Obesity and its associated metabolic and cardiovascular disorders are among the most challenging health problems confronting developed countries. Not only is obesity remarkably common, affecting more than one-third of U.S. adults, and very challenging to treat, but it is also tightly linked to type 2 diabetes and related metabolic disorders. A major obstacle to effective obesity treatment is that lost weight tends to be regained over time (1). Although the mechanisms underlying recovery of lost weight are incompletely understood, a large literature suggests that body fat stores are subject to homeostatic regulation, and that this process occurs in obese as well as normal-weight individuals. From this perspective, obesity can be viewed as a disorder in which the biologically defended level of body fat mass is increased. Recent findings implicate inflammation in key hypothalamic areas for body weight control in this process. In this review, we present an overview of energy homeostasis—the biological process that underlies the control of body fat mass—and describe evidence that defects in this regulatory system contribute to obesity pathogenesis. We then address molecular characteristics of hypothalamic inflammation and their implications for obesity pathogenesis (detailed more extensively in refs. 2,3), followed by evidence linking high-fat diet (HFD) feeding to neuropathological alteration of key hypothalamic areas controlling energy balance. We conclude by considering how cell-cell interactions may contribute to this deleterious hypothalamic response and the implications of these interactions for obesity pathogenesis.
The case for energy homeostasis
Homeostatic response to weight loss.
Following a period of caloric restriction, lost weight is gradually but inexorably recovered in most individuals. This effect involves the capacity of the brain to sense the reduction of body energy stores and activate responses to compensate for this deficit. In simple terms, voluntary weight loss triggers increases of both appetite and energy efficiency, such that both sides of the energy balance equation shift in favor of weight gain (4,5). Perhaps the clearest example of this compensatory response to lost weight comes from the large clinical literature of diet studies in human subjects. Some 20 years ago, Safer (6) reported that the vast majority (>90%) of weight lost through hypocaloric dieting in humans is regained over a 5-year period. Even with the addition of exercise (which slows the rate of weight regain [7]), caloric restriction through lifestyle modification is a Sisyphean task over the long term. As an example, obese subjects enrolled in the lifestyle intervention arm of the landmark Diabetes Prevention Program (DPP) study averaged ∼7% weight loss during the trial but regained virtually all of the weight lost over the full 10 years of postintervention monitoring (8). Lifestyle intervention in the follow-up Look AHEAD trial similarly failed to produce durable weight loss (9).
Robust defense of body energy stores is similarly observed in conditions that promote weight loss by increasing energy expenditure rather than reducing intake, such as housing mice in a cold environment. To survive, a marked increase of energy expenditure is required to support thermogenesis and maintain core body temperature. Increased heat production occurs initially at the expense of modest body fat depletion, but as fat loss proceeds, food intake increases in a commensurate manner to offset further loss of body fat stores (10). Consequently, graded decreases of environmental temperature trigger proportionate increases of energy expenditure that are compensated by increased energy intake so as to maintain body fat stores.
Adaptive responses to weight gain.
From the foregoing, it is evident that the energy homeostasis system responds robustly to states of negative energy balance, whether secondary to caloric restriction or increased energy expenditure. Does the energy homeostasis system also protect against weight gain? Available data from both animal models and humans support this possibility. A classic demonstration of this phenomenon was provided by the “Experimental Obesity in Man” studies performed at the University of Vermont almost 50 years ago (11). In these experiments, lean human volunteers were challenged to increase their body weight by 20% through overeating for a period of up to 6 months. Although overeating was initially accomplished without difficulty, the subjects’ capacity to do so soon began to wane, presumably because counterregulatory mechanisms were engaged that reduce appetite. After reaching the prescribed 20% increase of body weight and being released from the experimental mandate to overeat, the subjects displayed several weeks of profound anorexia that caused the excess pounds to be shed. Only when body weight began to converge on preintervention values did food intake also return to normal levels. Similar observations were made in subsequent human overfeeding studies (12), and across a variety of animal models as well (13).
