Abstract
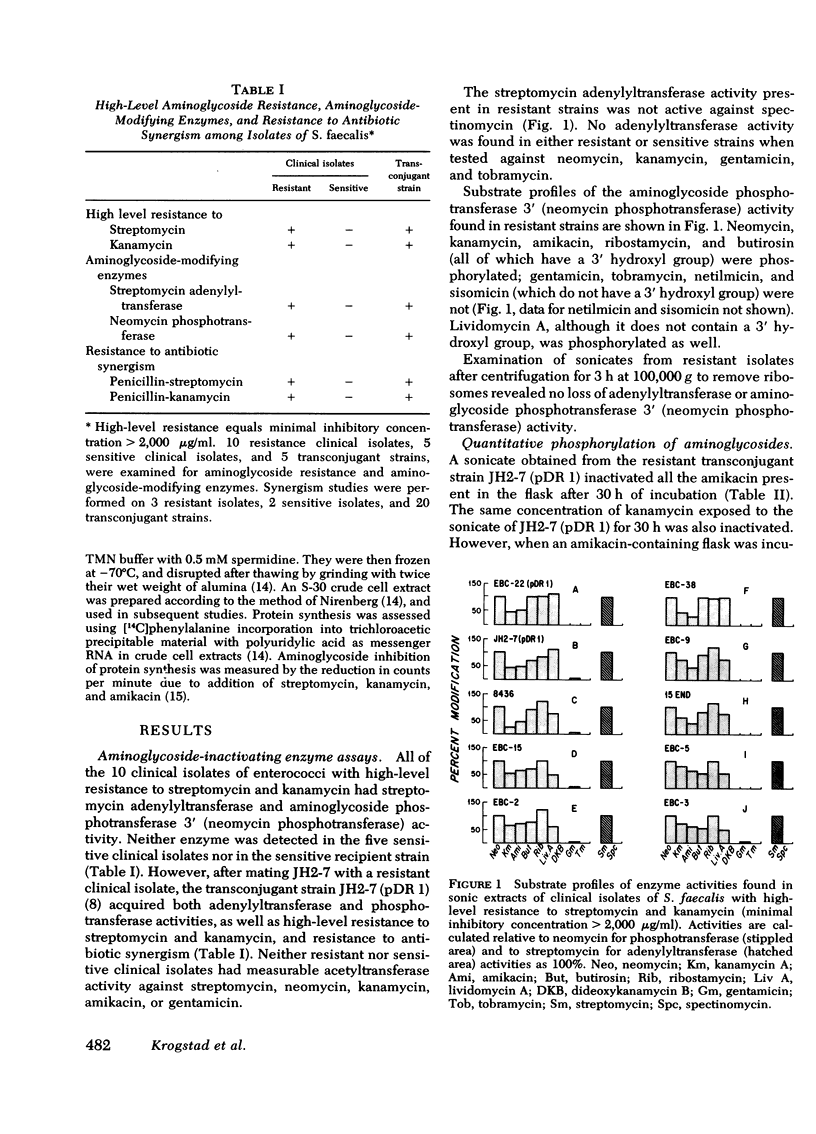
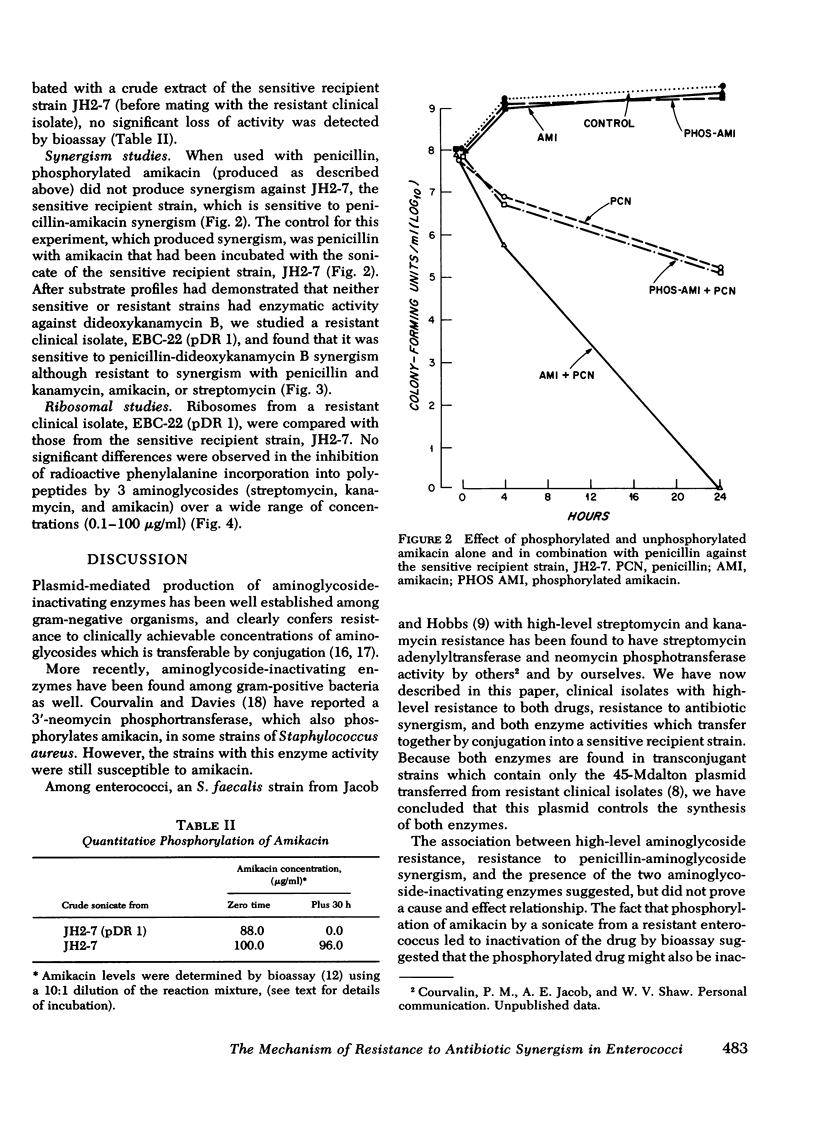
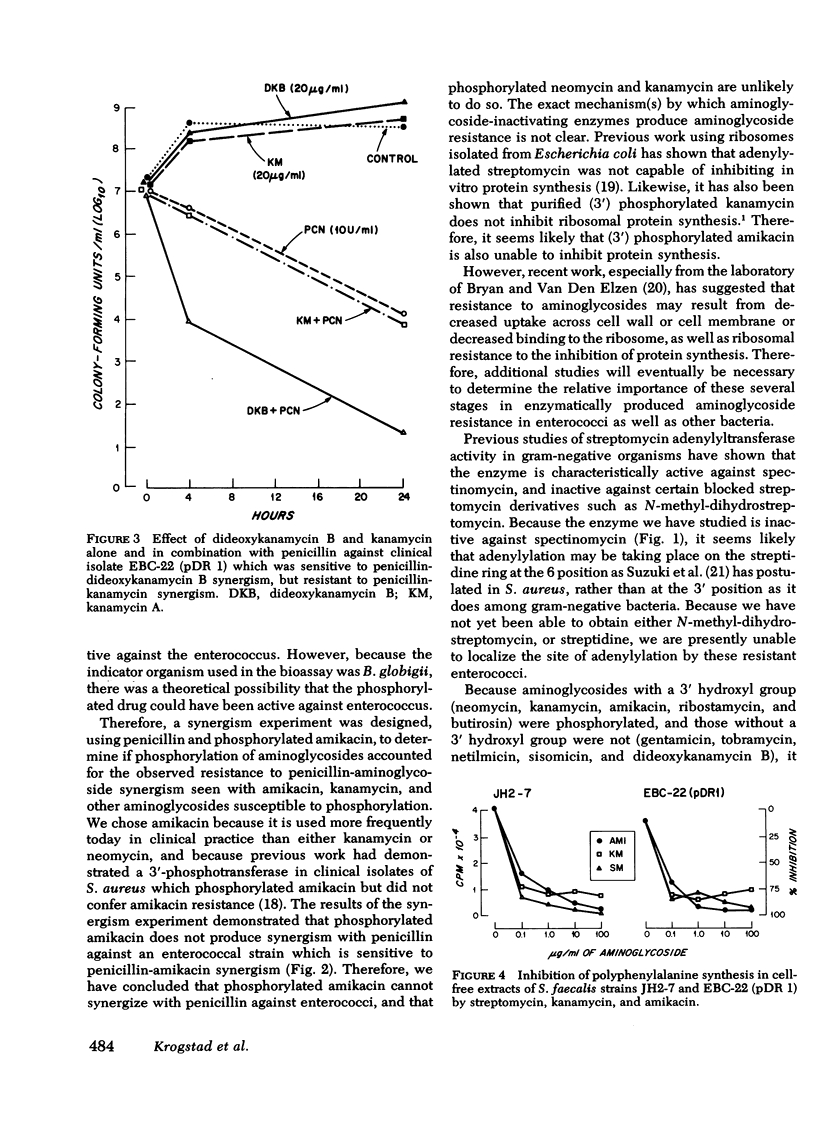
Clinical isolates of enterococci (Streptococcus faecalis) with high-level resistance to both streptomycin and kanamycin (minimal inhibitory concentration >2,000 μg/ml), and resistant to synergism with penicillin and streptomycin or kanamycin were examined for aminoglycoside-inactivating enzymes. All of the 10 strains studied had streptomycin adenylyltransferase and neomycin phosphotransferase activities; the latter enzyme phosphorylated amikacin as well as its normal substrates, such as kanamycin. Substrate profiles of the neomycin phosphotransferase activity suggested that phosphorylation occurred at the 3′-hydroxyl position, i.e., aminoglycoside 3′-phosphotransferase. A transconjugant strain, which acquired high-level aminoglycoside resistance and resistance to antibiotic synergism after mating with a resistant clinical isolate, also acquired both enzyme activities. Quantitative phosphorylation of amikacin in vitro by a sonicate of the transconjugant strain inactivated the antibiotic, as measured by bioassay, and the phosphorylated drug failed to produce synergism when combined with penicillin against a strain sensitive to penicillin-amikacin synergism.
