-cells. Old mice parabiosed to young mice have increased β-cell replication compared with unjoined old mice or old mice parabiosed to old mice. In addition, we demonstrate that old β-cells transplanted into young recipients have increased replication rate compared with cells transplanted into old recipients; conversely, young β-cells transplanted into old mice decrease their replication rate compared with young cells transplanted into young recipients. The expression of p16/INK4A mRNA did not change in heterochronic parabiosis, suggesting the involvement of other pathways. We conclude that systemic factors contribute to the replicative decline of old pancreatic β-cells.
Aging lowers the replicative potential of most cells and the capacity for tissue regeneration (1,2). The mechanisms responsible for this phenomenon are intensely investigated but are still poorly understood. In some cases, the decline in proliferation reaches an irreversible state called cellular senescence, which is characterized by a total lack of cell divisions and altered cellular morphology, gene expression profile, and chromatin organization (3). The gradual decrease in the rate of cell division seen already in early postnatal life, before any evidence of senescence, is even less well-understood.
The prevalence of replicating pancreatic β-cells declines dramatically with age in both rodents and humans (4,5). The potential of β-cells to enter the cell division cycle in response to injury also becomes restricted in old age (6,7); however, we have recently found that a residual replicative potential is retained even in β-cells of very old mice (8). The molecular mechanisms that underlie these phenotypes are usually thought to converge on enhanced expression of the p16/INK4A seen in old age. The p16 deficiency reduces and p16 overexpression enhances the age-related decline in β-cell replication (9). The increase in β-cell p16/INK4A expression was found to result from an age-related decline in the expression of polycomb genes Ezh2 and Bmi1 levels, which act to repress p16/INK4A in young age, and a decrease in the platelet-derived growth factor receptor (10,11). Beyond an increase in p16/INK4A expression, multiple other cyclin kinase inhibitors are upregulated with old age (7), but the functional significance of these genes is less clear. The increase in the expression of cell-cycle inhibitors, including p16/INK4A and others, was linked to upregulated p38MAPK signaling with age (12).
Notably, these studies suggest that the age-related decline in β-cell proliferation stems from a cell-autonomous increase in β-cell cycle inhibitors. However, outside the β-cell field, accumulating evidence suggests that systemic circulating factors are important determinants of aging in general and age-related decline of cell replication in particular. Some striking examples include muscle satellite cells (13), liver, skin (14), and neuronal stem cells (15). In all these cases, it was shown that factors present in the circulation of young animals are able to rejuvenate old cells and bring the rate of proliferation to the levels seen in young animals. The nature of the factors mediating the systemic effect of replication in old age remains largely unknown. Roles for Notch and transforming growth factor-β signaling were proposed in satellite muscle cells (13,16), and circulating chemokines were implicated in the age-related decline of neurogenesis (15). More generally, it is proposed that blood-borne factors altered in age may control cell replication via modulation of stem cell niches (16). A particularly powerful methodology used in many of these studies is heterochronic parabiosis, whereby the circulation of young and old mice is joined, allowing for the study of the effect of systemic factors on aging (13).
Recent studies have provided indirect evidence for the importance of systemic factors in the age-related decline of β-cell replication. In one study, both young and old islets were grafted into a hyperglycemic mouse. This work showed that when exposed to high levels of glucose, both young and old β-cells replicate at a similar rate (17). Additionally, when human islets were exposed to high levels of glucose in NOD-SCID mice, no connection was found between donor age and the β-cell proliferation rate (18). Most recently, an article by El Ouaamari et al. (19) used parabiosis to demonstrate the presence of a systemic factor emanating from the liver in the liver-specific insulin receptor knockout model of β-cell hyperplasia. To directly investigate the role of systemic factors in the age-related decline of β-cell proliferation, we established two experimental models in which old β-cells were exposed to a young environment. Using both heterochronic parabiosis and heterochronic islet transplantation, we showed the presence of a circulating factor that regulates the age-related β-cell proliferation rate.
