All forms of life share the ability to sense environmental change and respond in real time by engaging adaptive homeostatic programs. In higher organisms, the composition of consumed meals presents a complex, constantly changing nutritional environment, and we are only beginning to understand the sensing and signaling systems that detect and coordinate the transcriptional, physiological, and behavioral responses to meal components. Although it has long been axiomatic that dietary composition plays a deterministic role in overall health, the growing prevalence of obesity and metabolic syndrome has prompted a refocus of attention on the significance of dietary macronutrients to health outcomes. Findings from earlier investigations of potential roles for dietary carbohydrates and fat in disease progression or remediation (1–4) are now being reframed by experimental evidence linking dilution of dietary protein to compensatory increases in ingestive behavior (5,6). Recent studies also illustrate that modifying dietary essential amino acid (EAA) composition has profound effects on lipid metabolism and energy balance (7–10). A consensus is emerging to support the view that protein intake is monitored through nutrient-sensing mechanisms that detect changes in EAAs and provide regulatory input to both energy balance and the integration of carbohydrate and lipid metabolism (5,6,11,12). We will examine the transcriptional and metabolic responses to EAA deprivation or restriction to make the broader case that EAA sensing and signaling play an essential role in coordinating metabolic responses to dietary composition, particularly the content and composition of dietary protein.
Nutrient sensing and protein nutrition
Protein leverage hypothesis.
Simpson and Raubenheimer (11) used an integrative modeling approach to develop a geometric framework for evaluating the impact of dietary macronutrients on response variables such as nutrient selection, body composition, longevity, and reproduction. The geometric framework model of nutrition was originally developed and tested in experiments in which life span or fecundity were measured in three different insect species given ad libitum access to 1 of 28 different diets varying in their protein-to-carbohydrate ratio. The authors summarized the responses to all 28 diets by plotting the protein consumed per day on the x axis, the carbohydrate consumed per day on the y axis, and the biological response (e.g., longevity, fecundity, etc.) to each nutritional combination as a heat map in the z plane (13,14). The heat maps identified the specific ratios of protein to carbohydrate that optimized each response, and in all three species, the ratio producing optimal life span differed from the ratio producing greatest fecundity (15). The effectiveness of this approach in describing biological responses to nutritional complexity led to its application in examining how varying dietary macronutrient composition affects ingestive behavior, and how animals prioritize macronutrient intake when given a choice (11,14,16,17). It was found that lowering the percentage of protein in the diet causes a concomitant increase in energy intake to maintain constant protein intake. This leveraging of carbohydrate and fat intake that occurs with dilution of dietary protein represents the conceptual basis for the protein leverage hypothesis (16). A key prediction of the hypothesis is that the overconsumption of total energy that occurs with low-protein diets has the potential to predispose to the development of obesity and metabolic disease (12). However, if the protein-leveraged overconsumption of energy were accompanied by a commensurate increase in energy expenditure (EE), the target protein intake could be achieved without effect on overall energy balance. Alternatively, the hypothesis predicts that high protein diets would be more satiating and reduce energy intake. This element of the prediction has experimental support from work with humans (12,18,19). Collectively, the emerging evidence is consistent with the conclusion that both quantitative and compositional measures of protein intake are being sensed and factor into the regulation of ingestive behavior. It is far less clear how protein nutrition is actually monitored on an ongoing basis, what indices of protein nutrition and amino acid metabolism are being sensed, and the extent to which leveraged changes in overall energy intake are coordinated with changes in EE.
Metabolic responses to protein restriction.
The protein leverage hypothesis infers the superimposition of a protein intake target to the regulation of ingestive behavior that is functionally linked to the animal’s protein requirements for maintenance and growth. Dietary protein and amino acid requirements are normally highest in young, growing animals and decline with age during the approach to maturity. The orexigenic responses to variations in dietary protein have been studied extensively, and several overall conclusions are supported by this body of work (6,20,21). First, mild to moderate protein restriction produces an increase in consumption of a protein-diluted diet, and the magnitude of the response is dependent upon the ongoing protein requirements when it is imposed. For example, the response to moderate restriction is much more robust in young growing animals (e.g., higher protein requirements) as compared with a mature animal when protein needs are lower (22). Second, protein restriction beyond a certain critical level causes food aversion in both young and mature animals (23–25). Considered together, these responses imply the operation of one or more sensing systems that have detection limits and function within certain concentration ranges for both the orexigenic and anorexigenic responses to protein restriction. And in the case of the orexigenic component, the amplitude of the response is incrementally modulated within a defined range of protein restriction that is a function of the individual amino acid requirements when the restriction is imposed (22).
Low-protein diets, particularly when given to young animals, also influence the expenditure component of energy balance. In 32-day-old rats, restricting dietary protein to 8% increases EE, circulating thyroid hormones, and produces changes in brown adipose tissue mass and function (24,26,27). Although the increase in EE functions to counterbalance the increase in energy intake, in many cases protein restriction still leads to increases in body fat and carcass energy stores (24,25,28). In contrast, protein restriction initiated after physical maturity is perceived as a less severe restriction (22) because of the lower protein requirements and greater protein mass available for mobilization and redeployment. However, it is exactly under these physiological circumstances that even a modest leveraging of additional energy intake by lower dietary protein could favor a systematic increase in adiposity. A significant unanswered question is how the determinants of protein nutrition are interpreted and translated relative to the physiological state when protein restriction is imposed.
Amino acid sensing and protein nutrition
EAAs as markers of protein nutrition.
A strong case can be made that the systems that monitor dietary protein provide regulatory input to the control of energy balance and metabolism, but the associated sensing and signaling mechanisms that coordinate the elements of the response are poorly understood. Given that a subgroup of the amino acids (e.g., EAAs) that make up proteins cannot be synthesized endogenously, the ability to detect and respond to dietary EAA deficiencies is clearly an indispensable survival mechanism. Molecular and cellular mechanisms of EAA sensing have been identified and well described (29–36). Although their existence does not preclude important roles for additional sensing mechanisms, the well-documented in vivo responses to perturbation of dietary EAA composition make a compelling case that EAAs play a dominant role as mediators of the effects of dietary protein on metabolism and energy balance (7–10,37–39). Thus, the focus of this perspective is on EAAs, with special emphasis on the EAA-sensing and -signaling mechanisms that have been characterized using dietary manipulation of EAAs as models. A feature common to these dietary models is that they produce significant changes in tissue-specific lipid metabolism, the integration of substrate utilization among tissues, and not surprisingly, overall energy balance.
Mechanisms of EAA sensing and signaling.
