Abstract
The Clinical Islet Transplantation 07 (CIT07) protocol uses antithymocyte globulin and etanercept induction, islet culture, heparinization, and intensive insulin therapy with the same low-dose tacrolimus and sirolimus maintenance immunosuppression as in the Edmonton protocol. To determine whether CIT07 improves engrafted islet β-cell mass, our center measured β-cell secretory capacity from glucose-potentiated arginine tests at days 75 and 365 after transplantation and compared those results with the results previously achieved by our group using the Edmonton protocol and normal subjects. All subjects were insulin free, with CIT07 subjects receiving fewer islet equivalents from a median of one donor compared with two donors for Edmonton protocol subjects. The acute insulin response to glucose-potentiated arginine (AIRpot) was greater in the CIT07 protocol than in the Edmonton protocol and was less in both cohorts than in normal subjects, with similar findings for C-peptide. The CIT07 subjects who completed reassessment at day 365 exhibited increasing AIRpot by trend relative to that of day 75. These data indicate that engrafted islet β-cell mass is markedly improved with the CIT07 protocol, especially given more frequent use of single islet donors. Although several peritransplant differences may have each contributed to this improvement, the lack of deterioration in β-cell secretory capacity over time in the CIT07 protocol suggests that low-dose tacrolimus and sirolimus are not toxic to islets.
Islet transplantation is an emerging cell therapy for the treatment of type 1 diabetes (1), particularly for those patients experiencing severe problems with hypoglycemia or who have already received a kidney transplant. The Edmonton protocol for islet transplantation established that glucocorticoid-free immunosuppression together with a subsequent islet infusion from a second donor pancreas could reproducibly render the recipient insulin independent (2). A multicenter trial using this approach resulted in insulin independence in 60% of recipients, although the majority of these patients returned to insulin therapy by 2 years posttransplant (3). Nonetheless, 80% of recipients maintained islet graft function as indicated by a reduction in insulin requirements and C-peptide production for the 2 years of follow-up (3). Using the Edmonton protocol, we previously reported that despite receiving almost 1 million islets, insulin-independent recipients had a β-cell secretory capacity only ∼25% that of normal (4). The lower functional islet β-cell mass for the numbers transplanted suggested early loss of transplanted islets before engraftment that might be attributable to nonspecific inflammatory and thrombotic mechanisms (5). This reduced engrafted islet β-cell mass is just at the margin of what is required to avoid hyperglycemia (6); therefore, it likely explains the eventual return to insulin therapy experienced by the majority of recipients treated by the Edmonton protocol.
More recent induction immunosuppression protocols introduced by the University of Minnesota have incorporated peritransplant anti-inflammatory and antithrombotic therapy with similar low-dose calcineurin inhibitor and mammalian target of rapamycin (mTOR) inhibitor maintenance therapy as in the Edmonton protocol, with improved rates of insulin independence occurring more frequently with islets isolated from a single donor (7) and being sustained for a longer duration (8,9). The multicenter Clinical Islet Transplantation 07 (CIT07) protocol uses components of the Minnesota approach, including the polyclonal T-cell–depleting antibody rabbit antithymocyte globulin (rATG) and the tumor necrosis factor-α (TNF-α) inhibitor etanercept at induction, an islet culture period of 36–72 h, full heparinization peritransplant, pentoxifylline and anticoagulation for 1 week (7), intensive insulin therapy for 2 months, and the same low-dose tacrolimus and sirolimus (formerly called rapamycin) for maintenance immunosuppression as in the Edmonton protocol (10). We sought to determine whether the peritransplant changes in the CIT07 protocol improved engrafted islet β-cell mass by measuring β-cell secretory capacity from glucose-potentiated arginine tests in CIT07 subjects who underwent transplantation at the University of Pennsylvania at 75 and 365 days after their final islet infusion, and comparing their results with those previously achieved at our center with the Edmonton protocol.
RESEARCH DESIGN AND METHODS
Subjects included in this study were historical and previously reported insulin-independent islet transplant recipients from the Penn-JDRF study of the Edmonton protocol (n = 5) who underwent islet transplantation between 2002 and 2003 (4,11), islet transplant recipients from the CIT07 protocol at the University of Pennsylvania (n = 11) who received islet transplants between 2008 and 2012, and normal control subjects (n = 11) studied in our laboratory between 2003 and 2011. Both study protocols were approved by the Institutional Review Board of the University of Pennsylvania, and all subjects gave their written informed consent to participate. All transplant recipients had long-standing C-peptide–negative type 1 diabetes complicated by hypoglycemia unawareness and frequent severe hypoglycemia events. Subjects underwent one or two intraportal infusions of ABO-compatible islets to achieve insulin independence. Although no attempt was made at HLA matching, each subject had a negative panel-reactive antibody assessment and a negative cross-match against donor T cells and B cells before each islet infusion as described previously (12).