RESEARCH DESIGN AND METHODS
Mice.
Animal experiments were approved by the Institutional Animal Care Committee of The Hebrew University. Parabiosis was performed as previously described (13) in C57/Bl6 male mice aged ∼1 month and ∼8 months. Mice were killed after 3 weeks. Parabiosed animals that lost >25% of body weight were not included. In transplantation experiments, ∼125 islets were transplanted under the mouse kidney capsule for 2.5 weeks as described (20). Insulin and IGF-1 were measured using ELISA (Crystal Chem and R&D Systems, respectively).
Immunostaining.
Pancreas paraffin sections were prepared as previously described (21). The following primary antibodies were used: guinea pig anti-insulin (1:500; DAKO), rabbit anti-Ki67 (1:200; Neomarkers), and mouse anti-Nkx6.1 (1:200; Beta Cell Biology Consortium). Propidum iodide (1:500) was used for all DNA stains. Secondary antibodies were from Jackson ImmunoResearch. Approximately 30 islets or 3,000 β-cells were counted per 1-month-old animal and 50 islets (8,000–10,000 cells) were counted in 3-month-old animals using ImageJ.
RT-PCR.
All experiments were performed on an Applied Biosystems 7300 Real Time PCR system using SYBER Green and primers specific for each gene.
Statistics.
Statistical analyses were performed using a two-tailed Student t test and a single-factor ANOVA in which more than two groups were compared. A post hoc Tukey test was performed when necessary. For both analyses, P < 0.05 and P < 0.01 were considered significant.
RESULTS
Establishment of a heterochronic parabiosis model.
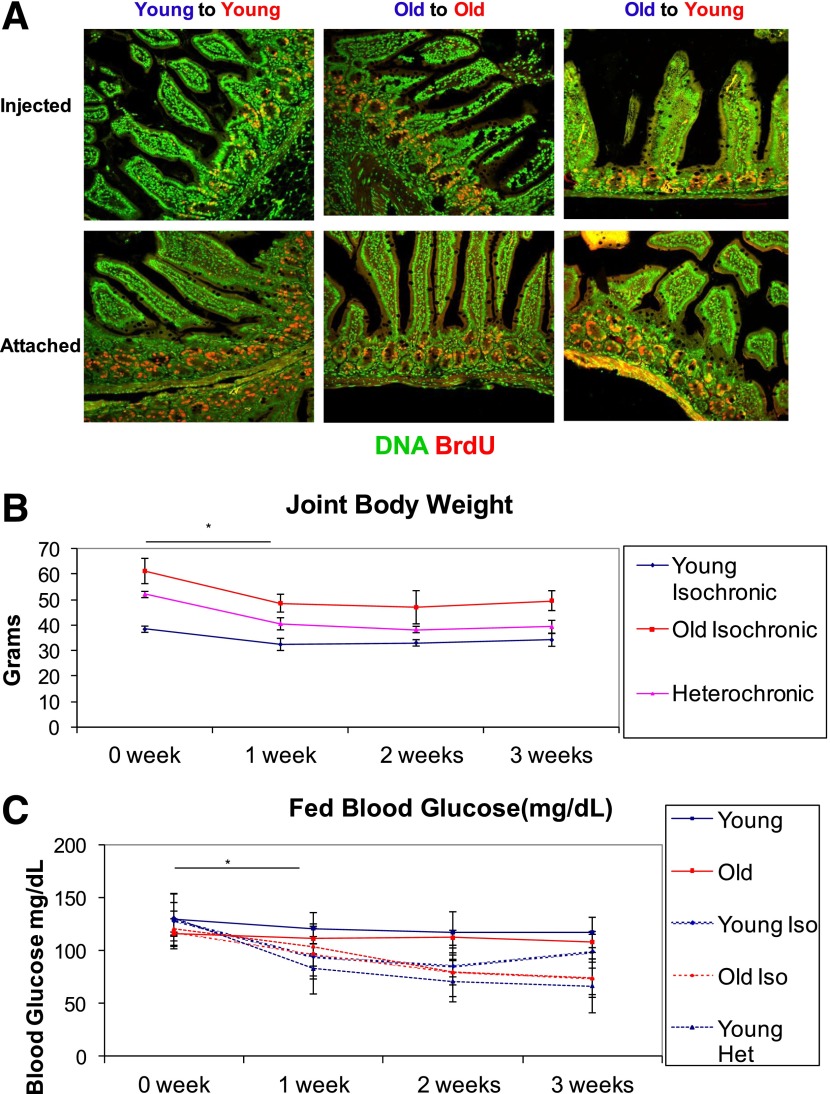
