Abstract
There is a need for plasma-based tests that can directly measure the extent of β-cell injury in vivo in patients receiving islet grafts and in animal models. In this study, we propose protein phosphatase 1, regulatory (inhibitor) subunit 1A (PPP1R1A) as a novel biomarker for acute β-cell destruction. Liquid chromatography–tandem mass spectrometry proteome analysis of fluorescence-activated cell sorter–purified β-cells, tissue-comparative Western blotting, and immunohistochemistry indicated relatively high molar abundance and selectivity of PPP1R1A in β-cells. PPP1R1A was discharged into the extracellular space of chemically injured rat and human islets in vitro, proportionate to the extent of β-cell death. Streptozotocin injection in rats led to a progressive PPP1R1A depletion from the cytoplasm of disintegrating β-cells and a marked surge in plasma levels detectable by an affinity-capture method. A similar massive PPP1R1A discharge in blood was also detected in three patients immediately after intraportal islet transplantation. Our findings provide first proof-of-principle for PPP1R1A as real-time biomarker of β-cell destruction in animal models and patients and warrant development of more sensitive methods for its further validation in clinical trials.
Islet transplantation has the potential to improve long-term metabolic control in patients with type 1 diabetes mellitus (T1DM), and further refinement of this technique may lead toward a lasting cure (1–3). Human donor organs, however, are scarce, limiting the number of primary islet grafts that can be composed. Moreover, a substantial fraction of isolated human islets are lost in culture before transplant. In addition, 50–75% of grafted β-cells are rapidly destroyed due to hypoxia, thrombosis, and inflammatory reactions (4–6). Optimizations of immune-modulatory, anti-inflammatory, and surgical protocols in islet transplantation can thus lead to better therapy for more patients. These optimizations require reliable biomarkers to monitor β-cell injury. Using classical indices of glucose homeostasis (HbA1c and glycemic variability) or dynamic assays of β-cell secretory capacity, long-term outcome of islet transplantation can be reliably evaluated (7–11). What is still lacking is a direct biomarker for real-time sensitive quantification of β-cell injury in vivo. Proof-of-principle for glutamic acid decarboxylase 65kDa (GAD65) as such a biomarker was provided by Waldrop et al. (12), who reported that streptozotocin (STZ)-injured β-cells discharge GAD65 into the plasma, proportionate to the degree of β-cell loss. We recently found that high plasma GAD65 levels after islet transplantation predict poor long-term functional graft outcome in patients (Z.L., unpublished observations). These studies, however, also revealed shortcomings of GAD65 as a biomarker: 1) GAD65 cannot be reliably used in the 30–40% of diabetic islet recipients that show high titers of circulating anti-GAD65 antibodies (13); and 2) its relatively low molar abundance in β-cells precludes detection of minor β-cell insults, despite highly sensitive immunoassays (14,15).
Therefore, we initiated a search for alternative biomarkers to be used complementary to GAD65. In a proteomics-based screening, we previously found that rat β-cells abundantly express protein phosphatase 1, regulatory (inhibitor) subunit 1A, alias protein phosphatase inhibitor-1 (PPP1R1A) (16). In combination with its reported high degree of β-cell selectivity in rats (16,17) and mice (18,19) and its cytoplasmic localization, PPP1R1A appeared an interesting candidate for validation as a biomarker of β-cell injury in vitro and in vivo.
RESEARCH DESIGN AND METHODS
In vitro and in vivo models of β-cell injury.
Freshly isolated rat islet cells (70% insulin+) and cryopreserved human (50% insulin+) islets were exposed to 5 mmol/L STZ (30 min) and or 2 mmol/L H2O2 (2 h), respectively, and cell death was monitored over the subsequent 6–24 h. In vivo biomarker discharge was measured in rats injected with 60 mg/kg STZ; EDTA-plasma and pancreas were sampled at 2–24 h after injection (n = 3/time point). Plasma PPP1R1A was also measured in four T1DM patients after intraportal infusion of 1.1–4.8 × 106 β-cells/kg body weight. Negative controls included patients suffering various acute organ injuries (pancreatitis, stroke, and kidney transplantation, sampled at intensive care unit <6 h from onset) and type 2 diabetic patients.