Induction therapy for the Edmonton protocol followed that reported by Shapiro et al. (2) and used the interleukin-2 (IL-2) receptor antagonist daclizumab: 1 mg/kg on day 0, repeated every 2 weeks for a total of 5 doses. Islets were isolated using a modified Ricordi method (13) with Liberase enzyme (Roche, Indianapolis, IN) (14) as previously described (15). Islets were cultured temporarily before transplantation and infused in <24 h via percutaneous transhepatic portal vein catheterization in the interventional radiology department. The islet product contained 35 units/kg heparin to prevent portal vein thrombosis. Insulin therapy was discontinued posttransplant and resumed if the fasting glucose was >125 mg/dL or if postprandial blood glucose was >200 mg/dL. The glucose values prompting insulin therapy also served as criteria for receiving a second islet infusion (2). If a second islet infusion occurred >10 weeks after the first, then the course of daclizumab was repeated.
Induction therapy for the CIT07 protocol followed that reported by Hering et al. (7) and included rATG up to 6 mg/kg in divided doses from day −2 to day 2 from transplantation and etanercept 50 mg intravenously 1 h pretransplantation and 25 mg subcutaneously on days 3, 7, and 10 after transplantation. Pentoxifylline slow-release was administered as 400 mg orally three times daily from day −2 to day 7 posttransplantation (7). Islets were again isolated using a modification of the Ricordi method (13), but with Serva collagenase (Heidelberg, Germany) (16) replacing the Liberase enzyme. Islets were cultured for 36–72 h to allow the first doses of rATG to be completed before transplantation and were subsequently infused via percutaneous transhepatic portal vein catheterization. The islet product contained 70 units/kg heparin to prevent portal vein thrombosis, and prophylactic anticoagulation was continued with intravenous heparin (target partial thromboplastin time 50–60 s) for 48 h, followed by enoxaparin 30 mg subcutaneously twice daily through day 7 (7). Subjects transitioned from subcutaneous insulin to intravenous insulin at least 2 h before transplantation and were transitioned back to subcutaneous insulin the next morning. Intensive insulin therapy was maintained for 2 months after an initial islet infusion and for at least 1 month in cases of a second islet infusion. For second islet infusions, 20 mg IL-2 receptor antagonist basiliximab was administered on days 0 and 4 from transplantation in place of rATG. One previously described subject (17) presented with rATG hypersensitivity after receiving 3 mg/kg of the drug and before islet infusion, which was cancelled; 5 months later, she underwent islet transplantation with basiliximab in place of rATG. After each islet infusion, insulin independence was determined at day 75 if, after tapering off insulin for >1 week, the fasting glucose was ≤126 mg/dL and if a 90-min mixed-meal tolerance test glucose was ≤180 mg/dL, among other criteria (10).
Maintenance immunosuppression was equivalent in both the Edmonton and CIT07 protocols, consisting of low-dose tacrolimus (12-h blood trough target 3–6 µg/L) and sirolimus (24-h blood trough target 10–15 µg/L for the first 3 months and 8–12 µg/L thereafter).
Glucose-potentiated arginine test.
All subjects were admitted to the University of Pennsylvania Clinical and Translational Research Center the afternoon before study and fasted overnight after 8:00 p.m. for 12 h before testing. By 7:00 a.m., one catheter was placed in an antecubital vein for infusions and one catheter was placed in a hand vein for blood sampling, with the hand placed in a thermoregulated box (∼50°C) or heating pad to promote arterialization of venous blood. After at least 20 min of acclimatization to the intravenous catheters, baseline blood samples were taken at −5 and −1 min before the injection of 5 g of 10% arginine over a 1-min period starting at 0 min. Additional blood samples were collected at 2, 3, 4, and 5 min after injection. Beginning at 10 min, a hyperglycemic clamp technique (18) using a variable rate infusion of 20% glucose was performed to achieve a plasma glucose concentration of ∼230 mg/dL. Blood samples were taken every 5 min, centrifuged, and measured at bedside with an automated glucose analyzer (YSI 2300; Yellow Springs Instruments, Yellow Springs, OH) to adjust the infusion rate and to achieve the desired plasma glucose concentration. After 45 min of the glucose infusion (at 55 min), a 5-g arginine pulse was injected again with identical blood sampling, ending at 60 min. It has been demonstrated that the first injection of arginine has no effect on the subsequent response to arginine using this protocol (19).
Biochemical analysis.
All samples were collected on ice into tubes containing EDTA, trasylol, and leupeptin (Protease Inhibitor Cocktail; Sigma-Aldrich, St. Louis, MO), centrifuged at 4°C, separated, and frozen at −80°C until subsequent analysis. Plasma glucose was measured in duplicate by the glucose oxidase method using an automated glucose analyzer (YSI 2300; Yellow Springs Instruments). Plasma insulin and C-peptide were measured in duplicate by double-antibody radioimmunoassay (Millipore, Billerica, MA).
Calculations and statistics.