To analyze the effect of the systemic environment on β-cell replication, we compared three groups of parabionts: connected young mice aged 1.5 months (isochronic young), connected old mice aged 7–8 months (isochronic old), and connected young mice and old mice (heterochronic). Mice were surgically joined by the muscles in the abdominal wall and the skin for a period of 2–3 weeks and then killed. To demonstrate a connected circulation between parabionts, BrdU was injected into one mouse and the pair was killed after 3 h. Intestinal crypts from the injected mice and the attached mice in all three groups of parabionts showed similar BrdU signals, indicating a joined circulatory system (Fig. 1A).
FIG. 1.
Heterochronic parabiosis model. A: One parabiosed partner was injected with BrdU and the joined pair was killed after 3 h. Both the injected mouse and attached mouse were stained for DNA (green) and BrdU (red). B: Parabiosed mice demonstrated a significant decline in body weight (*P < 0.05 for Student t test and ANOVA) in all three groups, after which their weight stabilized. C: Fed blood glucose levels of connected mice (all three groups) decreased significantly after 1 week (*P < 0.05 ANOVA) and remained lower throughout the experiment (N = 5). Het, heterochronic; Iso, isochronic.
We next examined the physiological effects of the parabiosis. After 1 week, all three groups of parabionts demonstrated a mild decrease in body weight (∼10–15%), which stabilized for the remainder of the experiment (Fig. 1B). Interestingly, parabiosed mice also demonstrated a decrease in their average blood glucose, stabilizing at 70–90 mg/dL after 2 weeks.
Heterochronic parabiosis increases old β-cell replication.
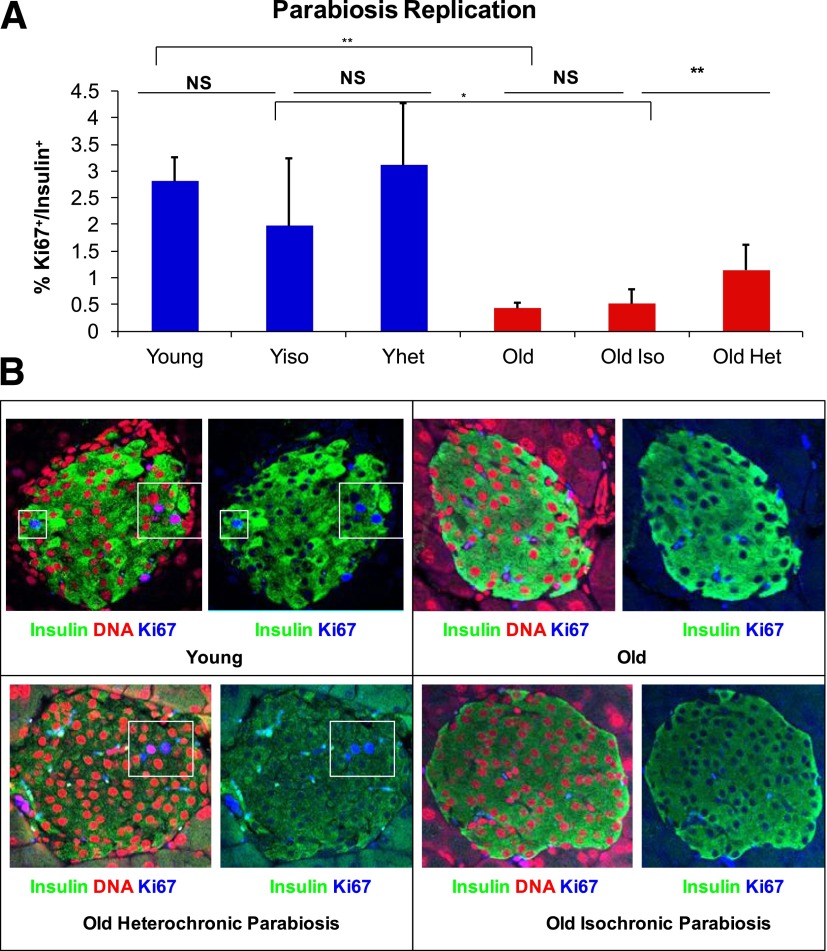
After 2–3 weeks, parabiont groups and age-matched nonconnected mice were killed. The β-cells then were analyzed for proliferation by staining with insulin and Ki67 (Fig. 2A and B). The frequency of β-cell replication in young isochronic, young heterochronic, and nonjoined mice was similar, at ∼3%. In contrast, old heterochronic mice demonstrated a