In the face of a challenge to body fat stores in either direction, therefore, robust homeostatic responses drive the return of body weight to its original, biologically defended level (sometimes referred to as a set-point). If normal-weight individuals defend against experimentally induced changes of body fat mass in either direction, it follows that obesity pathogenesis should involve impairment of the energy homeostasis system. Since overweight individuals appear, by and large, to defend their body fat mass as robustly as normal weight individuals (14), obesity does not appear to reflect a failure of energy homeostasis so much as the biological defense of an elevated level of body fat mass.
Obesity and energy homeostasis
An important strength of the homeostasis model for understanding obesity pathogenesis is that it offers a plausible explanation for why conservative approaches to weight loss fail over time. To understand how a slow, progressive increase in the defended level of body fat stores might occur, it is helpful to consider how the energy homeostasis system operates in normal-weight individuals.
How does energy homeostasis work?
Energy homeostasis involves an adiposity negative feedback system through which circulating signals such as the hormone leptin inform key brain centers about body energy stores (15). In response to a change of body fat mass, corresponding changes of plasma leptin levels and hence of leptin signaling in these brain areas elicit changes of both energy intake and energy expenditure that favor the return of body weight to preintervention levels. In response to weight loss due to caloric restriction, this model predicts that falling plasma levels of leptin and other adiposity negative feedback signals (e.g., insulin) elicit brain responses that minimize further weight loss and promote the eventual recovery of lost weight. Leptin also responds robustly to short-term changes of energy balance, prior to major changes in adiposity, and therefore integrates information about both long-term and short-term energy status (16,17).
A growing number of leptin-responsive neuronal subsets have been identified, and their roles in adaptive changes of energy balance are increasingly well understood. Perhaps best studied of these are two leptin-sensitive neuronal subsets situated in the hypothalamic arcuate nucleus (ARC). Activation of neuropeptide Y and agouti-related peptide (NPY/AgRP) neurons potently increases food intake and reduces energy expenditure through the paracrine action of the neuropeptides NPY and AgRP themselves and through inhibition of downstream neuronal activity via GABAergic signaling (15,18,19). Conversely, reduced intake and increased expenditure occurs with activation of proopiomelanocortin (POMC) neurons largely through the action of the anorexigenic neuropeptide α-melanocyte–stimulating hormone (15) (although direct neurotransmitter action likely contributes as well [20]). Weight loss reduces leptin levels causing activation of NPY/AgRP neurons and inhibition of POMC neurons (15). This combination potently favors positive energy balance and is sustained until lost weight has been regained.
A large literature now indicates that proper functioning of these neurocircuits is required for normal energy homeostasis in rodent models, and many models of obesity caused by impairment of the leptin-melanocortin pathway now exist. A similar role was recently invoked for a distinct subset of leptin-sensitive ARC neurons containing neuronal nitric oxide synthetase. Deletion of leptin receptors from these neurons causes hyperphagia and obesity even more severe than that induced by defective melanocortin signaling (21). Neurons in other hypothalamic areas, including the ventromedial, lateral hypothalamic, and paraventricular nuclei, and in extrahypothalamic areas, including hindbrain (e.g., nucleus of the solitary tract), midbrain (ventral tegmental area), and forebrain (nucleus accumbens), are also critical for normal energy homeostasis.
That the aforementioned hypothalamic neurocircuits are essential for normal energy homeostasis in humans is supported by insights gained from rare monogenic forms of human obesity. Loss-of-function mutations affecting the leptin-melanocortin pathway, including genes encoding leptin, melanocortin-4 receptor (Mc4r), leptin receptor, and the prohormone convertase enzyme that processes POMC, together constitute the most prevalent, highly penetrant genetic causes of human obesity (22). However, ∼95% of human obesity is due not to a single, highly penetrant mutation but rather to complex interactions between genetic and environmental factors that favor both weight gain and the defense of elevated body weight. Impaired leptin responsiveness is a feature of these more common forms of obesity as well and hence may contribute to obesity pathogenesis in susceptible individuals.
Hypothalamic inflammation and leptin resistance.