The acute insulin response to arginine (AIRarg) and acute C-peptide response to arginine (ACRarg) were calculated as the mean of the 2-, 3-, 4-, and 5-min values minus the mean of the prestimulus values (18,19). Acute responses during the ∼230 mg/dL glucose clamp enable determination of the acute insulin response to glucose-potentiated arginine (AIRpot) and the acute C-peptide response to glucose-potentiated arginine (ACRpot). We previously have shown in islet transplant recipients that AIRpot is highly predictive of the maximal acute insulin response during a ∼340 mg/dL glucose clamp (r2 = 0.98; P < 0.0001) (20), and therefore represents a valid measure of β-cell secretory capacity in this population.
All data are expressed as mean ± SE. Comparison of results across groups was performed by one-way ANOVA or Kruskal-Wallis ANOVA for nonparametric data; when significant differences were found, comparisons between groups were performed with unpaired Student t tests or the Mann-Whitney U test as appropriate using Statistica software (StatSoft, Tulsa, OK). Statistical significance was defined as P < 0.05 (two-tailed).
RESULTS
Subject characteristics.
The Edmonton cohort had undergone islet transplantation between 2002 and 2003 and the CIT07 cohort had undergone islet transplantation between 2008 and 2012 at the Hospital of the University of Pennsylvania. The Edmonton, CIT07, and normal control groups were of comparable sex distribution, age, BMI, and kidney function (Table 1). All transplant recipients were free from exogenous insulin use at the time of study 90 and 75 days after the last islet infusion for the Edmonton and CIT07 cohorts, respectively, with one subject in the Edmonton cohort initially studied 365 days posttransplantation. The two cohorts of islet transplant recipients had a similarly long duration of type 1 diabetes, but the Edmonton cohort received more islet equivalents, with four out of five subjects requiring a second islet infusion compared with only four of 11 subjects in the CIT07 cohort (P < 0.05; Table 1). Islet donors used during the Edmonton protocol were older (P < 0.05), with isolations resulting in fewer islet equivalents per gram of donor pancreas (P < 0.05), although measures of islet preparation purity, viability, and glucose-stimulated insulin release were not different between the Edmonton and CIT07 groups (Table 1). The HbA1c in the Edmonton cohort was higher than normal (P < 0.01; Table 1) but not statistically different from that of the CIT07 cohort, which was comparable with the normal group. Fasting glucose was greater than normal in both the Edmonton and CIT07 cohorts (P < 0.01 for both; Table 1) but was not different between the Edmonton and CIT07 cohorts. Fasting insulin, but not C-peptide, was higher in the CIT07 cohort than in both the Edmonton cohort and normal group (P < 0.01 for both; Table 1). There was no difference in blood trough concentrations of tacrolimus or sirolimus between the Edmonton and CIT07 cohorts. One of the CIT07 subjects had conversion from sirolimus to mycophenolate mofetil 500 mg twice daily with a tacrolimus trough target of 6–9 µg/L because of the development of interstitial pneumonia (21) 4 weeks posttransplantation that subsequently resolved.
TABLE 1.
Characteristics of islet transplant recipients and control subjects at the time of initial glucose-potentiated arginine testing after the final islet infusion and islet preparations
Glucose, insulin, and C-peptide.
Fasting glucose, insulin, and C-peptide levels given in Table 1 are the prestimulus levels before the first injection of arginine at the initial time of assessment. The prestimulus glucose during the 230 mg/dL glucose clamp was not different across the Edmonton, CIT07, and normal groups (230 ± 15 vs. 236 ± 5 vs. 236 ± 5 mg/dL). The prestimulus insulin and C-peptide levels during the 230-mg/dL glucose clamp provide measures of second-phase β-cell responsiveness (Fig. 1). Second-phase insulin was less in the Edmonton group (11) than in the CIT07 group, and in the CIT07 group it was greater than that of the normal group (18 ± 3 vs. 40 ± 6 vs. 23 ± 3 µU/mL; P < 0.01 for Edmonton and normal vs. CIT07). Second-phase C-peptide was also less in Edmonton than in CIT07, but in CIT07 was not different from normal (1.4 ± 0.4 vs. 3.7 ± 0.6 vs. 3.5 ± 0.4 ng/mL; P < 0.05 for both vs. Edmonton).
FIG. 1.
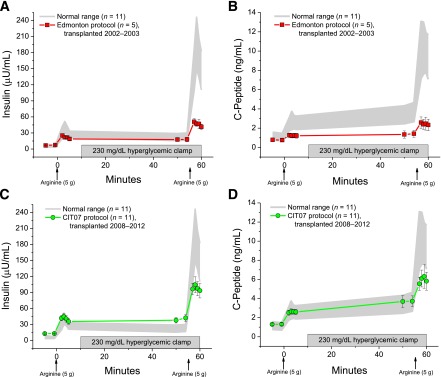
Plasma insulin (A and C) and C-peptide (B and D) levels in response to bolus injections of arginine (arrows) administered under fasting and 230 mg/dL hyperglycemic clamp conditions at day 90 posttransplantation in the Edmonton protocol subjects (A and B) and at day 75 posttransplantation in the CIT07 (C, D) protocol subjects. The Edmonton cohort underwent islet transplantation between 2002 and 2003, and the CIT07 cohort underwent islet transplantation between 2008 and 2012. The C-peptide (4) and insulin (11) data for the Edmonton group previously have been reported. The gray shaded area gives the 95% CI for levels in the normal control group.