significant increase in β-cell proliferation when compared with old nonattached and old isochronic mice (Fig. 2A). Although old nonjoined and old isochronic mice had ∼0.5% β-cell replication, ∼1.25% of β-cells in old heterochronic mice were replicating. These results demonstrate that exposure of old mice to a young systemic environment significantly increases β-cell replication.
FIG. 2.
Heterochronic parabiosis increased β-cell replication of old mice. A: Parabiosis was performed in young mice and old mice to create isochronic and heterochronic groups. After 3 weeks, mice were killed and proliferation was measured using Ki67. Student t test demonstrated a significant increase in replication between old isochronic mice and old heterochronic mice (**P < 0.01, whereas ANOVA analysis of the experiment yielded P < 0.001). Post hoc Tukey honestly significant difference test also showed a significant difference between old isochronic mice and heterochronic mice (*P < 0.005). B: Representative images of the islets from four different groups of parabiosed mice stained for insulin (green), DNA (red), and Ki67 (blue) (N ≥ 4). Het, heterochronic; Iso, isochronic; NS, not significant; Yhet, young heterochronic; Yiso, young isochronic.
β-Cell replication affected by recipient age in heterochronic islet transplants.
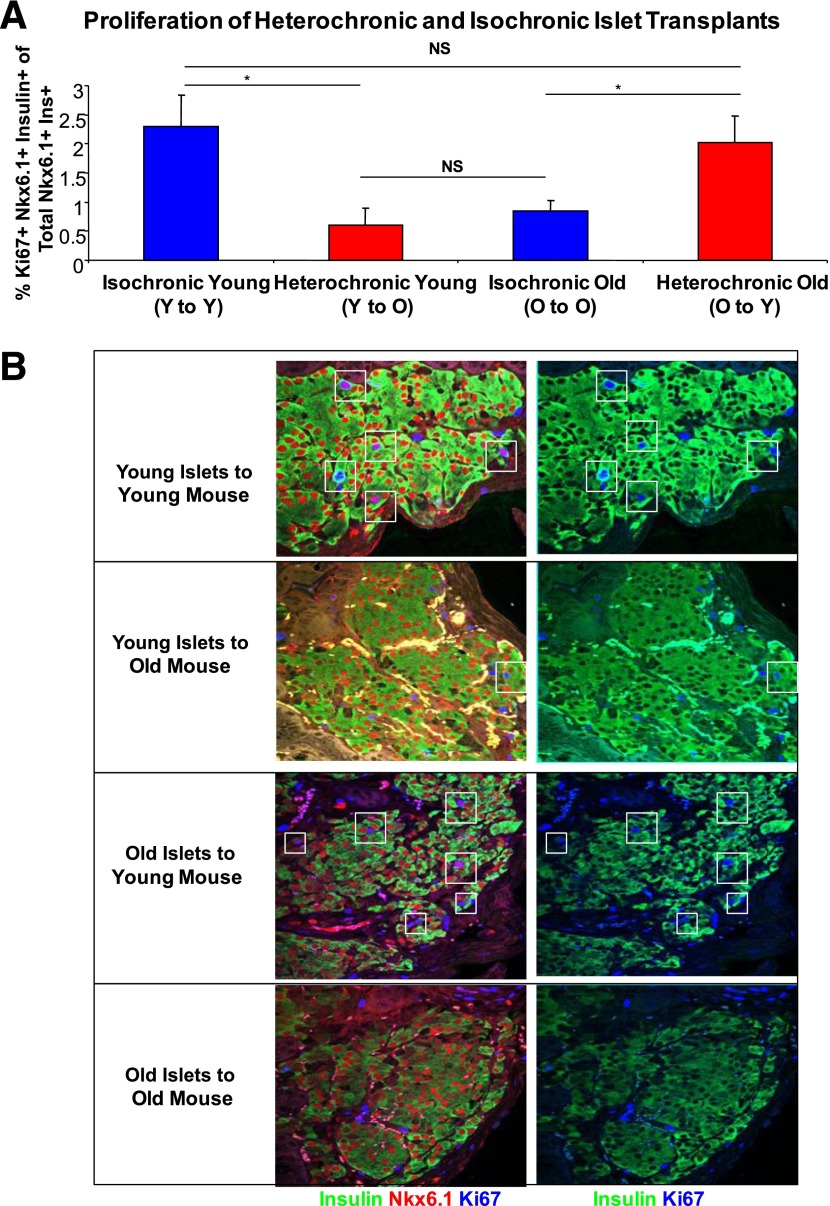
To further investigate the role of systemic factors in the age-related decline of β-cell proliferation, we established a heterochronic transplant assay. Four groups of mice were created: 150 young islets transplanted to young (1.5 months old) mice, 150 young islets transplanted to old mice (7–8 months old), 150 old islets transplanted to young mice, and 150 old islets transplanted to old mice. A small number of transplanted islets were used to preserve normal glucose homeostasis, which may independently