Leptin resistance can be defined simply as an acquired or inherited defect in the response to leptin. This simplistic definition is problematic, however, since such leptin resistance can potentially arise from a long list of defects—not only those affecting leptin receptor signal transduction, but at innumerable points downstream of or in parallel to leptin-mediated effects (23). For example, obesity due to Mc4r mutation, the most common monogenic form of human obesity, is characterized by leptin resistance, since leptin action on energy balance requires that it activate the melanocortin pathway. From this perspective, the term leptin resistance is easily misused, since a blunted feeding response to leptin is present in virtually all forms of obesity, save for that caused by leptin deficiency (23). This conundrum has to some extent confounded efforts to delineate underlying mechanisms.
Despite this caveat, growing evidence suggests a causal role for leptin resistance in obesity pathogenesis and that obesity-associated hypothalamic inflammation can underlie this resistance. This hypothesis originated following clear evidence that inflammatory signaling contributes to obesity-associated insulin resistance in peripheral tissues such as liver, muscle, and adipose tissue (24). Among neuronal consequences of proinflammatory signaling is a disruption of intracellular signal transduction downstream of both insulin and leptin receptors via the insulin receptor substrate–phosphatidylinositol 3-kinase pathway (2). Leptin signaling via the janus kinase–signal transducer and activator of transcription pathway can also be impaired by cellular inflammation (25,26), suggesting that this mechanism may contribute not only to obesity-associated leptin and insulin resistance, but also to the associated increase in the defended level of body fat stores. However, dissociating cause and effect has been challenging, and evidence of leptin resistance caused by inflammation independently of obesity is lacking (though studies of aging, injury, and endotoxin-induced leptin resistance are suggestive of this possibility [27–29]). We favor the hypothesis that a vicious cycle exists involving obesity, leptin resistance, and inflammation. According to this model, diet-induced increases of inflammation beget a state of leptin resistance that promotes weight gain, which in turn triggers further inflammation and leptin resistance, eventually resulting in the biological defense of an elevated level of body fat. This perspective highlights the challenges inherent in determining the extent to which leptin resistance and inflammation are causes or consequences of weight gain, a challenge that cannot be met without methodologies that can distinguish cellular responses to diet from metabolic alterations induced by obesity itself.
Does hypothalamic inflammation cause obesity?
The first report of obesity-associated hypothalamic inflammation was in a rat model of diet-induced obesity (DIO) published in 2005 (30), and many investigators have since replicated this finding (2,3,31–36). The observation that genetic interventions that disrupt neuronal inflammation can block both obesity and hypothalamic leptin resistance during HFD feeding implicates this inflammation in obesity pathogenesis. For this to be the case, however, hypothalamic inflammation would have to occur prior to obesity onset, unlike the later onset of inflammation in peripheral tissues. This hypothesis is supported by our recent observation that in rats predisposed to DIO, expression of proinflammatory biomarkers increases in the mediobasal hypothalamus within 24 h of HFD onset (37). The mediobasal hypothalamus level of these markers increases more by day 3 and is followed by a transient normalization of inflammation, only to rise again in 2–3 weeks and remain elevated thereafter. Interestingly, this initial rise and fall of hypothalamic inflammatory markers follows a temporal pattern similar to that of caloric intake following the switch to an HFD.
Several molecules and pathways have been identified as candidate mediators of hypothalamic inflammation during HFD feeding. Among these are the serine kinases c-Jun N-terminal kinase (Jnk) and inhibitor of nuclear factor-κB kinase (IKKβ) as well as Toll-like receptor 4 (TLR4), the ceramide biosynthesis pathway, and the endoplasmic reticulum (ER) stress pathway (30–36). Each of these is upregulated in the rodent hypothalamus during DIO (30,36,38), and Jnk and IKKβ can inhibit insulin and leptin signaling by at least two mechanisms: induction of the signal termination molecule suppressor of cytokine signaling-3 and serine phosphorylation of the essential pathway intermediate insulin receptor substrate (3,24). Targeted disruption of each of these pathways in a hypothalamus-specific manner (by pharmacologic, viral gene transfer, or genetic means) limits the extent of HFD-induced obesity, hypothalamic leptin resistance, and systemic insulin resistance (30–36), implying a contributory role for these mechanisms of hypothalamic inflammation in the pathogenesis of DIO and its metabolic sequelae. Conversely, although acute or robust central nervous system inflammation is typically associated with anorexia and weight loss, interventions that generate more modest levels of hypothalamic inflammation (e.g., virally mediated neuronal expression of constitutively active IKKβ, or central administration of either low-dose tumor necrosis factor-α or the ER stress inducer thapsigargin) can produce mild obesity/metabolic syndrome phenotypes (34,36,39,40). These data collectively suggest that in some contexts, hypothalamic inflammation is both necessary and sufficient for DIO. As such, an important research priority is to determine the relative importance of the many inflammatory signaling molecules and pathways implicated in promoting leptin resistance and obesity.