Immunohistochemistry.
After antigen retrieval in 10 mmol/L citric acid (pH 6.0), 5-µm sections of paraffin-embedded rat and human pancreas were stained with monoclonal rabbit anti-PPP1R1A (OriGene, Rockville, MD; 1/800 for rat, 1/200 for human), monoclonal mouse anti-glucagon (Sigma-Aldrich, St. Louis, MO; 1/500), and/or polyclonal guinea pig anti-insulin (1/1,000). Secondary antibodies (1/500) were from Jackson ImmunoResearch Laboratories (West Grove, PA). Pictures were taken using a Zeiss Axioplan fluorescence microscope (Carl Zeiss, Thornwood, NY) at fixed exposure time and processed with Smartcapture software (Digital Scientific Ltd., Cambridge, U.K.).
PPP1R1A and GAD65 measurements.
PPP1R1A release was measured by immunoprecipitation (IP): plasma and concentrated culture medium (Microcon 10kD spin columns; Millipore, Billerica, MA) were incubated overnight at 4°C with Dynabeads (Invitrogen, Carlsbad, CA) carrying anti-PPP1R1A (OriGene) noncovalently coupled to Protein A Dynabeads (Invitrogen, Carlsbad, CA) (culture medium, 0.8 μg Ab/1.5 mg beads) or covalently coupled to M-270 Epoxy Dynabeads (Invitrogen, Carlsbad, CA) (6 μg Ab/1.25 mg beads/500 μL plasma). Captured PPP1R1A was eluted with 0.1 mol citrate (pH 3.1), detected using a polyclonal rabbit anti-PPP1R1A [from F. Schuit (18)]. Intracellular PPP1R1A was quantified using recombinant human PPP1R1A as calibrator (Abcam, Cambridge, MA). Intensities of bands were quantified with Scion image software (Scion, Frederick, MD). This assay showed intra-assay (interassay) coefficient of variation percentage of 17% (26%), good linearity (R2 = 0.96; Supplementary Fig. 1), acceptable recovery (80 ± 16%), but limited sensitivity (±2 nmol/L in plasma).
Solid-phase detection of anti-PPP1R1A autoantibodies was done exactly as described (20) using 50 ng PPP1R1A as bait in sera from 20 C-peptide–negative T1DM patients, all showing antibodies against GAD65 and at least one other T1DM epitope, and 20 age-/sex-matched healthy control subjects, obtained from the Belgian Diabetes Registry.
Statistical analysis.
Data are expressed as mean ± SD and analyzed with one-way ANOVA or t test. P < 0.05 was considered significant.
RESULTS
PPP1R1A abundance and selectivity in β-cells.