alter the β-cell replication rate. There were no significant differences between fed and fasting glucose levels in any of the groups (Supplementary Fig. 1A and B). Two weeks after operation, the frequency of replication in transplanted β-cells was determined by staining for insulin, Nkx6.1, and Ki67 (Fig. 3A and B). Both insulin and Nkx6.1 were used as β-cell markers to provide accurate detection of proliferating transplanted cells; 2.5% of young β-cells grafted in young mice were dividing. However, when young islets were transplanted to old mice, only 0.5% of β-cells were dividing. Conversely, 0.5% of β-cells in old islets transplanted into old mice were dividing, yet old β-cells transplanted into young mice had a significantly higher replication rate of 2%. These results further show that the age of the systemic environment regulates the proliferation rate of β-cells.
FIG. 3.
Heterochronic islet transplants increase proliferation of old β-cells and decrease proliferation of young β-cells. A: Islet grafts were placed under the kidney capsule of young and old mice to create isochronic and heterochronic transplant groups. After 2 weeks, mice were killed and proliferation was measured using Ki67. Heterochronic transplant succeeded in increasing β-cell proliferation of old islets and in decreasing proliferation of young islets using Student t test (*P < 0.05) and ANOVA (P < 0.01). Post hoc Tukey honestly significant difference test also showed a significant difference in both heterochronic transplant groups (P < 0.007; N = 4). B: A representative image of β-cells transplanted under the kidney capsule from four different groups of transplanted mice stained for insulin (green), Nkx6.1 (red), and Ki67 (blue). Ins, insulin; NS, not significant; O, old; Y, young.
Analysis of serum and local factors in the heterochronic parabiosis assay.