For the initial time of assessment in the Edmonton cohort, the previously reported acute insulin (11) and C-peptide (4) responses are given in Fig. 1A and B; the acute insulin and C-peptide responses for the CIT07 cohort are given in Fig. 1C and D. The AIRarg was not different across the groups (15 ± 2 vs. 28 ± 4 vs. 28 ± 3 µU/mL), whereas the AIRpot was less in the Edmonton cohort compared with the CIT07 cohort (29 ± 3 vs. 58 ± 7 µU/mL; P < 0.05), and less in both transplant cohorts than in the normal group (143 ± 15 µU/mL; P < 0.01 for both comparisons). In Fig. 1B, the ACRarg was lower in the Edmonton group than in the CIT07 group or normal group (0.5 ± 0.1 vs. 1.3 ± 0.2 vs. 1.5 ± 0.2 ng/mL; P < 0.01 for both comparisons); the ACRpot was less in the Edmonton group than in the CIT07 group (1.1 ± 0.2 vs. 2.2 ± 0.3 ng/mL; P < 0.05), and less in both transplant cohorts compared with the normal group (6.4 ± 0.8 ng/mL; P < 0.01 for both comparisons).
Reassessment at 365 days.
For all islet recipients who underwent reassessment, there was no difference in the prestimulus glucose during the 230 mg/dL glucose clamp (228 ± 5 vs. 224 ± 5 mg/dL), with the between-subject difference <2% of the mean. Of the Edmonton protocol subjects, one returned to insulin soon after the initial study and lost all islet graft function by 365 days, one returned to insulin at 9 months and received a second islet infusion, two returned to insulin at 11 months and exhibited declines in β-cell secretory capacity at 365 days, and one initially studied at 365 days remained insulin independent at 730 days with stable β-cell secretory capacity (Fig. 2A). One of these subjects had discontinued sirolimus because of associated colitis and was previously reported to have developed islet donor–specific alloantibodies coincident with the decline in β-cell secretory capacity (22). All of the CIT07 protocol–treated subjects have remained insulin-independent, with all nine who have completed the day 365 assessment demonstrating stable β-cell secretory capacity (Fig. 2B). Reproducibility of the hyperglycemic clamp conditions from day 75 to day 365 in the CIT07 cohort has been demonstrated (Fig. 3A and B). In Fig. 3C, the AIRarg at days 75 and 365 remained normal (29 ± 5 vs. 35 ± 6 vs. 28 ± 3 µU/mL), and the AIRpot increased by trend from day 75 to day 365 (60 ± 9 vs. 79 ± 11 µU/mL; P = 0.05), although both were less than normal (143 ± 15 µU/mL; P < 0.01 for both comparisons). In Fig. 3D, similar results are seen for ACRarg (1.3 ± 0.2 vs. 1.4 ± 0.3 vs. 1.5 ± 0.2 ng/mL) and ACRpot (2.4 ± 0.4 vs. 2.7 ± 0.5 vs. 6.4 ± 0.8 ng/mL; P < 0.01 for both comparisons), again exhibiting a notable absence of deterioration with time.
FIG. 2.
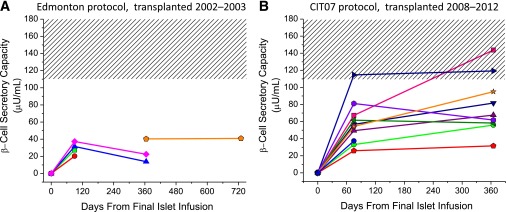
β-Cell secretory capacity measured as the AIRpot in the Edmonton protocol subjects (A) and in the CIT07 protocol subjects (B) over time after transplantation. The Edmonton cohort underwent islet transplantation between 2002 and 2003, and the CIT07 cohort underwent islet transplantation between 2008 and 2012. The hashed area gives the 95% CI for the β-cell secretory capacity in the normal control group. Of the Edmonton protocol subjects, one returned to insulin soon after initial study and lost all islet graft function by 365 days (□), one returned to insulin at 9 months and received a second islet infusion (○), two returned to insulin at 11 months and exhibited declines in β-cell secretory capacity at 365 days (△, ◇), and one initially studied at 365 days remained insulin independent at 730 days with stable β-cell secretory capacity (⌂). All nine of the CIT07 protocol subjects to have reached 365 days remained insulin independent.
FIG. 3.