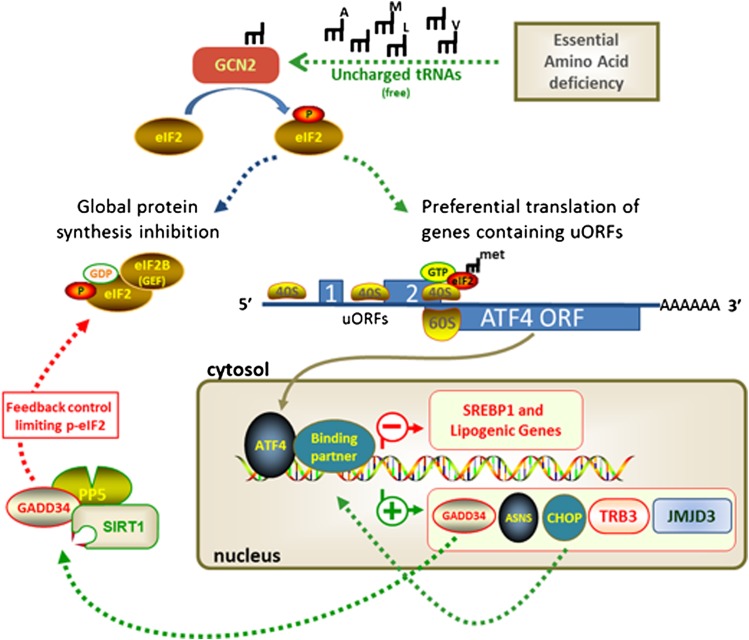
Restricting the availability of EAAs effectively limits charging of tRNA with its cognate amino acid and triggers a cascade of signaling events and transcriptional responses collectively referred to as the amino acid response (AAR) (Fig. 1). The transcriptional responses linked to the AAR are modulated in timing and magnitude by the perceived intensity of the nutritional stress (40–45). Evaluation of the in vivo responses to limitation of dietary EAAs makes a compelling case that the sequence of signaling and gene expression events occurs as part of an adaptive program to reestablish homeostasis. Among the well-documented responses of particular interest are the coordinated, tissue-specific effects on genes involved in lipid metabolism (7–9,39,46–48). This illustrates that the adaptive response to the perception of protein deprivation includes an additional integrative program involving the remodeling of lipid metabolism.
FIG. 1.
A model showing both global and gene-specific control of mRNA translation and gene transcription following sensing of EAA deficiency. One of the earliest sensing events involves phosphorylation of the protein factor eukaryotic initiation factor 2 (eIF2) by the highly conserved and ubiquitously expressed protein kinase GCN2. Phosphorylation of eIF2α by GCN2 produces a coordinated response that limits ribosomal translation of most mRNAs (35,43,52), while selectively derepressing the translation of specific genes containing specific upstream open reading frames. Through increased duration of ribosomal scanning and reinitiation efficiency (79), proteins such as activating transcription factor 4 (ATF4) are recruited to promoter regions of DNA, altering chromatin structure to enhance gene expression of other transcription factors (C/EBP homologous protein [CHOP], CCAAT/enhancer-binding protein-β, ATF3, ATF2) plus additional regulatory proteins such as asparagine synthetase, Tribbles homolog 3 (TRB3), and the histone demethylase Jumanji domain containing protein (JMJD3) (41,45,80–82). ATF4 can heterodimerize with both CCAAT/enhancer-binding protein family members and other ATF members, forming multimeric complexes that bind CCAAT/enhancer-binding protein–ATF response elements, which serve as AAR elements (40,41). uORF, upstream open reading frame; ASNS, asparagine synthetase (ASNS); GADD34, growth arrest and DNA damage gene; PP5, protein phosphatase; SIRT1, sirtuin 1; GEF, guanine nucleotide exchange factor.
In vitro responses to EAA deprivation on lipogenic genes.
The transcriptional effects of EAA deprivation on lipogenic genes were initially identified in human HepG2 cells, where media lacking any single EAA rapidly decreases transcriptional initiation and expression of fatty acid synthase (FAS) (49). FAS is also partially reduced in cells deprived of cysteine or glutamate, but not with any other non-EAAs (49). Since this initial report, transcriptional profiling with HepG2, HepG2/C3a, and mouse embryonic fibroblasts has been used to explore the overall systems biology of the AAR (35,50–52). Though it is beyond the scope of the current perspective to explore the profiles in detail, a common feature of the response in each cell line is a negative effect on genes associated with lipid metabolism (35,51,53). Loss of function approaches show that downregulation of many lipogenic genes is dependent on general control nonderepressible 2 (GCN2) (35). However, the findings from this and other studies (39) make a strong case for the involvement of additional GCN2-independent signaling mechanisms in mediating the in vitro responses to EAA deprivation. As will become apparent in subsequent sections, a similar conclusion is supported from in vivo studies using GCN2 loss-of-function approaches. It should also be noted that in vitro models of EAA deprivation evaluate an extreme cellular situation incompatible with life and in the absence of interorgan amino acid metabolism. The transcriptional responses of peripheral tissues to in vivo restriction of dietary protein or single EAAs reflect the cumulative and highly integrated inputs following both central and peripheral detection of the restriction. In a similar sense, net changes in adiposity also reflect how the perceived changes in dietary protein or specific EAAs affect the intake and expenditure components of energy balance. A deeper understanding of how gene expression patterns in individual tissues relate to substrate utilization and EE will help determine how dietary restriction of single EAAs may benefit overall metabolism.
In vivo responses to dietary limitations of EAAs
Dietary models of EAA deprivation versus restriction.
The conserved nature and ubiquitous expression of molecules associated with EAA sensing and signaling underscores the high priority placed on defending against EAA deficiency in the organism, but the broader and more difficult task is to understand how EAA sensing systems are anatomically organized to produce the highly integrated physiological and behavioral responses that occur when an EAA deficiency is detected. The primary experimental strategy used to study EAA deficiency involves formulation of diets with individual amino acids as the source of protein, and removing or limiting the amount of specific EAAs in the diet. The two most common models involve 1) deprivation of an EAA or 2) restriction of a specific EAA to low but not devoid levels in the diet. Much of the early work on EAA deprivation examined the acute behavioral responses to diets devoid of threonine (34,54–56), whereas later work used diets devoid of leucine to examine the transcriptional, translational, and associated signaling events regulating protein balance, insulin sensitivity, and lipid metabolism (30,57–59). The second approach involves dietary formulations in which an EAA is reduced but not absent (e.g., EAA restricted). This model has received far less attention than EAA deprivation, but that changed after an initial report showed that restricting dietary methionine from normal levels (e.g., ∼0.8 g/100 g diet) to 0.17% (e.g., 0.17 g /100 g diet) produced a remarkable improvement in the metabolic phenotype of rats while increasing their life span by ∼25% (60). In contrast, dietary methionine deprivation, like leucine deprivation, produces a rapid loss of weight, adiposity, and deterioration in health of the animal. Thus, the relatively small difference in methionine produced by dietary methionine deprivation (0%) versus restriction (0.17%) has a profound difference on the physiological responses to the respective diets. Essentially, all work to date on dietary restriction of an EAA has focused on methionine, but it will be important in future studies to restrict other EAAs and determine whether the beneficial responses to methionine restriction (MR) are specific to methionine or can be reproduced in whole or part by restricting other EAAs.