Although these findings collectively offer evidence of a causal role for hypothalamic inflammation in the pathogenesis of DIO in rodent models, there are conflicting results that cast doubt on this hypothesis. First, several models of increased tissue inflammation (e.g., fasting in adipose tissue [41] and p50 knockout in liver [42]) show improved rather than worsened insulin sensitivity, suggesting that the relationship between hormone signaling and inflammation is context dependent. More importantly, a number of total-body proinflammatory cytokine and cytokine receptor knockout models (e.g., interleukin [IL]-1, IL-6, IL-1 receptor, and tumor necrosis factor-α receptors [43–46]) display obesity phenotypes rather than being protected from DIO. Conversely, IL-1 receptor antagonist–deficient mice show a lean, hypermetabolic phenotype with increased leptin sensitivity despite higher levels of IL-1 activity (47). Reconciling these results with the literature supporting a role for hypothalamic inflammation in DIO is inherently difficult because these are largely whole-body gene knockouts that affect multiple tissues and may cause developmental compensation. In many instances, the degree of change in inflammatory signaling in these models is much greater than that observed with HFD-induced obesity. Lastly, the hypothalamus was not specifically examined in these models. However, two recent studies have demonstrated dissociation between weight changes and alterations in hypothalamic inflammation. In the first, DIO-sensitive HFD-fed rats switched back to regular chow for 8 weeks were observed to lose all their excess weight despite sustained elevations in hypothalamic inflammatory gene expression (48). Conversely, neuronal peroxisome proliferator–activated receptor-δ knockout mice are resistant to HFD-induced hypothalamic inflammation yet are more susceptible to DIO than wild-type controls (49). Thus, the evidence supporting a direct role of hypothalamic inflammation in promoting weight gain is clearly mixed. These considerations raise the possibility that HFD-induced hypothalamic inflammation is but one element of a more comprehensive injury process that itself contributes to obesity pathogenesis, a hypothesis that we explore further below.
Hypothalamic gliosis and neuron injury
At the cellular level, exposure of neurons to nutrient excess represents a significant stress that not only engages adaptive mechanisms such as autophagy and ER stress that limit neuronal damage, but also involves neighboring cell populations. More than 50% of the cellular composition of the brain is nonneuronal, including glial, vascular, and periventricular constituents (50). Astrocytes and microglia are the most abundant of these specialized cell types and, in addition to comprising much of the basic architecture of brain parenchyma, they maintain the blood-brain barrier, support neuronal metabolism, and both guard against and react to local tissue injury. To accomplish these various functions, both astrocytes and microglia are capable of remarkable plasticity, altering both their genetic programs and cellular morphologies (called reactive gliosis) to combat infection, support or consume damaged neurons, and direct the restorative process (51,52). Interestingly, rats receiving systemic leptin administration 3 h prior to cerebral ischemia had reduced infarct volume with signal transducer and activator of transcription-3 activation in astrocytes of the ischemic penumbra (53). Similarly, leptin administration and fasting reciprocally modulate glucose and glutamate transporter expression in hypothalamic astrocytes. These observations are suggestive of an interaction between nutritional status and glial cell function (54).