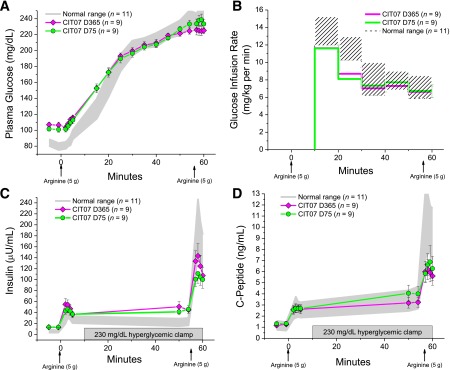
Plasma glucose (A) and glucose infusion rates (B) during conduct of the glucose-potentiated arginine test, and insulin (C) and C-peptide (D) levels in response to bolus injections of arginine (arrows) administered under fasting and 230 mg/dL hyperglycemic clamp conditions at day 75 and day 365 posttransplantation in the CIT07 protocol subjects. The gray shaded and hashed areas give the 95% CIs for levels in the normal control group.
DISCUSSION
The current study demonstrates a significant improvement in β-cell secretory capacity after islet transplantation using the CIT07 protocol compared with historical use of the Edmonton protocol. Because β-cell secretory capacity provides the best estimate of functional β-cell mass (23,24), these results provide evidence for improved islet engraftment with the CIT07 protocol, an even more remarkable finding when one considers that fewer islets were transplanted, most often from a single donor pancreas, using the CIT07 protocol. Importantly, and in contrast to experience with the Edmonton protocol, there was a distinct absence of deterioration of the β-cell secretory capacity in the CIT07 protocol subjects between 75 and 365 days posttransplantation. These findings suggest that a reserve capacity for insulin secretion more than that necessary to achieve initial insulin independence is required to maintain long-term islet graft function. This reserve capacity may protect islets from excessive metabolic demand inducing endoplasmic reticulum stress (25) or amyloid deposition (26), which have been described in preclinical islet transplant models. Moreover, this sustainable β-cell secretory capacity in the CIT07 protocol subjects is evident despite the use of the same low-dose tacrolimus and sirolimus maintenance immunosuppression as in the Edmonton protocol. We previously have shown that the β-cell secretory capacity is fully normal in uncomplicated whole pancreas transplant recipients who have received 100% of an islet β-cell mass despite treatment with tacrolimus-based immunosuppression (11). Thus, these current data are consistent with the interpretation that modern dosing of currently available immunotherapy is not toxic to islets.
Because the maintenance immunosuppression therapy was identical in both protocols, the superior β-cell secretory capacity with CIT07 is best explained by its peritransplant differences from the Edmonton protocol. The rATG has proved more efficacious at suppressing alloimmunity than an IL-2 receptor antagonist (27), and it has been shown to affect autoimmunity in recent-onset type 1 diabetes (28). However, its use in islet transplantation previously had been limited by the requirement for glucocorticoid before the first dose to ameliorate symptoms of cytokine release from lysed T cells, both known to be detrimental to islets by increasing secretory demand and by direct toxicity, respectively (29,30). With the introduction of a period of islet culture, Hering et al. (7) showed that by administering the first dose of rATG with glucocorticoid >36 h before islet infusion, a high rate of single-donor insulin independence could be achieved. In addition, initiation of the TNF-α inhibitor etanercept before islet infusion also is likely to mitigate any residual cytokine effects or nonspecific inflammation related to intraportal islet delivery (31). In fact, Bellin et al. (9) demonstrated that induction regimens based on a T-cell–depleting antibody resulted in superior long-term insulin independence when compared with use of an IL-2 receptor antagonist, but only when combined with a TNF-α inhibitor. It is also possible that peritransplant administration of pentoxifylline, which can inhibit TNF-α production (32), may contribute to anti-inflammatory efficacy. Our data support that the prolonged insulin independence reported with combined T-cell–depletion and TNF-α inhibitor is, at least in part, attributable to improved engraftment of a sufficient islet β-cell mass to sustain long-term function.
Another peritransplant difference in the CIT07 protocol is the extension of anticoagulation beyond the islet infusion procedure. Because isolated islets express tissue factor that may induce an instant blood-mediated inflammatory reaction (33,34), anticoagulation beyond what is required to prevent portal vein thrombosis may further decrease localized inflammation and microthrombus formation during islet revascularization and engraftment. Koh et al. (35) showed by multivariate analysis that the combined use of peritransplant infusions of heparin and insulin was associated with a higher rate of single-donor insulin independence. In CIT07, the peritransplant heparin and insulin infusions were followed by low-molecular-weight heparin until day 7 and intensive insulin therapy for 2 months before attempting to taper. Because complete functional recovery of transplanted islets is not expected for several weeks after transplantation (36), we believe that intensive insulin therapy to avoid glucotoxicity (37,38) and to reduce β-cell demand during this relatively hypoxic period before revascularization is essential to optimizing islet survival during the engraftment process. Although this study cannot determine to what extent each of these peritransplant differences may have contributed to the improved β-cell secretory capacity with the CIT07 protocol, the findings nonetheless demonstrate that marked improvements in islet β-cell engraftment may be achieved through refinements in peritransplant management.