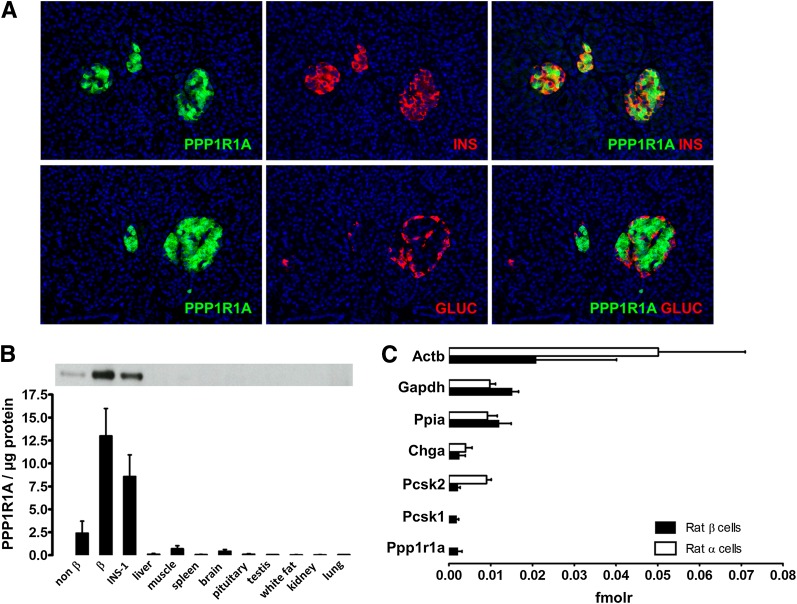
In human pancreas, PPP1R1A expression was restricted to the β-cells, with no protein detected in α-cells, nor in exocrine tissue (Fig. 1A). Also at whole body level in rat, PPP1R1A showed a reasonable degree of β-cell selectivity (Fig. 1B). Quantitative liquid chromatography–tandem mass spectrometry proteome analysis indicated that the molar abundance of PPP1R1A was similar to that of endocrine markers prohormone convertase 1 and chromogranin A and amounted to 20% of abundant housekeeping proteins such as cyclophilin-A and glyceraldehyde-3-phosphate dehydrogenase (Fig. 1C). Quantitative SDS-PAGE using recombinant PPP1R1A standards indicated that rat and human β-cells express 24 ± 10 and 36 ± 10 attomol/cell (n = 4), respectively (Supplementary Fig. 1) comparing favorably to their respective GAD65 contents (0.8 ± 0.1 and 1.5 ± 0.1 attomol/cell), as measured by time-resolved fluorescence immunoassay (TRFIA) (14).
FIG. 1.
Selectivity and abundance of PPP1R1A in rat and human β-cells. A: Human pancreas stained for PPP1R1A (green) and insulin (INS) or glucagon (GLUC) (red). Selected images are representative for three different organs. B: Western blotting of PPP1R1A expression in rat tissues. Bars represent mean ± SD of three biological replicates. C: Relative molar protein abundances in rat α- and β-cells (n = 3) measured by label-free quantitative liquid chromatography–tandem mass spectrometry (16). Proteins are denoted by their official National Center for Biotechnology Information gene symbol: Actb, β-actin; Chga, chromogranin A; fmolr, relative femtomolar amount; Gapdh, glyceraldehyde-3-phosphate dehydrogenase; Pcsk1, prohormone convertase 1; Pcsk2, prohormone convertase 2; Ppia, cyclophilin-A.