The mechanistic basis for the fundamental difference between the responses to MR versus methionine deprivation represents a critical gap in our understanding of the underlying sensing systems that detect and respond to changes in dietary EAAs. In the years since the initial report of the beneficial responses to restricting dietary methionine to 0.17% (60), significant effort has been devoted to understanding the mechanistic basis for the effects of dietary MR on energy balance, lipid metabolism, and insulin sensitivity. In the subsequent sections, the similarities and differences between leucine deprivation and MR will be presented to illustrate potential future lines of inquiry.
Physiological and transcriptional responses to dietary leucine deprivation.
Metabolic phenotyping of mice subjected to short-term (e.g., 7 or 17 days) dietary leucine, valine, or isoleucine deprivation shows a strong anorexigenic response (30,39) in conjunction with a significant increase in EE (9,10). Studies of EAA deprivation beyond 3 weeks are typically not permitted or possible because of extensive weight loss and increased mortality, so most work on leucine deprivation examines responses after 7 days on the diet. Mice presented with diets lacking leucine decrease intake by 20–30% in the first 4 h after introduction of the imbalanced diet (34,36) and maintain the lowered rate of consumption after 7 days and 17 days (30,39). The significant decrease in energy intake is accompanied by increased sympathetic outflow to adipose tissues and a concomitant increase in lipid mobilization, oxidation, and uncoupled respiration (9,10). The combination of decreased energy intake and increased EE has a profound effect on energy balance such that after 7 days body weight is reduced by ∼15–20% (30) and adipose tissue mass is two- to threefold lower (10,39). After 17 days of leucine deprivation, body weight is 30% lower and virtually no dissectable fat mass remains (39). The effects of 7 days of leucine deprivation on energy balance are accompanied by a significant reduction in fasting insulin (30,39), which is reflective of increases in tissue-specific and overall insulin sensitivity (58). The inclusion of a pair-fed control group supports the conclusion that the beneficial effects of the diet on insulin sensitivity are independent of the effects of the diet on energy balance (58). The authors propose a mechanism for the diet-induced increase in hepatic insulin sensitivity involving increased AMP-activated protein kinase activation and a GCN2-dependent decrease in mammalian target of rapamycin (mTOR)/S6 kinase 1 (S6K1) signaling (30,58). However, previous studies show that the ability of leucine deprivation to decrease fasting insulin is not compromised in GCN2-null mice (30,39).
The transcriptional effects of leucine deprivation include targeted effects on genes involved in lipid metabolism in both liver and white adipose tissue (WAT). In liver, 7 days on the diet produces significant decreases in genes associated with fatty acid and triglyceride synthesis but not fatty acid transport or oxidation (39). In WAT, leucine deprivation increases expression of genes involved in fatty acid oxidation and thermogenesis while simultaneously repressing lipogenic genes (9). In a subsequent study using mice lacking β-adrenergic receptors, the ability of the diet to increase uncoupling protein 1, activate thermogenesis, increase EE, and reduce adiposity is compromised (10), suggesting that the sympathetic nervous system (SNS) is an essential mediator of the effect of leucine deprivation on fat deposition. As expected, the diet-induced effects on genes associated with oxidative and thermogenic responses in WAT and brown adipose tissue are blocked in β-less mice, but it remains unclear whether the previously observed downregulation of lipogenic genes in both liver and WAT requires intact signaling through β-adrenergic receptors (10).
Physiological and transcriptional responses to dietary MR.
Dietary MR produces a highly integrated series of behavioral, physiological, and biochemical responses that improve biomarkers of metabolic health and increase longevity in rodents (60–62) and flies (63). The metabolic responses are rapid in onset but persist indefinitely and include hyperphagia, increased EE, reduced fat deposition, reduced circulating lipids and leptin, and increased plasma adiponectin (7,8,64). The diet-induced reduction in fat deposition is particularly interesting because it occurs despite a paradoxical increase in food intake (60,64,65). Subsequent studies establish that dietary MR produces a simultaneous increase in EE that compensates for the increase in energy intake (7,8). More importantly, the diet enhances metabolic flexibility (7,8) and produces a significant increase in overall insulin sensitivity that develops quickly after introduction of the diet and persists after long-term MR (64). In most studies, MR is initiated after weaning in young growing animals, but the beneficial metabolic responses are also observed when MR is initiated after physical maturity (7). In this case, initiation of dietary MR at 6 months of age prevents any further increase in adiposity over the following 6 months by producing a coordinated increase in energy intake and expenditure (7). Dietary MR also increases metabolic flexibility, and after 3 and 6 months on the diet, fasting insulin, plasma triglycerides, and liver triglycerides are two- to threefold lower than that in rats on the control diet (7; T.W. Gettys, unpublished observation). It is unclear whether the reductions in hepatic and circulating neutral lipids are products of dietary effects on energy balance, direct effects of dietary MR in the liver, or some combination of both mechanisms.