Whereas the link between hypothalamic inflammation and obesity pathogenesis is the focus of a sizeable literature, the role of astrocytes and microglia in energy homeostasis is comparatively unexplored. Both cell types play crucial roles in synaptic remodeling (55), metabolic coupling at active synapses (56), and neurotransmitter reuptake (57), functions that ultimately influence neuronal activity and behavior. Astrocytes and microglia play central roles in the central nervous system response to injury but can provide either neuroprotection or perpetuate neurotoxicity depending on the nature of the underlying insult (51,58). In neurodegenerative conditions such as Alzheimer’s disease, amyotrophic lateral sclerosis, and multiple sclerosis, microglia and astrocytes often accelerate the disease process by generating cytokines, reactive oxygen species, and other toxic mediators involved in clearing degenerating cells. By amplifying inflammatory signals in the hypothalamus analogously to the recruitment and activation of proinflammatory immune cells in adipose and other peripheral tissues (59,60), both cell types have the potential to impair neuronal function in ways that favor leptin resistance and associated weight gain. Lending support to this argument, TLR4, a putative mediator of saturated fatty acid–induced inflammatory signaling, is abundantly expressed by microglia (33), and acute inactivation of TLR4 by central administration of an anti-TLR4 antibody can reduce weight gain during HFD feeding (33). In addition, reactive astrocytes and microglia accumulate in the hypothalamus during long-term HFD consumption at times when hypothalamic inflammation is clearly evident (33,37). In contrast, HFD-fed mice subjected to an involuntary exercise regimen exhibit modest improvements in glucose tolerance along with reduced hypothalamic microglial activation (61), suggesting a link between microglial phenotype and obesity-associated metabolic impairment. Finally, in a nonhuman primate model, microglial activation along with increased expression of inflammatory pathway genes was observed in fetuses from HFD-fed mothers, raising the possibility that hypothalamic microglia contribute to the effect of intrauterine programming to influence the adult phenotype (62).
Other studies, however, suggest a more complex picture in which microglia and astrocytes play a protective role by limiting the deleterious hypothalamic consequences of consuming an HFD. First, mice with moderately increased production of IL-6 from astrocytes were protected from DIO, rather than being more susceptible (63). Moreover, adult rats overfed during the neonatal period manifest hypothalamic microglial activation (as evidenced by major histocompatibility complex class II expression) without local inflammation (64), and hypothalamic microglia from mice fed an HFD accumulate the generally anti-inflammatory molecule IgG (65). Finally, our recent work suggests that effects of short-term HFD feeding on hypothalamic inflammation and reactive gliosis are separable from one another (37). Specifically, whereas both processes were evident within the first week of HFD consumption, hypothalamic inflammation subsided over the next 2 weeks despite accumulation of enlarged microglia in the ARC that continued unabated.
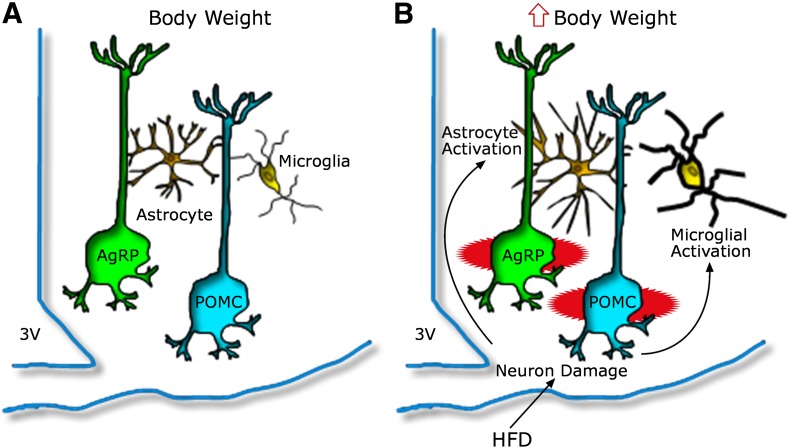
Although definitive answers are still awaited, these results are consistent with a model (Fig. 1) in which gliosis develops initially as a neuroprotective response to cope with neuronal stress induced by HFD feeding. In this scenario, glial responses initially constrain hypothalamic inflammatory signaling. With prolonged exposure to an HFD, however, astrocytes and/or microglia may eventually convert to a more proinflammatory, neurotoxic phenotype. Detailed cellular phenotyping and carefully timed functional interventions should help clarify these possibilities.