An important limitation to the current study is that the comparison of CIT07 protocol subjects with a historical group treated with the Edmonton protocol does not account for immeasurable confounding factors that were different during the two periods of transplantation and may have affected the results. One likely confounder is potential differences in the quality rather than quantity of the islet preparations. Although there was no difference in the in vitro purity, viability, or glucose-stimulated insulin release measures between the islet preparations transplanted during the Edmonton protocol compared with the CIT07 protocol, the younger donor age and modestly increased islet yield per gram of donor pancreas in the CIT07 group might indicate higher-quality pancreata or more efficient islet isolation related to changes in the available collagenase enzymes used for digestion. Other reports have demonstrated better in vivo islet function results with use of younger (younger than 40–45 years of age) relative to older donors (39,40), and with higher islet yield per gram of donor pancreas with the newer collagenase enzymes used in CIT07 compared with those used with the Edmonton protocol (41). Finally, the introduction of a pretransplant islet culture period with the CIT07 protocol may have resulted in a less immunogenic islet graft because previous work has demonstrated an effect of islet culture on decreasing the number of passenger leukocytes and islet expression of class I HLA (42).
Interestingly, fasting and second-phase insulin, but not C-peptide, levels were increased in CIT07 subjects; this finding was not present in the Edmonton subjects. We (43) and others (44) have shown that insulin sensitivity is normal in islet transplant recipients receiving low-dose tacrolimus and sirolimus immunosuppression, a finding confirmed in our CIT07 subjects (manuscript under revision); therefore, insulin resistance leading to increased insulin secretion cannot explain this finding. Moreover, the normal fasting and second-phase C-peptide levels in the CIT07 subjects support an absence of increased insulin secretory demand on the β-cell and suggest a decrease in insulin clearance. A previous study that assessed hepatic extraction of insulin from intraportally transplanted islets did not detect a difference from normal (45), although the number of subjects studied was small and, similar to our Edmonton protocol group, likely had low engrafted islet β-cell masses that may not secrete a sufficient quantity of insulin to detect variations in hepatic extraction. In a canine auto-islet transplant model, treatment with sirolimus was associated with a 13% reduction in insulin clearance and no change in insulin sensitivity (46), a modest effect consistent with our results. Whether a potential decrease in hepatic insulin extraction in human islet recipients may be attributable to sirolimus or the intrahepatic site of transplantation requires further study.
In conclusion, β-cell secretory capacity, a measure of functional islet β-cell mass, is markedly improved with current use of the CIT07 protocol. These functional results are superior to what has been previously reported and were achieved with a lower number of islets, more often from a single donor pancreas, and have been stable for 1 year posttransplantation. Reducing the number of donor pancreata required to achieve insulin independence is important because the use of multiple donors with a greater number of HLA class I mismatches appears to be associated with an increased risk for HLA sensitization if a reduction or discontinuation of immunosuppression is required in the future (12,47). Although several peritransplant differences between the present CIT07 and historical Edmonton protocols each may have contributed to the improved engrafted islet β-cell mass, the lack of deterioration in β-cell secretory capacity over time in the CIT07 protocol suggests the achievement of a sufficient reserve capacity for insulin secretion necessary to resist metabolic exhaustion of the islet graft and any purported toxicity from the required immunosuppression.
Acknowledgments
This work was performed as a project of the Clinical Islet Transplantation Consortium, a collaborative clinical research program headquartered at the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Allergy and Infectious Diseases, and was supported by Public Health Services Research Grants U01 DK070430 (to A.N.), UL1 RR024134 (University of Pennsylvania Clinical and Translational Research Center), and P30 DK19525 (University of Pennsylvania Diabetes Research Center), by the Schiffrin award in autoimmune research (to M.R.R.), and by the Humpton Fellowship award (to S.A.S.).
No potential conflicts of interest relevant to this article were reported.
M.R.R. designed and conducted the study, researched data, and wrote the manuscript. C.L. participated in the conduct of the study, researched data, and revised the manuscript critically for important intellectual content. R.D.S.-G., S.A.S., K.V., M.K., Z.M., E.M., M.P., C.D.-B., C.F., and A.J.C. participated in the conduct of the study, researched data, and reviewed and edited the manuscript. C.F.B. contributed to the design of the study and the discussion and reviewed and edited the manuscript. E.T.L.P. contributed to the design of the study, participated in its conduct, researched data, and revised the manuscript critically for important intellectual content. A.N. designed and conducted the study, researched data, and revised the manuscript critically for important intellectual content. M.R.R. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are indebted to the islet recipients for their participation; to the nursing staff of the Transplant Unit, Interventional Radiology Suite, and Clinical and Translational Research Center for their subject care and technical assistance; to Dr. Heather Collins of the University of Pennsylvania Diabetes Research Center for performance of the radioimmunoassays; and to Huong-Lan Nguyen at the Monell Chemical Senses Center for laboratory assistance.