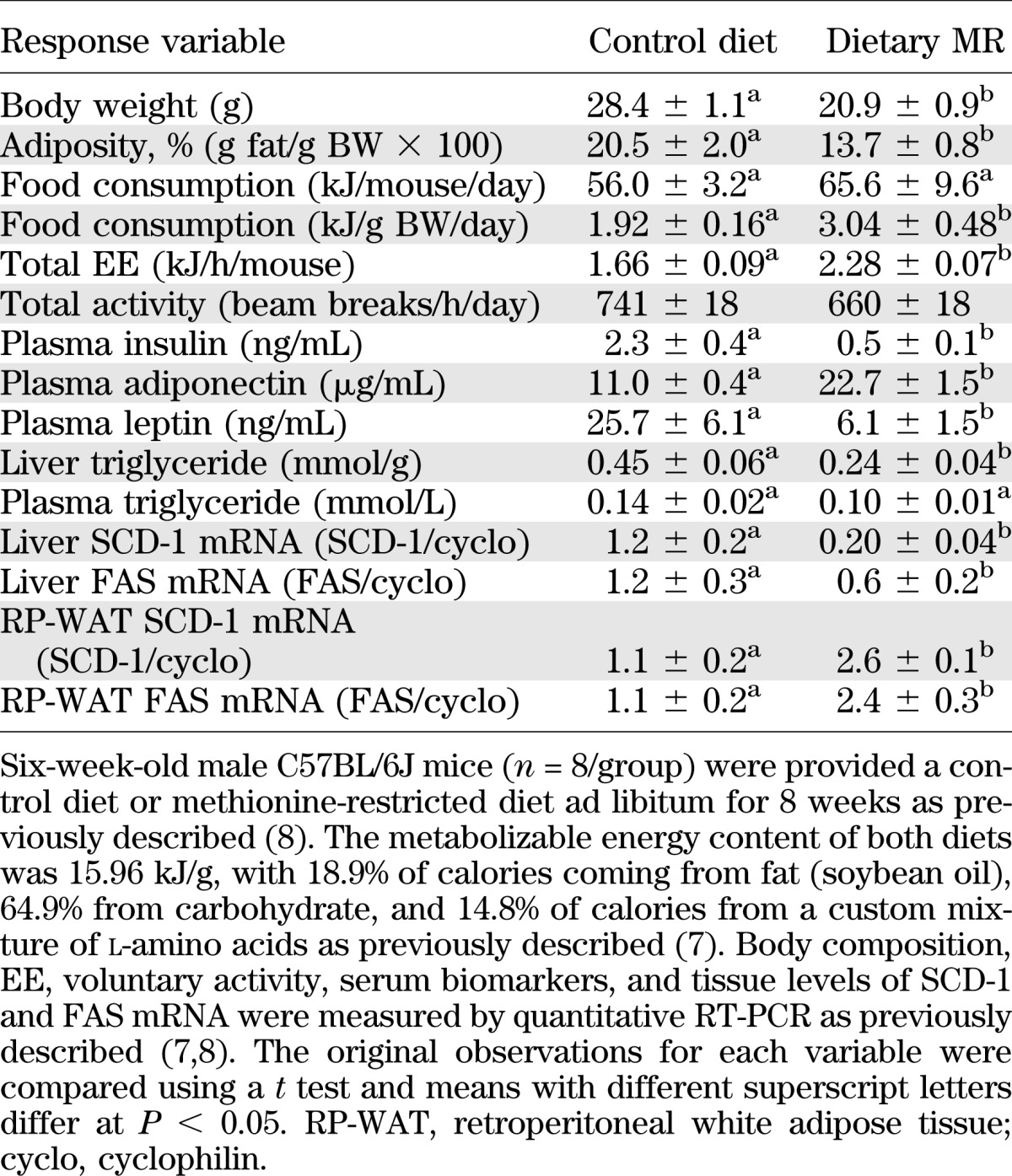
Most of the early work on MR was conducted with rats, but the responses to dietary MR in mice are comparable in almost every respect (8). A summary of the metabolic profile of mice after short-term (8 week) consumption of dietary MR from our work illustrates that the offsetting effects on energy intake and expenditure effectively limit fat deposition while producing significant changes in endocrine function of WAT (Table 1). WAT is also a transcriptional target of dietary MR, with the diet increasing expression of genes involved in both lipid oxidation and synthesis (8) (Table 1). The upregulation of FAS and stearoyl-CoA desaturase-1 (SCD-1) in WAT is complemented by downregulation of these lipogenic genes in the liver, potentially compromising its capacity to synthesize and export triglyceride. This prediction is supported by data from both preclinical studies (Table 1) and in patients with metabolic syndrome, in which dietary MR for 16 weeks produced a significant reduction in hepatic lipid content (66). These data illustrate the complex but highly beneficial effects that accrue from consumption of a diet in which methionine is limited but not absent. Although the combined effects of dietary MR on energy homeostasis, WAT endocrine function, and remodeling of tissue-specific lipid metabolism contribute to the observed increase in metabolic flexibility, the relative importance of the component responses to the improvement in overall insulin sensitivity remains to be established.
TABLE 1.
Responses of male C57BL/6J mice to dietary MR for 8 weeks
Role of GCN2 in in vivo responses to EAA deprivation.
Diets deficient in an EAA activate GCN2 in the anterior piriform cortex and produce an aversive feeding response within 20–40 min (34,36). Loss of function studies using either global or neuron-specific GCN2-null mice establish that the acute aversive response to EAA deprivation is mediated by GCN2 (34,36). EAA deprivation also produces a chronic anorexigenic response that remains evident after 7 and 17 days (30,39) on the diet. However, both wild-type and GCN2-null mice reduce consumption of a diet lacking leucine by 20–30% over the 1–2 weeks (30,39) following its introduction, indicating that GCN2 is not required for the chronic anorexigenic response to leucine deprivation.
The hypothalamus is also an important target and mediator of the effects of EAA deprivation on energy intake because it both receives axonal projections from the anterior piriform cortex (67,68) and responds directly to depletion and repletion of EAAs (69). Recent studies have shown that EAA deprivation produces rapid changes in anorexigenic neuropeptides and signaling in hypothalamic feeding centers (70–72) and that the hypothalamus is responsive to repletion of the limiting EAA or altered neuropeptide (71). The mediobasal hypothalamus is thought to play a role in responding to changes in protein quality and quantity through leucine-dependent activation of mammalian target of rapamycin complex 1 (73) and provide corresponding input to the control of energy intake (74). An integrated picture of the anatomical organization and temporal development of hypothalamic responses to EAA deprivation has yet to emerge but will clearly be essential to understanding the full range of adaptive responses.
The coordinated downregulation of the lipogenic gene program in both liver and WAT by leucine deprivation is mediated in part by a decrease in expression of sterol regulatory element binding protein-1c (SREBP-1c) in both tissues (39,75). In wild-type mice, these changes are associated with a 40% decrease in serum triglyceride but no change in liver triglyceride (39). The diet-induced decrease in hepatic SREBP-1c and associated lipogenic genes (e.g., FAS, SCD-1, acetyl-CoA carboxylase-1, ATP-citrate lyase) is absent in GCN2-null mice, but the decrease in serum triglycerides is unaltered, as is the decrease in fasting insulin (39). However, compared with wild-type mice, hepatic triglycerides are increased twofold by leucine deprivation in GCN2-null mice (39). Although the question has not been addressed experimentally, these findings suggest the interesting possibility that leucine deprivation limits lipid export into VLDLs secreted from the liver, and the additional absence of GCN2 may further compromise lipoprotein signal peptide abundance, apoliprotein B synthesis, and VLDL secretion. Considered together, these findings make a compelling case that the overall effects of leucine deprivation are mediated through both GCN2-dependent and -independent mechanisms. Studies evaluating the role of GCN2 as a mediator of the responses to dietary MR are ongoing and suggest that the orexigenic response to MR does not require GCN2 (T.G. Anthony, T.W. Gettys, unpublished data).
Fundamental differences between dietary MR and EAA deprivation.