FIG. 1.
Model depicting the hypothalamic response to an HFD in animals predisposed to DIO. A: AgRP and POMC neurons are integral components of energy balance neurocircuitry located in the ARC nucleus, situated adjacent to the third cerebral ventricle (3V) along the floor of the hypothalamus. Activity of these neurons is sensitive to input from circulating hormones (e.g., leptin and insulin) and nutrients, and it plays an important role to establish the defended level of body weight. B: Recent evidence suggests that during HFD feeding, these neurons may be injured via unknown mechanisms, and that this injury triggers activation of local glial cell populations (astrocytes and microglia). This neuron injury and reactive gliosis can in turn impair homeostatic control of body fat stores, leading to increased body weight.
Integrated model of neuronal injury and obesity pathogenesis
Like cells in peripheral tissues of animals fed an HFD, hypothalamic neurons confronted by nutrient excess are proposed to respond by engaging mechanisms that limit excessive hormone- and nutrient-related signaling, manage organellar stress, and maintain structural integrity. At the same time, these neurons also integrate signaling inputs from neurons in other brain regions that may themselves be reacting to the change in diet. These various challenges to neuronal function are compatible with evidence that in rats predisposed to DIO, hypothalamic neurons initiate a stress response (as measured by up-regulation of the chaperone protein hsp72) within the first week of HFD exposure, close to the onset of hypothalamic inflammation (37). The accumulation of reactive astrocytes and microglia in the mediobasal hypothalamus occurs around the same time and precedes a subsequent reduction in the level of inflammation (Fig. 1).
With prolonged HFD exposure, however, POMC neurons exhibit increased autophagic activity and eventually (after 8 months) appear to decrease in number by 25% (37), consistent with prior reports of apoptosis of these neurons in mice with DIO (66). Based on these considerations, we speculate that hypothalamic inflammation during HFD feeding originates primarily within neurons rather than glial cells, and that the associated gliosis occurs in response to neuron injury and is initially neuroprotective. This hypothesis does not exclude the possibility that glial cells that respond directly to dietary components and/or nutrient excess are also drivers of inflammation and/or neuronal injury, but to date we are unaware of data that directly support this view. If chronic exposure to an HFD causes permanent damage to or death of neurons comprising energy balance neurocircuits, obesity susceptibility and other phenotypic outcomes may depend on the degree to which these neuronal subsets are susceptible to this type of stress. For example, if neuron injury and loss occur preferentially in POMC over AgRP neurons, the balance of functional anorexigenic to orexigenic inputs should shift so as to favor leptin resistance, excess weight gain, and an upward resetting of the defended level of body fat mass.
Although the extent to which obesity and/or HFD feeding impacts various hypothalamic cell populations in humans remains uncertain, early insights from brain imaging studies are beginning to support this type of neuropathological model. A retrospective analysis of magnetic resonance images from 34 subjects (BMI range 17.7–44.1 kg/m2) revealed evidence of gliosis in the mediobasal hypothalamus that correlated with BMI (37), and a separate study reported an inverse correlation between systemic inflammation (measured as serum fibrinogen) and the integrity of brain structures involved in food reward and feeding behavior measured by magnetic resonance imaging in 44 overweight/obese subjects (67). Brain imaging (including new approaches to the use of PET imaging to detect microglial activation) and other modalities have important potential to ascertain whether human obesity involves cumulative ARC neuronal damage and whether such an effect is reversible and/or predictive of either future weight gain or weight regain following voluntary weight loss.