In vitro validation: PPP1R1A discharge by injured rat and human β-cells.
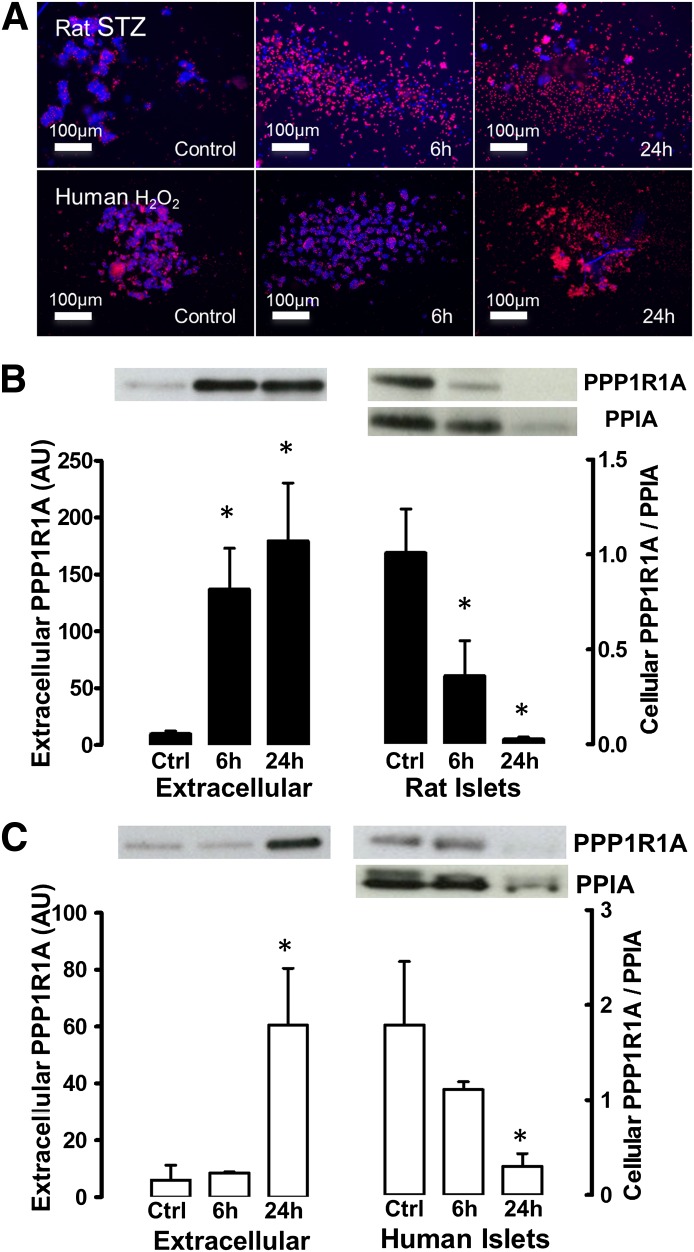
Rat islets exposed to 5 mmol/L STZ disintegrated during the subsequent 24-h culture into 60–70% single dead cells (Fig. 2A). This led to a depletion of the intracellular PPP1R1A (Fig. 2B) and a parallel increase of extracellular PPP1R1A: 24 h after injury, 20-fold more PPP1R1A was immunoprecipitated from the extracellular space than in control cultures. Similar results were obtained using cryopreserved human islets: exposure to H2O2 (2 h, 2 mmol/L) caused islet disintegration with 40–50% dead cells after 24 h of culture (Fig. 2A). This led to a 10-fold higher PPP1R1A discharge (Fig. 2C; P < 0.01) concomitant with depletion of intracellular PPP1R1A. The PPP1R1A discharge at 6 h after injury was only detected in rat cells (P < 0.01), reflecting the more severe cellular injury than in human islets at this time point (Fig. 2A).
FIG. 2.
In vitro discharge of PPP1R1A protein by injured rat and human β-cells. Panel A illustrates the in vitro cytotoxicity model: pulse exposure of rat islets to STZ and human cryopreserved islets to H2O2 induced a progressive disintegration of living islets (Hoechst+, blue) into single dead (propidium iodide+, red) cells. This caused increase of PPP1R1A in culture medium of both rat (B) and human (C) islets, quantified by IP and Western blotting. Increase of precipitated PPP1R1A discharge was accompanied by progressive depletion of intracellular PPP1R1A (right y-axis, normalized to cyclophilin-A [PPIA]). Bars indicate mean ± SD. *P < 0.01. AU, arbitrary units; Ctrl, control.
In vivo validation in rat: STZ injection causes a surge of plasma PPP1R1A.