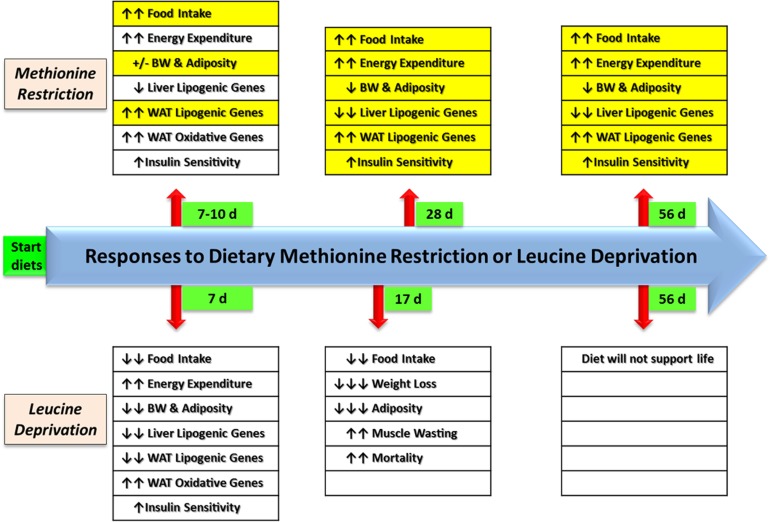
In contrast to the acute and chronic anorectic responses to EAA deprivation, dietary MR produces a hyperphagic response within 6–7 days after introduction of the diet, and the 20–25% increase in consumption of the diet continues indefinitely (7,64). A compilation of the short-term responses to dietary MR and leucine deprivation are summarized in Fig. 2, with differences highlighted in yellow. The acute responses to the diets also share several similarities, including comparable transcriptional effects on lipogenic genes in the liver, increased oxidative genes in WAT, increased EE, and enhanced insulin sensitivity (7–10,30,39,48,58,76,77). A key similarity is that both diets increase SNS stimulation of adipose tissue, which induces oxidative and thermogenic gene programs, resulting in increased EE. Recent studies provide evidence that leucine deprivation activates the SNS by increasing expression of corticotropin-releasing hormone in the hypothalamic paraventricular nucleus (10) through a mechanism involving inhibition of S6K1 (76). The central sensing and signaling mechanisms of dietary MR have received far less attention to date, so it is unknown whether dietary MR acts through a similar mechanism to activate the SNS. In contrast to their similar effects on the SNS, the two diets have opposite effects on ingestive behavior, and not surprisingly, different effects on body weight and adiposity (Fig. 2). Whereas the effect of MR is to slow the deposition of adipose tissue relative to body weight, leucine deprivation causes mobilization and loss of adipose tissue at a rate exceeding the loss of body weight (39). A third major difference between the responses in adipose tissue is that leucine deprivation produces a significant downregulation of SREBP-1c and the primary genes involved in de novo lipogenesis and triglyceride synthesis (9). In contrast, dietary MR produces a significant and persistent increase in lipogenic gene expression in WAT depots (47), which suggests retention of the capacity to conduct de novo lipogenesis in the fed state to compensate for the loss of lipogenic potential in the liver (Fig. 2). Recently we assessed the physiological significance of this by measuring 2H-enrichment in palmitate 12 h after injecting control and MR mice with 2H2O (T.W. Gettys, unpublished data). As expected, the calculated rates of de novo lipogenesis in WAT from control mice were quite low but were increased almost fourfold by MR. These findings are consistent with the exaggerated increases in respiratory quotient and EE observed in rats on the MR diet in the fed state (7). The associated increase in metabolic flexibility comes at the expense of metabolically inefficient conversion of glucose to lipid (78), but this may be a key component of the mechanism through which dietary MR produces a disproportionate increase in EE in the fed state (7). Conversely, MR reduced hepatic de novo lipogenesis by fivefold compared with controls, consistent with a remodeling of the integration of lipid metabolism between liver and adipose tissue. Collectively, the tissue-specific changes in de novo lipogenesis are fully consistent with the MR-induced changes in lipogenic gene expression among the tissues, as well as the relative retention of adipose tissue mass after chronic consumption of the diet. Lastly, the fundamental differences in the adaptive responses between diets should be viewed in the context that dietary MR extends life span, producing a beneficial metabolic phenotype in the process; in contrast EAA deprivation cannot sustain life beyond a few weeks.
FIG. 2.
Responses of male C57BL/6J mice to dietary MR vs. dietary leucine deprivation at comparable time points after introduction of the respective diets. Data on MR is from ref. 8 and unpublished data. Data on leucine deprivation was compiled from refs. 9, 10, 30, 39, and 58. BW, body weight; d, days.
Perspectives and future directions
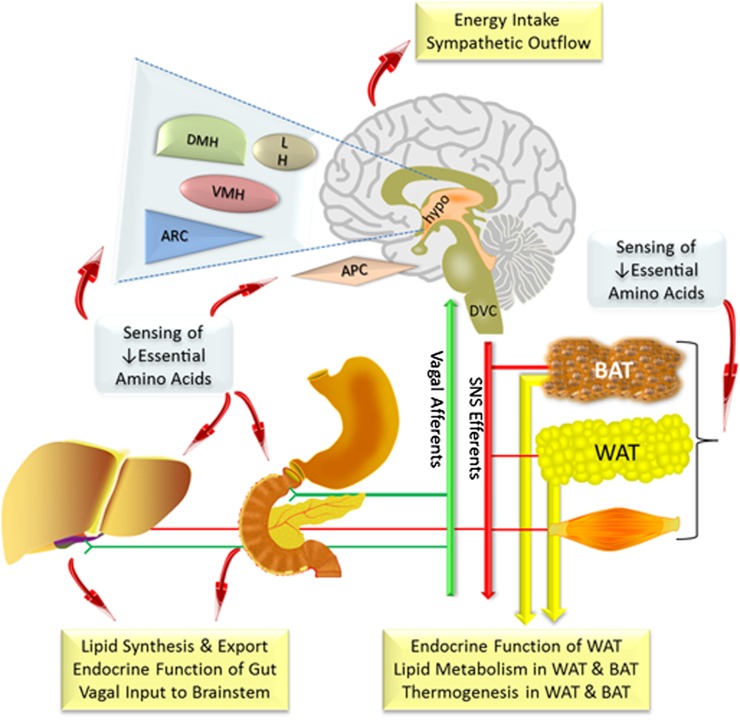
A comparison of the responses to leucine deprivation and MR has been provided to illustrate their tissue-specific and physiological responses and to better understand the sensing and signaling mechanisms being engaged. A conceptual model of the anatomical organization of the communication networks that are either known or proposed to be involved in coordinating the complex physiological responses to EAA sensing are presented in Fig. 3. The illustration is equally applicable to EAA deprivation or restriction, and can also be used to guide a comparison of the temporal relationships among responses to leucine deprivation, MR, or other dietary modifications of EAAs (Fig. 2). Irrespective of dietary model, it is difficult to distinguish the direct effects of an EAA limitation in a particular tissue from responses that are modulated by detection of the deficiency in another anatomical site that may then provide secondary signaling or endocrine input to the initial site. Thus, in addition to their spatial organization, the individual components of the response to an EAA deficiency are also temporally organized, developing in a reproducible progression after introduction of the diet. Our model proposes that there are at least four potential sites of sensing amino acid depletion or restriction: 1) Direct sensing of luminal or absorbed EAAs by the gut; 2) sensing of EAAs in the portal circulation and/or liver, perhaps through the afferent vagus; 3) direct sensing of EAAs in the general circulation by tissues; and 4) sensing of EAAs in various regions of the brain. The model is also presented as a way to illustrate gaps in our understanding of how these and as yet unknown sensing components function together to mediate the integrated physiological responses to changes in dietary EAA content. Several areas needing further exploration include the following: 1) To what extent does the gut participate in sensing dietary changes in protein or amino acid content and does such a sensing system use endocrine signals (e.g., GLP-1, cholecystokinin, ghrelin, peptide YY) or vagal afferents to communicate this information to the brainstem and other central sites for integration? 2) How are the central amino acid sensing systems organized to provide integrated regulation of the components of energy balance and communication to peripheral tissues? 3) How are other AAR signaling mechanisms besides GCN2 (e.g., mTOR, S6K1, leucyl-tRNA synthetases, mitogen-activated protein kinases) involved in detecting changes in dietary amino acids? 4) What components of the adaptive response to EAA deprivation or restriction mediate the tissue-specific and overall enhancement of insulin sensitivity? 5) Are the beneficial metabolic responses to MR unique to methionine or will restriction of other EAAs reproduce the phenotype? The overall metabolic phenotype produced by EAA deprivation versus restriction is the product of a series of responses that are anatomically and temporally organized and in many cases, interdependent. Therefore, a significant ongoing challenge within the field will be to develop experimental approaches that distinguish between the direct, tissue-specific responses to EAA deprivation versus restriction and the responses perceived in one anatomical site and modulated in another.