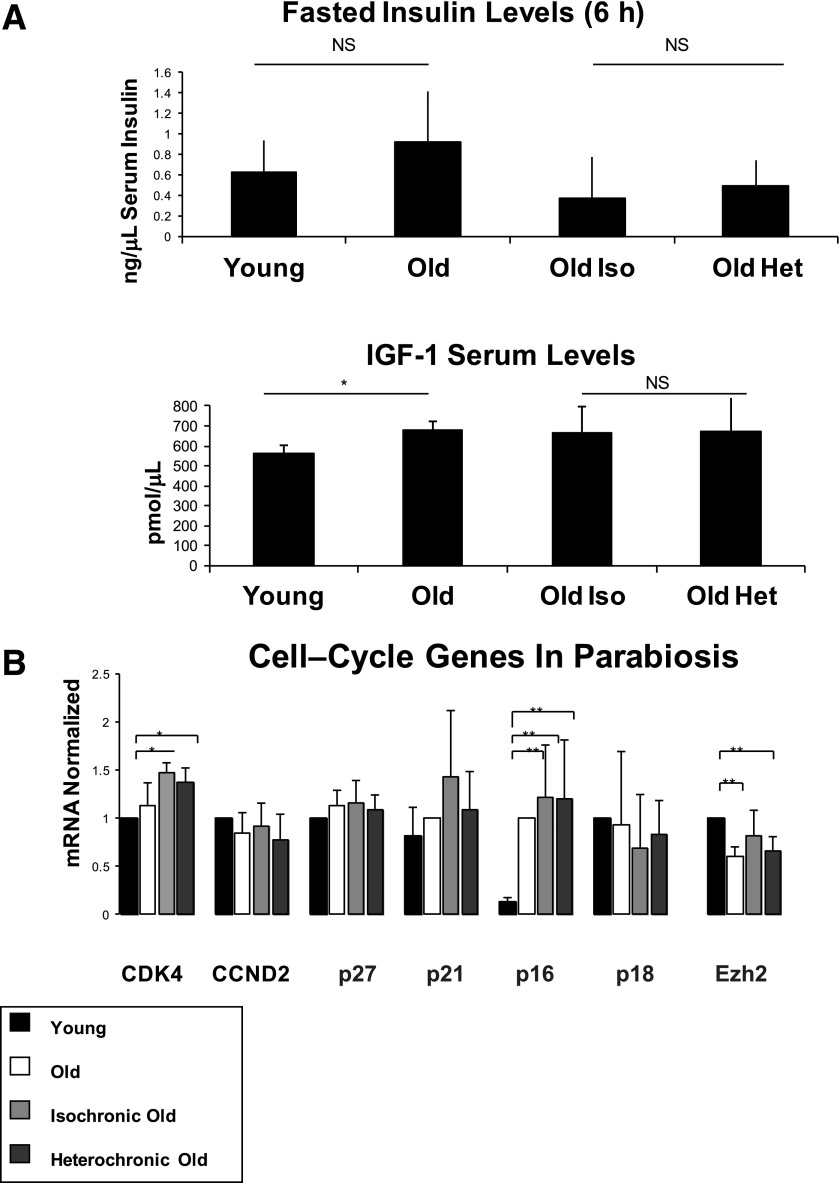
We next investigated the heterochronic parabiosis model to identify possible factors regulating the upregulation of β-cell replication in the heterochronic parabionts. Fasting insulin levels did not differ significantly between young mice, old mice, old isochronic mice, and old heterochronic mice (Fig. 4A). Interestingly, we found that serum IGF-1 levels increased between young and old mice; however, there was no difference between old isochronic and old heterochronic mice (Fig. 4A). We further analyzed mRNA levels of selected genes known to effect cell-cycle progression and β-cell physiology. Although the expression of previously identified cell-cycle genes (p16/INK4A and Ezh2) was significantly different between young and old mice, no difference was found in these or other factors between old isochronic mice and old heterochronic mice (Fig. 4B).