REFERENCES
- 1.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care 2012;35:1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343:230–238 [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AMJ, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318–1330 [DOI] [PubMed] [Google Scholar]
- 4.Rickels MR, Schutta MH, Markmann JF, Barker CF, Naji A, Teff KL. β-Cell function following human islet transplantation for type 1 diabetes. Diabetes 2005;54:100–106 [DOI] [PubMed] [Google Scholar]
- 5.Eriksson O, Eich T, Sundin A, et al. Positron emission tomography in clinical islet transplantation. Am J Transplant 2009;9:2816–2824 [DOI] [PubMed] [Google Scholar]
- 6.Greenbaum CJ, Prigeon RL, D’Alessio DA. Impaired beta-cell function, incretin effect, and glucagon suppression in patients with type 1 diabetes who have normal fasting glucose. Diabetes 2002;51:951–957 [DOI] [PubMed] [Google Scholar]
- 7.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA 2005;293:830–835 [DOI] [PubMed] [Google Scholar]
- 8.Bellin MD, Kandaswamy R, Parkey J, et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes [corrected in Am J Transplant 2010;10:1337]. Am J Transplant 2008;8:2463–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellin MD, Barton FB, Heitman A, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant 2012;12:1576–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CIT website [Internet], 2011. Available from http://www.isletstudy.org/ Accessed 1 May 2012
- 11.Rickels MR, Mueller R, Teff KL, Naji A. β-Cell secretory capacity and demand in recipients of islet, pancreas, and kidney transplants. J Clin Endocrinol Metab 2010;95:1238–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rickels MR, Kearns J, Markmann E, et al. HLA sensitization in islet transplantation. In Clinical Transplants 2006 Cecka JM, Terasaki PI, Angles L, Eds. Los Angeles, CA, UCLA Tissue Typing Laboratory, 2007, p. 413–420 [PMC free article] [PubMed] [Google Scholar]
- 13.Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes 1989;38(Suppl. 1):140–142 [DOI] [PubMed] [Google Scholar]
- 14.Linetsky E, Bottino R, Lehmann R, Alejandro R, Inverardi L, Ricordi C. Improved human islet isolation using a new enzyme blend, liberase. Diabetes 1997;46:1120–1123 [DOI] [PubMed] [Google Scholar]
- 15.Markmann JF, Deng S, Huang X, et al. Insulin independence following isolated islet transplantation and single islet infusions. Ann Surg 2003;237:741–749; discussion 749–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bucher P, Mathe Z, Bosco D, et al. Serva collagenase NB1: a new enzyme preparation for human islet isolation. Transplant Proc 2004;36:1143–1144 [DOI] [PubMed] [Google Scholar]
- 17.Soleimanpour SA, Sekiguchi DR, LaRosa DF, Luning Prak ET, Naji A, Rickels MR. Hypersensitivity to rabbit antithymocyte globulin in an islet transplant recipient: a case report. Transplant Proc 2011;43:3302–3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward WK, Halter JB, Beard JC, Porte D., Jr Adaptation of B and A cell function during prolonged glucose infusion in human subjects. Am J Physiol 1984;246:E405–E411 [DOI] [PubMed] [Google Scholar]
- 19.Larsson H, Ahrén B. Glucose-dependent arginine stimulation test for characterization of islet function: studies on reproducibility and priming effect of arginine. Diabetologia 1998;41:772–777 [DOI] [PubMed] [Google Scholar]
- 20.Rickels MR, Naji A, Teff KL. Acute insulin responses to glucose and arginine as predictors of β-cell secretory capacity in human islet transplantation. Transplantation 2007;84:1357–1360 [DOI] [PubMed] [Google Scholar]
- 21.Digon BJ, 3rd, Rother KI, Hirshberg B, Harlan DM. Sirolimus-induced interstitial pneumonitis in an islet transplant recipient (Letter). Diabetes Care 2003;26:3191. [DOI] [PubMed] [Google Scholar]
- 22.Rickels MR, Kamoun M, Kearns J, Markmann JF, Naji A. Evidence for allograft rejection in an islet transplant recipient and effect on β-cell secretory capacity. J Clin Endocrinol Metab 2007;92:2410–2414 [DOI] [PubMed] [Google Scholar]
- 23.Robertson RP. Estimation of beta-cell mass by metabolic tests: necessary, but how sufficient? Diabetes 2007;56:2420–2424 [DOI] [PubMed] [Google Scholar]
- 24.Kahn SE, Carr DB, Faulenbach MV, Utzschneider KM. An examination of beta-cell function measures and their potential use for estimating beta-cell mass. Diabetes Obes Metab 2008;10(Suppl. 4):63–76 [DOI] [PubMed] [Google Scholar]