FIG. 3.
Model of proposed anatomical organization of sites contributing to sensing and coordinating tissue-specific and overall physiological responses to dietary EAA deprivation or dietary MR. The model illustrates the sites where EAA sensing has been detected (liver, WAT, brown adipose tissue, hypothalamus, anterior piriform cortex) or is suspected (gut) as well as target tissues where responses have been measured. The model proposes a potential role for vagal afferents (green lines) in providing sensory input to the brainstem from the gut about decreases in methionine or EAA intake. The model proposes that increased SNS outflow (red lines) serves as a motor arm linking hypothalamic EAA sensing to peripheral target tissues, where the effects include tissue-specific transcriptional responses, changes in endocrine function (WAT and brown adipose tissue), and thermogenic activity (WAT and brown adipose tissue). Central detection of EAAs is proposed to impact energy balance through integrated effects on energy intake and expenditure. MR increases both energy intake and expenditure, whereas leucine deprivation decreases energy intake and increases EE. Together, Figs. 2 and 3 illustrate the anatomical and temporal organization of the responses to MR and leucine deprivation. APC, anterior piriform cortex; DMH, dorsomedial hypothalamus; DVC, dorsal vagal complex; LH, lateral hypothalamus; hypo, hypothalamus; VMH, ventromedial hypothalamus.
The comparison of the physiological responses to dietary MR and leucine deprivation suggest that the EAAs are in a sense functioning as ligands with responses determined by the degree of the restriction. It follows from this observation that an important future objective will be to examine systematically the physiological responses to incremental restriction of methionine, leucine, and other EAAs. In addition to identifying a range of restrictions that are most beneficial, this approach could also provide important mechanistic insights regarding EAA-specific sensing mechanisms and temporal interdependence among components of the overall phenotype. Returning to the concept of the geometric framework, Piper et al. (14) proposed to evaluate biological responses (e.g., longevity, fecundity, etc.) to nutritional complexity by representing variations in dietary composition in the x-y plane and plotting individual responses to the nutritional matrix in the z plane. We suggest that this may be a particularly productive approach to examine the physiological responses to variations in the dietary content of specific amino acids as part of a comprehensive strategy to assess the impact of EAA nutrition in model organisms. In our work on MR, we have identified a range of dietary methionine concentrations that produces profound improvements in biomarkers of metabolic health. The important next steps are to refine our understanding of the degree of MR linked to each component and extend this work to the other EAAs and their collective roles in the mechanistic basis of nutrient sensing in protein nutrition. Another important unexplored area involves the effects of EAA restriction on protein turnover within narrow ranges of EAA restriction such as those identified in studies of MR. Understanding the signals that trigger remodeling of protein metabolism alongside that of lipid and energy metabolism will provide an integrative view of the benefits of EAA restriction.
Lastly, the translational potential of the concepts developed in preclinical studies of dietary MR were recently evaluated in a human cohort meeting the criteria for metabolic syndrome (66). Dietary MR was accomplished using the semisynthetic medical food, Hominex-2 (Abbott Nutrition, Columbus, OH) in a short-term study (16 weeks) to evaluate the metabolic consequences of limiting dietary methionine from 35 mg/kg body weigh/day to 2 mg/kg body weight/day. The experimental diet (Hominex-2) is a commercial food designed to provide nutritional support for patients with pyridoxine-unresponsive homocystinuria or hypermethioninemia. However, it is comprised in part of elemental amino acids, and their associated low palatability resulted in high withdrawal rates and raised questions about compliance and achieving the desired degree of MR. Notwithstanding these experimental limitations, we were able to conclude that dietary MR has metabolic responses that are beneficial in subjects with metabolic disease. An important remaining challenge will be to develop palatable solutions that will allow the full potential of dietary MR to be evaluated in a variety of translational contexts.
ACKNOWLEDGMENTS
This work was supported in part by the National Institutes of Health ([NIH] RO1 HD-070487 to T.G.A.; RO1 DK-081563 to C.D.M.; RO1 DK-096311 to T.W.G.), the American Diabetes Association (1-12-BS-58 to T.W.G.), and a subcontract from the Mouse Metabolic Phenotyping Center Consortium (NIH U24 DK-076169 to T.W.G.). This work made use of the Genomics and Cell Biology and Bioimaging core facilities supported by NIH P20-GM-103528 (T.W.G.) and NIH P30 DK-072476.
No potential conflicts of interest relevant to this article were reported.
T.G.A. and C.D.M. contributed to the writing and editing of the manuscript. T.W.G. contributed to the writing and editing of the manuscript and analyzed the data used to produce illustrations.
The authors acknowledge Drs. Jean-Marc Schwarz of the University of California, San Francisco Diabetes Center and Jennifer Rood of the Pennington Stable Isotope core facility for conducting the de novo lipogenesis analysis.