FIG. 4.
Serum factors and gene expression in heterochronic parabiosis. A: Fasting insulin was analyzed in young, old, old isochronic, and old heterochronic mice. There was no significant difference between the groups (using both t test and ANOVA). Although IGF-1 levels were significantly higher in old mice (*P < 0.05; t test), no difference was detected between old isochronic mice and old heterochronic mice (no significant difference was found in ANOVA; N = 4). B: Expression of selected cell-cycle genes in islets of young, old, and parabiosed mice. All genes were normalized to the young mouse group except p21 and p16, which were normalized to the old mouse group. Parabiosis to a young partner did not change the expression of any gene in old mice (N = 3). Het, heterochronic; Iso, isochronic; NS, not significant.
DISCUSSION
We investigated whether the age-related decline in β-cell replication was controlled by local or systemic factors. Using two surgical assays, we showed a blood-borne contribution to this decline. In heterochronic parabiosis, β-cell replication in old mice significantly increased when joined to a young partner. These results are supported by heterochronic islet transplantation experiments. Here, we found that old islets transplanted into young mice have increased β-cell replication, whereas young islets transplanted into old mice have decreased β-cell replication. These latter results suggest that a systemic factor involved in the age-related decline of β-cell replication is also a major regulator of β-cell replication in young mice.
Our results parallel those of similar studies of other organs that have described a contribution of a systemic factor to the age-related decline in muscle, liver, and skin cell replication and the age-related decline in neurogenesis (13,15,22). Moreover, our work is consistent with the recent article by El Ouaamari et al. (19) showing the ability of a systemic factor to act as a β-cell mitogen in a parabiosis experiment. Recent studies have suggested that the age-related decline in β-cell replication results from increased expression of cell-cycle inhibitors, particularly p16/INK4A (10). Our results suggest that the systemic factor regulating the decline in β-cell proliferation with age is unlikely acting through this pathway because the mRNA levels of p16/INK4A, p18, p27, and p21 cyclin kinase inhibitors in old mice have not changed on exposure to young circulation that led to increased replication (Fig. 4B).
We also examined the potential contribution of insulin or IGF-1 to the increase in β-cell proliferation in heterochronic parabiosis. However, serum levels of these factors did not change in heterochronic parabiosis, providing no support for this idea (Fig. 4A). Further studies are needed to identify circulating factors that are mitogenic or cytostatic to β-cells and are altered with aging, with platelet-derived growth factor, hepatocyte growth factor, and epidermal growth factor being primary targets.
One provocative possibility is that glucose itself is a determinant of the age-related decline in β-cell proliferation. Glucose acting via glucose metabolism is a key physiological β-cell mitogen (21,23,24). Furthermore, two recent islet transplantation studies showed that young and old β-cells replicate at a similar rate when grafted in a hyperglycemic host (17,18). Importantly, blood glucose levels significantly decrease in mice between 4 and 9 months of age (25), and we have observed this phenomenon (127 ± 11.878 mg/dL [young] and 88.25 ± 4.787 mg/dL [old]; P < 0.0001; N = 8). Thus, it is possible that the mild reduction in blood glucose levels seen as mice age is mediating at least part of the decline in β-cell replication.
In summary, we show that the age-related decline in β-cell replication is affected by circulating factors, and that exposure of old β-cells to a young environment can rejuvenate β-cell replication. Identifying the factors responsible for this effect may suggest new directions for regenerative therapy in diabetes.
ACKNOWLEDGMENTS
This work was supported by grants from the Juvenile Diabetes Research Foundation, European Union (European Research Council and the Seventh Framework Programme under grant agreement 241883), the Leona M. and Harry B. Helmsley Charitable Trust, the Dutch Friends of Hebrew University, the Diabetes Research Foundation Netherlands (DON), and the Israel Center of Research Excellence (I-CORE) Program of the Planning and Budgeting Committee and The Israel Science Foundation (#41.11 to Y.D.).