No differences were found in the sensitivity of ribosomes from a sensitive and resistant strain when examined in vitro using polyuridylic acid directed [14C]-phenylalanine incorporation in the presence of streptomycin, kanamycin, or amikacin. Therefore, we conclude that aminoglycoside-inactivating enzymes are responsible for the aminoglycoside resistance, and resistance to antibiotic synergism observed in these strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Effects of membrane-energy mutations and cations on streptomycin and gentamicin accumulation by bacteria: a model for entry of streptomycin and gentamicin in susceptible and resistant bacteria. Antimicrob Agents Chemother. 1977 Aug;12(2):163–177. doi: 10.1128/aac.12.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. A., Wennersten C., Moellering R. C., Jr, Kunz L. J., Krogstad D. J. Resistance to six aminoglycosidic aminocyclitol antibiotics among enterococci: prevalence, evolution, and relationship to synergism with penicillin. Antimicrob Agents Chemother. 1977 Sep;12(3):401–405. doi: 10.1128/aac.12.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Davies J. Plasmid-medicated aminoglycoside phosphotransferase of broad substrate range that phosphorylates amikacin. Antimicrob Agents Chemother. 1977 Apr;11(4):619–624. doi: 10.1128/aac.11.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannini P. B., Ehret J., Eickhoff T. C. Effects of ampicillin-amikacin and ampicillin-rifampin on enterococci. Antimicrob Agents Chemother. 1976 Mar;9(3):448–451. doi: 10.1128/aac.9.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawetz E., Gunnison J. B., Coleman V. R. The Combined Action of Penicillin with Streptomycin or Chloromycetin on Enterococci in Vitro. Science. 