REFERENCES
- 1.Fogelholm M, Anderssen S, Gunnarsdottir I, Lahti-Koski M. Dietary macronutrients and food consumption as determinants of long-term weight change in adult populations: a systematic literature review. Food Nutr Res 2012;56:19103–19148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kratz M, Baars T, Guyenet S. The relationship between high-fat dairy consumption and obesity, cardiovascular, and metabolic disease. Eur J Nutr 2012;51:418. [DOI] [PubMed] [Google Scholar]
- 3.Lê KA, Bortolotti M. Role of dietary carbohydrates and macronutrients in the pathogenesis of nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care 2008;11:477–482 [DOI] [PubMed] [Google Scholar]
- 4.Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr 2007;86:285–300 [DOI] [PubMed] [Google Scholar]
- 5.Bosse JD, Dixon BM. Dietary protein in weight management: a review proposing protein spread and change theories. Nutr Metab (Lond) 2012;9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison CD, Reed SD, Henagan TM. Homeostatic regulation of protein intake: in search of a mechanism. Am J Physiol Regul Integr Comp Physiol 2012;302:R917–R928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasek BE, Stewart LK, Henagan TM, et al. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am J Physiol Regul Integr Comp Physiol 2010;299:R728–R739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plaisance EP, Henagan TM, Echlin H, et al. Role of β-adrenergic receptors in the hyperphagic and hypermetabolic responses to dietary methionine restriction. Am J Physiol Regul Integr Comp Physiol 2010;299:R740–R750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y, Meng Q, Wang C, et al. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 2010;59:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Y, Zhang Q, Meng Q, et al. Leucine deprivation stimulates fat loss via increasing CRH expression in the hypothalamus and activating the sympathetic nervous system. Mol Endocrinol 2011;25:1624–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson SJ, Raubenheimer D. Geometric analysis of macronutrient selection in the rat. Appetite 1997;28:201–213 [DOI] [PubMed] [Google Scholar]
- 12.Gosby AK, Conigrave AD, Lau NS, et al. Testing protein leverage in lean humans: a randomised controlled experimental study. PLoS ONE 2011;6:e25929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raubenheimer D, Simpson SJ. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr Res Rev 1997;10:151–179 [DOI] [PubMed] [Google Scholar]
- 14.Piper MD, Partridge L, Raubenheimer D, Simpson SJ. Dietary restriction and aging: a unifying perspective. Cell Metab 2011;14:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson SJ, Raubenheimer D. The Nature of Nutrition. Princeton, Princeton University Press, 2012 [Google Scholar]
- 16.Simpson SJ, Raubenheimer D. Obesity: the protein leverage hypothesis. Obes Rev 2005;6:133–142 [DOI] [PubMed] [Google Scholar]
- 17.Wilder SM, Le Couteur DG, Simpson SJ. Diet mediates the relationship between longevity and reproduction in mammals. Age (Dordr) 2013;35:921–927 [DOI] [PMC free article] [PubMed]
- 18.Martinez-Cordero C, Kuzawa CW, Sloboda DM, Stewart J, Simpson SJ, Raubenheimer D. Testing the Protein Leverage Hypothesis in a free-living human population. Appetite 2012;59:312–315 [DOI] [PubMed] [Google Scholar]
- 19.Griffioen-Roose S, Mars M, Siebelink E, Finlayson G, Tomé D, de Graaf C. Protein status elicits compensatory changes in food intake and food preferences. Am J Clin Nutr 2012;95:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson GH, Moore SE. Dietary proteins in the regulation of food intake and body weight in humans. J Nutr 2004;134:974S–979S [DOI] [PubMed] [Google Scholar]
- 21.Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr 2009;29:21–41 [DOI] [PubMed]
- 22.White BD, Porter MH, Martin RJ. Effects of age on the feeding response to moderately low dietary protein in rats. Physiol Behav 2000;68:673–681 [DOI] [PubMed] [Google Scholar]
- 23.Whitedouble dagger BD, Porter MH, Martin RJ. Protein selection, food intake, and body composition in response to the amount of dietary protein. Physiol Behav 2000;69:383–389 [DOI] [PubMed] [Google Scholar]
- 24.Bell RR, McGill TJ, Digby PW, Bennett SA. Effects of dietary protein and exercise on brown adipose tissue and energy balance in experimental animals. J Nutr 1984;114:1900–1908 [DOI] [PubMed] [Google Scholar]
- 25.Du F, Higginbotham DA, White BD. Food intake, energy balance and serum leptin concentrations in rats fed low-protein diets. J Nutr 2000;130:514–521 [DOI] [PubMed] [Google Scholar]
- 26.Rothwell NJ, Stock MJ, Tyzbir RS. Energy balance and mitochondrial function in liver and brown fat of rats fed “cafeteria” diets of varying protein content. J Nutr 1982;112:1663–1672 [DOI] [PubMed] [Google Scholar]
- 27.Rothwell NJ, Stock MJ, Tyzbir RS. Mechanisms of thermogenesis induced by low protein diets. Metabolism 1983;32:257–261 [DOI] [PubMed] [Google Scholar]
- 28.Aparecida de França S, Dos Santos MP, Garófalo MA, et al. Low protein diet changes the energetic balance and sympathetic activity in brown adipose tissue of growing rats. Nutrition 2009;25:1186–1192 [DOI] [PubMed] [Google Scholar]
- 29.Anthony TG, Reiter AK, Anthony JC, Kimball SR, Jefferson LS. Deficiency of dietary EAA preferentially inhibits mRNA translation of ribosomal proteins in liver of meal-fed rats. Am J Physiol Endocrinol Metab 2001;281:E430–E439 [DOI] [PubMed] [Google Scholar]
- 30.Anthony TG, McDaniel BJ, Byerley RL, et al. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem 2004;279:36553–36561 [DOI] [PubMed] [Google Scholar]
- 31.Kimball S, Anthony TG. Cavener DR, Jefferson LS. 5 Nutrient signaling through mammalian GCN2. Top Curr Genet 2004;7:113–130 [Google Scholar]
- 32.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 2006;34:7–11 [DOI] [PubMed] [Google Scholar]
- 33.Zhang P, McGrath BC, Reinert J, et al. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol 2002;22:6681–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hao S, Sharp JW, Ross-Inta CM, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 2005;307:1776–1778 [DOI] [PubMed] [Google Scholar]
- 35.Deval C, Chaveroux C, Maurin AC, et al. Amino acid limitation regulates the expression of genes involved in several specific biological processes through GCN2-dependent and GCN2-independent pathways. FEBS J 2009;276:707–718 [DOI] [PubMed] [Google Scholar]
- 36.Maurin AC, Jousse C, Averous J, et al. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab 2005;1:273–277 [DOI] [PubMed] [Google Scholar]
- 37.Du Y, Meng Q, Zhang Q, Guo F. Isoleucine or valine deprivation stimulates fat loss via increasing energy expenditure and regulating lipid metabolism in WAT. Amino Acids 2012;43:725–734 [DOI] [PubMed] [Google Scholar]
- 38.Han JM, Jeong SJ, Park MC, et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 2012;149:410–424 [DOI] [PubMed] [Google Scholar]
- 39.Guo F, Cavener DR. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab 2007;5:103–114 [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Pan YX, Dudenhausen EE, Kilberg MS. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive basic region/leucine zipper transcription factors as well as localized histone acetylation. J Biol Chem 2004;279:50829–50839 [DOI] [PubMed] [Google Scholar]
- 41.Pan Y, Chen H, Siu F, Kilberg MS. Amino acid deprivation and endoplasmic reticulum stress induce expression of multiple activating transcription factor-3 mRNA species that, when overexpressed in HepG2 cells, modulate transcription by the human asparagine synthetase promoter. J Biol Chem 2003;278:38402–38412 [DOI] [PubMed] [Google Scholar]