Summary
Our understanding of obesity-associated hypothalamic inflammation—its underlying causes, the contributions made by distinct cell types, the extent to which it is reflective of tissue injury versus repair, and its implications for obesity pathogenesis and treatment—remains incomplete, but the field is evolving rapidly. Although hypothalamic inflammation shares several features with responses observed in peripheral tissues (e.g., comparable increases of some of the same inflammatory biomarkers, activation of local immune cells), its many unique features identify it as a fundamentally distinct process. For one, inflammation occurs much more rapidly in the hypothalamus than in peripheral tissues following the switch to an obesogenic HFD, such that it precedes weight gain. Available data are compatible with a model in which the initial cause of hypothalamic inflammation induced by HFD feeding involves injury to neurons that comprise energy balance neurocircuits. In turn, this injury may undermine homeostatic responses that protect against weight gain, thereby contributing to obesity pathogenesis. Additional studies are warranted to critically test this model and determine if therapeutic interventions targeting this process have a role in the future of obesity treatment.
ACKNOWLEDGMENTS
This work was supported by a National Institutes of Health (NIH) Fellowship Training Program Award (T32DK007247), NIH National Research Service Award (F32DK091989), the NIH-funded University of Washington Nutrition Obesity Research Center and the Diabetes Research Center, and NIH grants to M.W.S. (DK090320, DK083042, and DK052989) and J.P.T. (DK088872). J.P.T. was also supported by a Beginning Grant-in-Aid from the American Heart Association.
M.W.S. has consulted for Santarus and Pfizer. No other potential conflicts of interest relevant to this article were reported.
J.P.T. and M.W.S. wrote the manuscript. S.J.G. and B.E.W. reviewed and edited the manuscript. M.D.D. contributed the figure and reviewed and edited the manuscript.
REFERENCES
- 1.Wadden TA, Sternberg JA, Letizia KA, Stunkard AJ, Foster GD. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five-year perspective. Int J Obes 1989;13(Suppl. 2):39–46 [PubMed] [Google Scholar]
- 2.Thaler JP, Schwartz MW. Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 2010;151:4109–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velloso LA, Schwartz MW. Altered hypothalamic function in diet-induced obesity. Int J Obes (Lond) 2011;35:1455–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr 2008;88:906–912 [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum M, Kissileff HR, Mayer LE, Hirsch J, Leibel RL. Energy intake in weight-reduced humans. Brain Res 2010;1350:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safer DJ. Diet, behavior modification, and exercise: a review of obesity treatments from a long-term perspective. South Med J 1991;84:1470–1474 [PubMed] [Google Scholar]
- 7.Pronk NP, Wing RR. Physical activity and long-term maintenance of weight loss. Obes Res 1994;2:587–599 [DOI] [PubMed] [Google Scholar]
- 8.Knowler WC, Fowler SE, Hamman RF, et al. Diabetes Prevention Program Research Group 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wing RR, Look AHEAD Research Group Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med 2010;170:1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao ZJ, Chi QS, Cao J, Han YD. The energy budget, thermogenic capacity and behavior in Swiss mice exposed to a consecutive decrease in temperatures. J Exp Biol 2010;213:3988–3997 [DOI] [PubMed] [Google Scholar]
- 11.Sims EA, Goldman RF, Gluck CM, Horton ES, Kelleher PC, Rowe DW. Experimental obesity in man. Trans Assoc Am Physicians 1968;81:153–170 [PubMed] [Google Scholar]
- 12.Dériaz O, Fournier G, Tremblay A, Després JP, Bouchard C. Lean-body-mass composition and resting energy expenditure before and after long-term overfeeding. Am J Clin Nutr 1992;56:840–847 [DOI] [PubMed] [Google Scholar]
- 13.Hagan MM, Rushing PA, Schwartz MW, et al. Role of the CNS melanocortin system in the response to overfeeding. J Neurosci 1999;19:2362–2367 [DOI] [PMC free article] [PubMed]
- 14.Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA. Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am J Clin Nutr 1992;56:641–655 [DOI] [PubMed] [Google Scholar]
- 15.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–671 [DOI] [PubMed] [Google Scholar]
- 16.Grinspoon SK, Askari H, Landt ML, et al. Effects of fasting and glucose infusion on basal and overnight leptin concentrations in normal-weight women. Am J Clin Nutr 1997;66:1352–1356 [DOI] [PubMed] [Google Scholar]