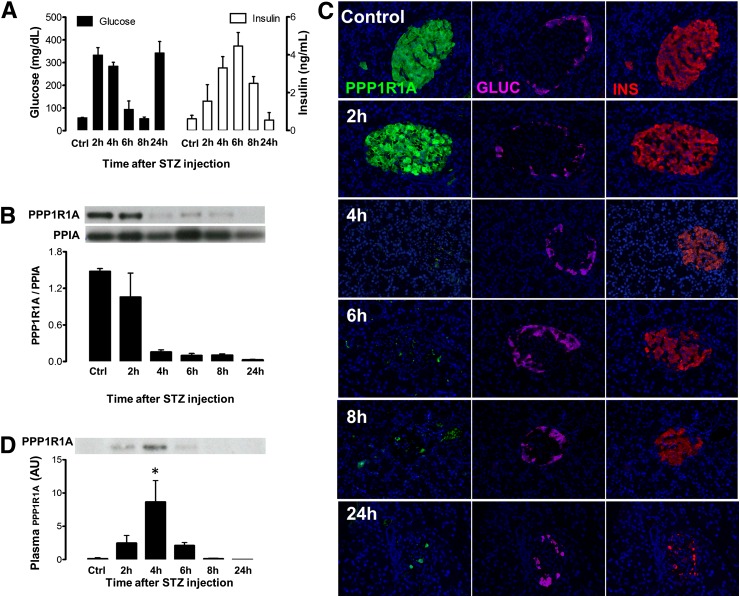
Injection of a diabetogenic dose of STZ (60 mg/kg) induced hyperglycemia within 2 h. Coinciding with a massive insulin discharge from the pancreas (peaking at 6 h), glycemia temporarily dropped to rise again and remain permanently elevated after 24 h (Fig. 3A). STZ induced progressive depletion of PPP1R1A protein from the pancreas, with little protein left after 4 h (Fig. 3B): in situ analysis showed that insulin+ cells became devoid of PPP1R1A at 4 h after STZ well before they disintegrated and were phagocytized (8–24 h) (Fig. 3C). PPP1R1A depletion from injured β-cells coincided with a sharp peak in plasma PPP1R1A between 2 and 6 h after STZ with a maximal level at 4 h (Fig. 3D). Clearance from the circulation was verified by injection of recombinant human PPP1R1A in rats, which indicated a half-life of ∼15 min (Fig. 4B), less than the 2.8 h reported for GAD65 (21).
FIG. 3.
In vivo discharge of PPP1R1A protein by STZ-injured rat β-cells. Panel A shows plasma glucose (black bars, left y-axis) and insulin (white bars, right y-axis) at the indicated time after STZ (60 mg/kg) injection (mean ± SD, n = 3/time point). STZ induced a progressive depletion of total pancreatic PPP1R1A content (B), attributed to a disappearance of PPP1R1A protein (green) from insulin (INS)-positive (red) β-cells (C) before the β-cell core of the islets disintegrates and can only be recognized by the residual α-cell mantle (magenta). Images representative for n = 3 animals/time point. This causes PPP1R1A to become detectable by IP (D) in plasma, with a peak discharge at 4 h after STZ injection. Bars indicate mean ± SD; n = 3. *P < 0.05 versus baseline. AU, arbitrary units; Ctrl, control; GLUC, glucagon.
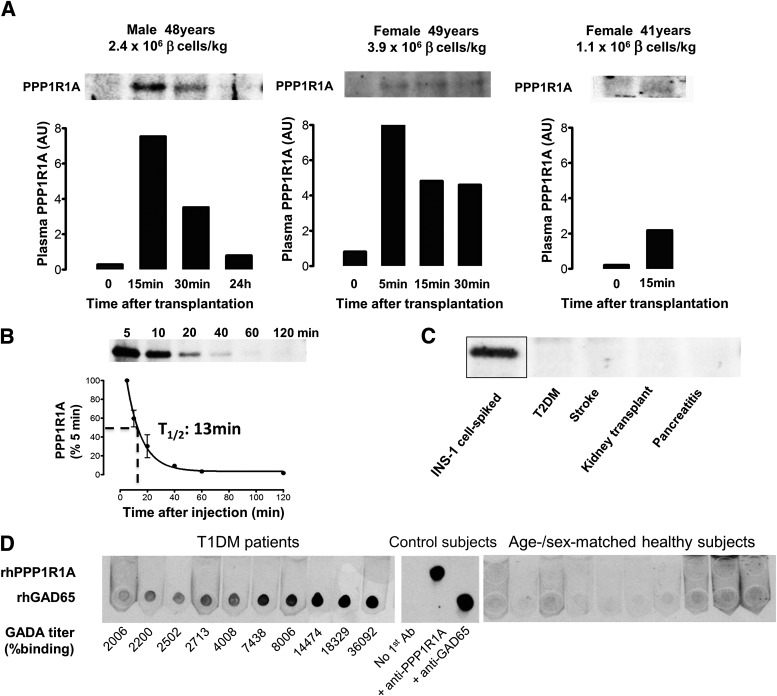
FIG. 4.