1950 Mar 10;111(2880):254–256. doi: 10.1126/science.111.2880.254. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Korfhagen T. R., Moellering R. C., Jr, Wennersten C., Swartz M. N. Plasmid-mediated resistance to antibiotic synergism in enterococci. J Clin Invest. 1978 Jun;61(6):1645–1653. doi: 10.1172/JCI109085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Watson B. K., Kunz L. J. Endocarditis due to group D streptococci. Comparison of disease caused by streptococcus bovis with that produced by the enterococci. Am J Med. 1974 Aug;57(2):239–250. doi: 10.1016/0002-9343(74)90448-3. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Benveniste R., Tipper D., Davies J. Aminoglycoside antibiotics: inactivation by phosphorylation in Escherichia coli carrying R factors. J Bacteriol. 1969 Nov;100(2):1144–1146. doi: 10.1128/jb.100.2.1144-1146.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhen R. W., Darrell J. H. Antibiotic synergism against group D streptococci in the treatment of endocarditis. Med J Aust. 1973 Jul 21;2(3):114–116. doi: 10.5694/j.1326-5377.1973.tb128693.x. [DOI] [PubMed] [Google Scholar]
- Smith D. H. R factors for aminoglycoside antibiotics. J Infect Dis. 1969 Apr-May;119(4):378–380. doi: 10.1093/infdis/119.4-5.378. [DOI] [PubMed] [Google Scholar]
- Standiford H. D., De Maine J. B., Kirby W. M. Antibiotic synergism of enterococci. Relation to inhibitory concentrations. Arch Intern Med. 1970 Aug;126(2):255–259. [PubMed] [Google Scholar]