- 42.Pan YX, Chen H, Thiaville MM, Kilberg MS. Activation of the ATF3 gene through a co-ordinated amino acid-sensing response programme that controls transcriptional regulation of responsive genes following amino acid limitation. Biochem J 2007;401:299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shan J, Ord D, Ord T, Kilberg MS. Elevated ATF4 expression, in the absence of other signals, is sufficient for transcriptional induction via CCAAT enhancer-binding protein-activating transcription factor response elements. J Biol Chem 2009;284:21241–21248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su N, Kilberg MS. C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J Biol Chem 2008;283:35106–35117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jousse C, Deval C, Maurin AC, et al. TRB3 inhibits the transcriptional activation of stress-regulated genes by a negative feedback on the ATF4 pathway. J Biol Chem 2007;282:15851–15861 [DOI] [PubMed] [Google Scholar]
- 46.Elshorbagy AK, Valdivia-Garcia M, Mattocks DA, et al. Cysteine supplementation reverses methionine restriction effects on rat adiposity: significance of stearoyl-coenzyme A desaturase. J Lipid Res 2011;52:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perrone CE, Mattocks DA, Plummer JD, et al. Genomic and metabolic responses to methionine-restricted and methionine-restricted, cysteine-supplemented diets in Fischer 344 rat inguinal adipose tissue, liver and quadriceps muscle. J Nutrigenet Nutrigenomics 2012;5:132–157 [DOI] [PubMed] [Google Scholar]
- 48.Perrone CE, Mattocks DA, Jarvis-Morar M, Plummer JD, Orentreich N. Methionine restriction effects on mitochondrial biogenesis and aerobic capacity in white adipose tissue, liver, and skeletal muscle of F344 rats. Metabolism 2010;59:1000–1011 [DOI] [PubMed] [Google Scholar]
- 49.Dudek SM, Semenkovich CF. Essential amino acids regulate fatty acid synthase expression through an uncharged transfer RNA-dependent mechanism. J Biol Chem 1995;270:29323–29329 [DOI] [PubMed] [Google Scholar]
- 50.Jousse C, Averous J, Bruhat A, Carraro V, Mordier S, Fafournoux P. Amino acids as regulators of gene expression: molecular mechanisms. Biochem Biophys Res Commun 2004;313:447–452 [DOI] [PubMed] [Google Scholar]
- 51.Shan J, Lopez MC, Baker HV, Kilberg MS. Expression profiling after activation of the amino acid deprivation response in HepG2 human hepatoma cells. Physiol Genomics 2010;41:315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palii SS, Kays CE, Deval C, Bruhat A, Fafournoux P, Kilberg MS. Specificity of amino acid regulated gene expression: analysis of genes subjected to either complete or single amino acid deprivation. Amino Acids 2009;37:79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sikalidis AK, Lee JI, Stipanuk MH. Gene expression and integrated stress response in HepG2/C3A cells cultured in amino acid deficient medium. Amino Acids 2011;41:159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gietzen DW, Erecius LF, Rogers QR. Neurochemical changes after imbalanced diets suggest a brain circuit mediating anorectic responses to amino acid deficiency in rats. J Nutr 1998;128:771–781 [DOI] [PubMed] [Google Scholar]
- 55.Gietzen DW. Neural mechanisms in the responses to amino acid deficiency. J Nutr 1993;123:610–625 [DOI] [PubMed] [Google Scholar]
- 56.Koehnle TJ, Russell MC, Gietzen DW. Rats rapidly reject diets deficient in essential amino acids. J Nutr 2003;133:2331–2335 [DOI] [PubMed] [Google Scholar]
- 57.Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr 2001;131:856S–860S [DOI] [PubMed] [Google Scholar]
- 58.Xiao F, Huang Z, Li H, et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes 2011;60:746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reiter AK, Anthony TG, Anthony JC, Jefferson LS, Kimball SR. The mTOR signaling pathway mediates control of ribosomal protein mRNA translation in rat liver. Int J Biochem Cell Biol 2004;36:2169–2179 [DOI] [PubMed] [Google Scholar]
- 60.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr 1993;123:269–274 [DOI] [PubMed] [Google Scholar]
- 61.Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 2005;4:119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun L, Sadighi Akha AA, Miller RA, Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Gerontol A Biol Sci Med Sci 2009;64:711–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 2009;462:1061–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 2006;5:305–314 [DOI] [PubMed] [Google Scholar]
- 65.Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp Gerontol 2003;38:47–52 [DOI] [PubMed] [Google Scholar]
- 66.Plaisance EP, Greenway FL, Boudreau A, et al. Dietary methionine restriction increases fat oxidation in obese adults with metabolic syndrome. J Clin Endocrinol Metab 2011;96:E836–E840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anthony TG, Gietzen DW. Detection of amino acid deprivation in the central nervous system. Curr Opin Clin Nutr Metab Care 2013;16:96–101 [DOI] [PubMed] [Google Scholar]
- 68.Gietzen DW, Aja SM. The brain’s response to an essential amino acid-deficient diet and the circuitous route to a better meal. Mol Neurobiol 2012;46:332–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gietzen DW, Hao S, Anthony TG. Mechanisms of food intake repression in indispensable amino acid deficiency. Annu Rev Nutr 2007;27:63–78 [DOI] [PubMed]
- 70.Blevins JE, Teh PS, Wang CX, Gietzen DW. Effects of amino acid deficiency on monoamines in the lateral hypothalamus (LH) in rats. Nutr Neurosci 2003;6:291–299 [DOI] [PubMed] [Google Scholar]
- 71.Goto S, Nagao K, Bannai M, et al. Anorexia in rats caused by a valine-deficient diet is not ameliorated by systemic ghrelin treatment. Neuroscience 2010;166:333–340 [DOI] [PubMed] [Google Scholar]
- 72.Nakahara K, Takata S, Ishii A, et al. Somatostatin is involved in anorexia in mice fed a valine-deficient diet. Amino Acids 2012;42:1397–1404 [DOI] [PubMed] [Google Scholar]
- 73.Cota D, Proulx K, Smith KA, et al. Hypothalamic mTOR signaling regulates food intake. Science 2006;312:927–930 [DOI] [PubMed] [Google Scholar]
- 74.Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci 2009;29:8302–8311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dyck JR, Berthiaume LG, Thomas PD, et al. Characterization of rat liver malonyl-CoA decarboxylase and the study of its role in regulating fatty acid metabolism. Biochem J 2000;350:599–608 [PMC free article] [PubMed] [Google Scholar]
- 76.Xia T, Cheng Y, Zhang Q, et al. S6K1 in the central nervous system regulates energy expenditure via MC4R/CRH pathways in response to deprivation of an essential amino acid. Diabetes 2012;61:2461–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perrone CE, Mattocks DA, Hristopoulos G, Plummer JD, Krajcik RA, Orentreich N. Methionine restriction effects on 11 -HSD1 activity and lipogenic/lipolytic balance in F344 rat adipose tissue. J Lipid Res 2008;49:12–23 [DOI] [PubMed] [Google Scholar]
- 78.Masoro EJ. Role of lipogenesis in nonshivering thermogenesis. Fed Proc 1963;22:868–873 [PubMed] [Google Scholar]
- 79.Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem 2011;286:10939–10949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bunpo P, Dudley A, Cundiff JK, Cavener DR, Wek RC, Anthony TG. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase. J Biol Chem 2009;284:32742–32749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shan J, Fu L, Balasubramanian MN, Anthony TG, Kilberg MS. ATF4-dependent regulation of the JMJD3 gene during amino acid deprivation can be rescued in Atf4-deficient cells by inhibition of deacetylation. J Biol Chem 2012;287:36393–36403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carraro V, Maurin AC, Lambert-Langlais S, et al. Amino acid availability controls TRB3 transcription in liver through the GCN2/eIF2α/ATF4 pathway. PLoS ONE 2010;5:e15716. [DOI] [PMC free article] [PubMed] [Google Scholar]