- 25.Negi S, Park SH, Jetha A, Aikin R, Tremblay M, Paraskevas S. Evidence of endoplasmic reticulum stress mediating cell death in transplanted human islets. Cell Transplant 2012;21:889–900 [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Koeberlein B, Feldman MD, et al. Accumulation of intrahepatic islet amyloid in a nonhuman primate transplant model. Endocrinology 2012;153:1673–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan DC, Schnitzler MA. Long-term results of rabbit antithymocyte globulin and basiliximab induction. N Engl J Med 2008;359:1736–1738 [DOI] [PubMed] [Google Scholar]
- 28.Eisenbarth GS, Srikanta S, Jackson R, et al. Anti-thymocyte globulin and prednisone immunotherapy of recent onset type 1 diabetes mellitus. Diabetes Res 1985;2:271–276 [PubMed] [Google Scholar]
- 29.Rabinovitch A, Sumoski W, Rajotte RV, Warnock GL. Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J Clin Endocrinol Metab 1990;71:152–156 [DOI] [PubMed] [Google Scholar]
- 30.Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab 1982;54:131–138 [DOI] [PubMed] [Google Scholar]
- 31.Farney AC, Xenos E, Sutherland DER, et al. Inhibition of pancreatic islet beta cell function by tumor necrosis factor is blocked by a soluble tumor necrosis factor receptor. Transplant Proc 1993;25:865–866 [PubMed] [Google Scholar]
- 32.Strieter RM, Remick DG, Ward PA, et al. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun 1988;155:1230–1236 [DOI] [PubMed] [Google Scholar]
- 33.Johansson H, Lukinius A, Moberg L, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes 2005;54:1755–1762 [DOI] [PubMed] [Google Scholar]
- 34.Moberg L, Johansson H, Lukinius A, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet 2002;360:2039–2045 [DOI] [PubMed] [Google Scholar]
- 35.Koh A, Senior P, Salam A, et al. Insulin-heparin infusions peritransplant substantially improve single-donor clinical islet transplant success. Transplantation 2010;89:465–471 [DOI] [PubMed] [Google Scholar]
- 36.Rickels MR. Recovery of endocrine function after islet and pancreas transplantation. Curr Diab Rep 2012;12:587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davalli AM, Ricordi C, Socci C, et al. Abnormal sensitivity to glucose of human islets cultured in a high glucose medium: partial reversibility after an additional culture in a normal glucose medium. J Clin Endocrinol Metab 1991;72:202–208 [DOI] [PubMed] [Google Scholar]
- 38.Dubois S, Madec AM, Mesnier A, et al. Glucose inhibits angiogenesis of isolated human pancreatic islets. J Mol Endocrinol 2010;45:99–105 [DOI] [PubMed] [Google Scholar]
- 39.Ihm SH, Matsumoto I, Sawada T, et al. Effect of donor age on function of isolated human islets. Diabetes 2006;55:1361–1368 [DOI] [PubMed] [Google Scholar]
- 40.Niclauss N, Bosco D, Morel P, et al. Influence of donor age on islet isolation and transplantation outcome. Transplantation 2011;91:360–366 [DOI] [PubMed] [Google Scholar]
- 41.Bucher P, Mathe Z, Morel P, et al. Assessment of a novel two-component enzyme preparation for human islet isolation and transplantation. Transplantation 2005;79:91–97 [DOI] [PubMed] [Google Scholar]
- 42.Markmann JF, Tomaszewski J, Posselt AM, et al. The effect of islet cell culture in vitro at 24 degrees C on graft survival and MHC antigen expression. Transplantation 1990;49:272–277 [DOI] [PubMed] [Google Scholar]
- 43.Rickels MR, Naji A, Teff KL. Insulin sensitivity, glucose effectiveness, and free fatty acid dynamics after human islet transplantation for type 1 diabetes. J Clin Endocrinol Metab 2006;91:2138–2144 [DOI] [PubMed] [Google Scholar]
- 44.Hirsch D, Odorico J, Radke N, et al. Correction of insulin sensitivity and glucose disposal after pancreatic islet transplantation: preliminary results. Diabetes Obes Metab 2010;12:994–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meier JJ, Hong-McAtee I, Galasso R, et al. Intrahepatic transplanted islets in humans secrete insulin in a coordinate pulsatile manner directly into the liver. Diabetes 2006;55:2324–2332 [DOI] [PubMed] [Google Scholar]
- 46.Kneteman NM, Lakey JR, Wagner T, Finegood D. The metabolic impact of rapamycin (sirolimus) in chronic canine islet graft recipients. Transplantation 1996;61:1206–1210 [DOI] [PubMed] [Google Scholar]
- 47.Naziruddin B, Wease S, Stablein D, et al. HLA class I sensitization in islet transplant recipients: report from the Collaborative Islet Transplant Registry. Cell Transplant 2012;21:901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]