Proof-of-principle in human islet graft recipients. PPP1R1A discharge in plasma was investigated by IP in C-peptide–negative T1DM patients after intraportal infusion of the indicated number of β-cells per kilogram body weight. Bar graphs represent the quantification of the captured PPP1R1A visualized on Western blot. Panel B shows the pharmacokinetic profile in rat circulation after injection of recombinant human PPP1R1A in tail vein (average ± SD for n = 3, representative blot). Panel C illustrates that no detectable PPP1R1A amounts could be affinity-captured from sera of patients with the indicated pathologies; serum spiked with INS-1 cell lysate served as positive control (representative for n = 3 patients/condition). Panel D shows a solid-phase adsorption test for anti-PPP1R1A–reactive immunoglobulins in sera of T1DM patients (left) and age-/sex-matched healthy control subjects (right). Twenty subjects were examined, and 10 are shown in figure. T1DM all showed anti-GAD65 antibodies (GADA), the titers of which (determined by percent radiobinding in certified assays) correlated well with solid-phase horseradish peroxidase (HRP) signal against spotted recombinant human (rh)GAD65. None of the samples showed any immunoglobulin G reactivity to spotted rhPPP1R1A. Technical control subjects (middle) include 1:1,000 dilutions of mouse monoclonal anti-PPP1R1A and anti-GAD65 or no primary antibody. AU, arbitrary units; T1/2, half-life; T2DM, type 2 diabetes mellitus.
Proof-of-principle in human islet transplantation.
Engrafted islets suffer ischemic, inflammatory, and mechanical stress shortly after infusion, likely causing plasma membrane disruption and inadvertent discharge of intracellular proteins. In view of its rapid clearance, we investigated plasma PPP1R1A in four diabetic patients after intraportal islet infusion. A posttransplant PPP1R1A surge was detected in three patients (Fig. 4A), ranging from borderline detectable, in a patient receiving only 1.1 × 106 β-cells/kg, to an unambiguous detection in two patients receiving larger grafts (2.4 and 3.9 × 106 β-cells/kg). Kinetic data in the latter two patients indicated a peak discharge directly after graft infusion, followed by rapid clearance roughly compatible with PPP1R1A’s pharmacokinetic properties in rats (Fig. 4B). PPP1R1A discharge appeared to be β-cell–selective, since no PPP1R1A could be detected at baseline before transplant nor in other types of acute tissue damage such as pancreatitis, stroke, or kidney transplantation (Fig. 4C). Presence of anti-PPP1R1A autoreactive antibodies in diabetic patients was ruled out using a solid-phase adsorption assay: no anti-PPP1R1A IgGs were detected in sera of 20 T1DM patients who showed B-cell reactivity to GAD65 and at least one other classical T1DM epitope nor in age-/sex-matched healthy control subjects (Fig. 4D).
DISCUSSION
This study presents proof-of-principle that PPP1R1A can be used as a real-time biomarker for pancreatic β-cell injury in vitro and in vivo, in rodents and human patients. During culture of human islets, extracellular PPP1R1A may thus be used to assess in vitro survival, while in islet graft recipients, plasma PPP1R1A may be envisaged as biomarker to evaluate the extent of β-cell graft destruction, complementary to GAD65.
PPP1R1A shows interesting properties in terms of β-cell-selectivity and molar abundance. In line with previous results (18,22), we also detected it in brain and muscle cells, albeit at a much lower level than in β-cells. This could constrain the use of PPP1R1A in patients but not invalidate it: we could confidently detect a surge of plasma PPP1R1A after STZ-induced β-cell injury in rodents but never in baseline conditions. Also in humans, PPP1R1A was detected in three patients just after islet transplantation, but not in control patients with acute damage to exocrine pancreas (pancreatitis), brain (stroke), or kidney (transplantation).