No potential conflicts of interest relevant to this article were reported.
S.J.S. designed the experiments, performed experiments, and wrote the manuscript. A.K. performed experiments. N.W.-C. performed experiments. O.Z. performed experiments. B.G. designed the experiments and wrote the manuscript. Y.D. designed the experiments and wrote the manuscript. Y.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Orr Spiegel of The Hebrew University for his assistance with statistical analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0160/-/DC1.
REFERENCES
- 1.Bonner-Weir S. Islet growth and development in the adult. J Mol Endocrinol 2000;24:297–302 [DOI] [PubMed] [Google Scholar]
- 2.Jones DL, Rando TA. Emerging models and paradigms for stem cell ageing. Nat Cell Biol 2011;13:506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol 2005;37:961–976 [DOI] [PubMed] [Google Scholar]
- 4.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 1997;138:1736–1741 [DOI] [PubMed] [Google Scholar]
- 5.Finegood DT, Weir GC, Bonner-Weir S. Prior streptozotocin treatment does not inhibit pancreas regeneration after 90% pancreatectomy in rats. Am J Physiol 1999;276:E822–E827 [DOI] [PubMed] [Google Scholar]
- 6.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes 2005;54:2557–2567 [DOI] [PubMed] [Google Scholar]
- 7.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes 2009;58:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stolovich-Rain M, Hija A, Grimsby J, Glaser B, Dor Y. Pancreatic beta cells in very old mice retain capacity for compensatory proliferation. J Biol Chem 2012;287:27407–27414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnamurthy J, Ramsey MR, Ligon KL, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 2006;443:453–457 [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Gu X, Su IH, et al. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev 2009;23:975–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Gu X, Liu Y, et al. PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature 2011;478:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong ES, Le Guezennec X, Demidov ON, et al. p38MAPK controls expression of multiple cell cycle inhibitors and islet proliferation with advancing age. Dev Cell 2009;17:142–149 [DOI] [PubMed] [Google Scholar]
- 13.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005;433:760–764 [DOI] [PubMed] [Google Scholar]
- 14.Song G, Nguyen DT, Pietramaggiori G, et al. Use of the parabiotic model in studies of cutaneous wound healing to define the participation of circulating cells. Wound Repair Regen 2010;18:426–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011;477:90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conboy IM, Rando TA. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle 2012;11:2260–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Zhang X, Chen F, Larson CS, Wang LJ, Kaufman DB. Comparative study of regenerative potential of beta cells from young and aged donor mice using a novel islet transplantation model. Transplantation 2009;88:496–503 [DOI] [PubMed] [Google Scholar]
- 18.Levitt HE, Cyphert TJ, Pascoe JL, et al. Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia 2011;54:572–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Ouaamari A, Kawamori D, Dirice E, et al. Liver-derived systemic factors drive β cell hyperplasia in insulin-resistant states. Cell Rep 2013;3:401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molano RD, Pileggi A, Berney T, et al. Long-term islet allograft survival in nonobese diabetic mice treated with tacrolimus, rapamycin, and anti-interleukin-2 antibody. Transplantation 2003;75:1812–1819 [DOI] [PubMed] [Google Scholar]
- 21.Salpeter SJ, Klein AM, Huangfu D, Grimsby J, Dor Y. Glucose and aging control the quiescence period that follows pancreatic beta cell replication. Development 2010;137:3205–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature 2008;454:528–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab 2011;13:440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salpeter SJ, Klochendler A, Weinberg-Corem N, et al. Glucose regulates cyclin D2 expression in quiescent and replicating pancreatic β-cells through glycolysis and calcium channels. Endocrinology 2011;152:2589–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leiter EH, Premdas F, Harrison DE, Lipson LG. Aging and glucose homeostasis in C57BL/6J male mice. FASEB J 1988;2:2807–2811 [DOI] [PubMed] [Google Scholar]