- Umezawa H., Okanishi M., Kondo S., Hamana K., Utahara R., Maeda K., Mitsuhashi S. Phosphorylative inactivation of aminoglycosidic antibiotics by Escherichia coli carrying R factor. Science. 1967 Sep 29;157(3796):1559–1561. [PubMed] [Google Scholar]
- Umezawa H., Yamamoto H., Yagisawa M., Kondo S., Takeuchi T. Letter: Kanamycin phosphotransferase. I. Mechanism of cross resistance between kanamycin and lividomycin. J Antibiot (Tokyo) 1973 Jul;26(7):407–411. doi: 10.7164/antibiotics.26.407. [DOI] [PubMed] [Google Scholar]
- Umezawa Y., Yagisawa M., Sawa T., Takeuchi T., Umezawa H. Aminoglycoside 3'-phosphotransferase III, a new phosphotransferase. Resistance mechanism. J Antibiot (Tokyo) 1975 Nov;28(11):845–853. doi: 10.7164/antibiotics.28.845. [DOI] [PubMed] [Google Scholar]
- Winters R. E., Litwack K. D., Hewitt W. L. Relation between dose and levels of gentamicin in blood. J Infect Dis. 1971 Dec;124 (Suppl):S90–S95. doi: 10.1093/infdis/124.supplement_1.s90. [DOI] [PubMed] [Google Scholar]
- Yamada T., Tipper D., Davies J. Enzymatic inactivation of streptomycin by R factor-resistant Escherichia coli. Nature. 1968 Jul 20;219(5151):288–291. doi: 10.1038/219288a0. [DOI] [PubMed] [Google Scholar]
- Zimmermann R. A., Moellering R. C., Jr, Weinberg A. N. Mechanism of resistance to antibiotic synergism in enterococci. J Bacteriol. 1971 Mar;105(3):873–879. doi: 10.1128/jb.105.3.873-879.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