PPP1R1A shows several advantages over GAD65, an established marker of β-cell injury. First, human and rat β-cells abundantly express PPP1R1A, apparently more than GAD65. Use of different analytical techniques precludes a direct comparison of PPP1R1A and GAD65 molar abundances in β-cells. Yet, the fact that PPP1R1A was detectable in plasma with an IP assay operating in the low nanomolar sensitivity, while the GAD65 assay operates at the picomolar range, is encouraging with regard to maximal attainable diagnostic sensitivity. Second, unlike GAD65, PPP1R1A is also expressed by mouse β-cells and can thus be applied in preclinical murine models (17,18). Finally, we showed that PPP1R1A is not a T1DM autoantigen, so it can be measured without interference by autoreactive antibodies. A possible disadvantage of PPP1R1A is its rapid clearance, likely a consequence of its limited molecular weight (20 kD) below the glomerular filtration threshold.
Future validation of the PPP1R1A’s sensitivity as biomarker of β-cell injury, defined in this study as the number of β-cells needed to be simultaneously disrupted before the biomarker plasma level rises significantly above baseline, will require a high-throughput assay that can operate at low picomolar sensitivity. Such an assay is needed to evaluate if baseline PPP1R1A levels can build up in plasma despite the relatively rapid PPP1R1A clearance in patients with neuronal or muscle damage. We calculated that our GAD65 TRFIA can detect the simultaneous destruction of 20 × 106 and 40 × 103 β-cells in humans and rats, respectively, amounting to ∼5% of their normal endogenous β-cell number and 15% of a typical islet graft. Providing that an equally sensitive PPP1R1A sandwich assay can be developed, PPP1R1A’s apparent higher abundance in β-cells could significantly improve this sensitivity. This could not only facilitate its application to monitor chronic allorejection of islet grafts, for which GAD65 showed borderline efficacy (5), but it could also create the experimental conditions to explore the largely enigmatic dynamics of preclinical autoimmune destruction T1DM, provided that these instances of β-cell destruction involve at least some degree of biomarker discharge into the plasma, either free or enclosed in cellular blebs or apoptotic bodies. A test that could give direct proof of ongoing β-cell destruction in subjects with high risk of progression to clinical T1DM based on their autoantibody and genetic profile would be valuable to select those subjects who could benefit from immune-reprogramming therapy with monoclonal antibodies such as anti-CD3 (10).
There is still a long way to go before this will be possible: apart from higher analytical sensitivity toward the proposed biomarkers, the ideal assay should also be run in high-throughput and in miniaturized mode (e.g., on dried blood spots obtained by capillary sampling during routine glycemic monitoring).
In conclusion, current pilot study establishes PPP1R1A as novel biomarker β-cell injury in vitro and in vivo, with favorable properties in terms of β-cell selectivity and abundance that warrant its further validation in (pre)clinical trials.
ACKNOWLEDGMENTS
This study was supported by research grants from the Research Foundation Flanders (FWO G.0492.12 project grant and Senior Clinical Investigator career support grant to G.A.M.) from the European Foundation for the Study of Diabetes (European Foundation for the Study of Diabetes/Juvenile Diabetes Research Foundation/Roche Young Investigator Award to G.A.M.), the China Scholarship Council (China Scholarship Council student grant to L.J.), the Fund for Scientific Research and Willy Gepts Foundation at Universitair Ziekenhuis Brussel (to G.A.M.), and the European Union (Center Grant 241883 to Z.L., D.P., and F.G. under FP7-Health-2009).
No potential conflicts of interest relevant to this article were reported.
L.J., B.B., G.K., and J.M.A. researched data. Z.L., F.S., B.K., D.P., and F.G. reviewed and edited the manuscript. G.A.M. designed the study, researched data, and wrote the manuscript. G.A.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-1507/-/DC1.
REFERENCES
- 1.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318–1330 [DOI] [PubMed] [Google Scholar]
- 2.Keymeulen B, Gillard P, Mathieu C, et al. Correlation between beta cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc Natl Acad Sci USA 2006;103:17444–17449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care 2012;35:1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emamaullee JA, Shapiro AM. Interventional strategies to prevent beta-cell apoptosis in islet transplantation. Diabetes 2006;55:1907–1914 [DOI] [PubMed] [Google Scholar]
- 5.Shapiro AM, Hao EG, Lakey JR, et al. Novel approaches toward early diagnosis of islet allograft rejection. Transplantation 2001;71:1709–1718 [DOI] [PubMed] [Google Scholar]
- 6.Piemonti L, Guidotti LG, Battaglia M. Modulation of early inflammatory reactions to promote engraftment and function of transplanted pancreatic islets in autoimmune diabetes. Adv Exp Med Biol 2010;654:725–747 [DOI] [PubMed] [Google Scholar]
- 7.Caumo A, Maffi P, Nano R, et al. Comparative evaluation of simple indices of graft function after islet transplantation. Transplantation 2011;92:815–821 [DOI] [PubMed] [Google Scholar]
- 8.Caumo A, Maffi P, Nano R, et al. Transplant estimated function: a simple index to evaluate beta-cell secretion after islet transplantation. Diabetes Care 2008;31:301–305 [DOI] [PubMed] [Google Scholar]
- 9.Ryan EA, Paty BW, Senior PA, Lakey JR, Bigam D, Shapiro AM. Beta-score: an assessment of beta-cell function after islet transplantation. Diabetes Care 2005;28:343–347 [DOI] [PubMed] [Google Scholar]
- 10.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 [DOI] [PubMed] [Google Scholar]
- 11.Vantyghem MC, Kerr-Conte J, Arnalsteen L, et al. Primary graft function, metabolic control, and graft survival after islet transplantation. Diabetes Care 2009;32:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldrop MA, Suckow AT, Marcovina SM, Chessler SD. Release of glutamate decarboxylase-65 into the circulation by injured pancreatic islet beta-cells. Endocrinology 2007;148:4572–4578 [DOI] [PubMed] [Google Scholar]
- 13.Baekkeskov S, Aanstoot HJ, Christgau S, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 1990;347:151–156 [DOI] [PubMed] [Google Scholar]
- 14.Rui M, Hampe CS, Wang C, et al. Species and epitope specificity of two 65 kDa glutamate decarboxylase time-resolved fluorometric immunoassays. J Immunol Methods 2007;319:133–143 [DOI] [PubMed] [Google Scholar]
- 15.Waldrop MA, Suckow AT, Hall TR, Hampe CS, Marcovina SM, Chessler SD. A highly sensitive immunoassay resistant to autoantibody interference for detection of the diabetes-associated autoantigen glutamic acid decarboxylase 65 in blood and other biological samples. Diabetes Technol Ther 2006;8:207–218 [DOI] [PubMed] [Google Scholar]
- 16.Martens GA, Jiang L, Verhaeghen K, et al. Protein markers for insulin-producing beta cells with higher glucose sensitivity. PLoS ONE 2010;5:e14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martens GA, Jiang L, Hellemans KH, et al. Clusters of conserved beta cell marker genes for assessment of beta cell phenotype. PLoS ONE 2011;6:e24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vander Mierde D, Scheuner D, Quintens R, et al. Glucose activates a protein phosphatase-1-mediated signaling pathway to enhance overall translation in pancreatic beta-cells. Endocrinology 2007;148:609–617 [DOI] [PubMed] [Google Scholar]
- 19.Lilja L, Meister B, Berggren PO, Bark C. DARPP-32 and inhibitor-1 are expressed in pancreatic beta-cells. Biochem Biophys Res Commun 2005;329:673–677 [DOI] [PubMed] [Google Scholar]
- 20.Jiang L, Brackeva B, Stangé G, et al. LC-MS/MS identification of doublecortin as abundant beta cell-selective protein discharged by damaged beta cells in vitro. J Proteomics 2013;80:268–280 [DOI] [PubMed] [Google Scholar]
- 21.Schlosser M, Walschus U, Klöting I, Walther R. Determination of glutamic acid decarboxylase (GAD65) in pancreatic islets and its in vitro and in vivo degradation kinetics in serum using a highly sensitive enzyme immunoassay. Dis Markers 2008;24:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolaou P, Rodriguez P, Ren X, et al. Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ Res 2009;104:1012–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]