- 17.Kolaczynski JW, Considine RV, Ohannesian J, et al. Responses of leptin to short-term fasting and refeeding in humans: a link with ketogenesis but not ketones themselves. Diabetes 1996;45:1511–1515 [DOI] [PubMed] [Google Scholar]
- 18.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 2011;14:351–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 2008;11:998–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhan C, Zhou J, Feng Q, et al. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci 2013;33:3624–3632 [DOI] [PMC free article] [PubMed]
- 21.Leshan RL, Greenwald-Yarnell M, Patterson CM, Gonzalez IE, Myers MG., Jr Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat Med 2012;18:820–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farooqi IS, O’Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab 2008;4:569–577 [DOI] [PubMed] [Google Scholar]
- 23.Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 2010;21:643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011;29:415–445 [DOI] [PubMed] [Google Scholar]
- 25.Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 2008;70:537–556 [DOI] [PubMed] [Google Scholar]
- 26.Shi X, Wang X, Li Q, et al. Nuclear factor κB (NF-κB) suppresses food intake and energy expenditure in mice by directly activating the Pomc promoter. Diabetologia 2013;56:925–936 [DOI] [PubMed] [Google Scholar]
- 27.Morrison CD, White CL, Wang Z, et al. Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology 2007;148:433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bigford GE, Bracchi-Ricard VC, Nash MS, Bethea JR. Alterations in mouse hypothalamic adipokine gene expression and leptin signaling following chronic spinal cord injury and with advanced age. PLoS ONE 2012;7:e41073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borges BC, Rorato R, Avraham Y, et al. Leptin resistance and desensitization of hypophagia during prolonged inflammatory challenge. Am J Physiol Endocrinol Metab 2011;300:E858–E869 [DOI] [PubMed] [Google Scholar]
- 30.De Souza CT, Araujo EP, Bordin S, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 2005;146:4192–4199 [DOI] [PubMed] [Google Scholar]
- 31.Holland WL, Bikman BT, Wang LP, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 2011;121:1858–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleinridders A, Schenten D, Könner AC, et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab 2009;10:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milanski M, Degasperi G, Coope A, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci 2009;29:359–370 [DOI] [PMC free article] [PubMed]
- 34.Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 2009;9:35–51 [DOI] [PubMed] [Google Scholar]
- 35.Posey KA, Clegg DJ, Printz RL, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 2009;296:E1003–E1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008;135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012;122:153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 2004;145:4880–4889 [DOI] [PubMed] [Google Scholar]
- 39.Arruda AP, Milanski M, Coope A, et al. Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion. Endocrinology 2011;152:1314–1326 [DOI] [PubMed] [Google Scholar]
- 40.Purkayastha S, Zhang H, Zhang G, Ahmed Z, Wang Y, Cai D. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc Natl Acad Sci USA 2011;108:2939–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kosteli A, Sugaru E, Haemmerle G, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest 2010;120:3466–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang T, Zhang J, Yin J, et al. Uncoupling of inflammation and insulin resistance by NF-kappaB in transgenic mice through elevated energy expenditure. J Biol Chem 2010;285:4637–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García MC, Wernstedt I, Berndtsson A, et al. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes 2006;55:1205–1213 [DOI] [PubMed] [Google Scholar]
- 44.Chida D, Osaka T, Hashimoto O, Iwakura Y. Combined interleukin-6 and interleukin-1 deficiency causes obesity in young mice. Diabetes 2006;55:971–977 [DOI] [PubMed] [Google Scholar]
- 45.Pamir N, McMillen TS, Kaiyala KJ, Schwartz MW, LeBoeuf RC. Receptors for tumor necrosis factor-alpha play a protective role against obesity and alter adipose tissue macrophage status. Endocrinology 2009;150:4124–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreyer SA, Chua SC, Jr, LeBoeuf RC. Obesity and diabetes in TNF-alpha receptor-deficient mice. J Clin Invest 1998;102:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somm E, Henrichot E, Pernin A, et al. Decreased fat mass in interleukin-1 receptor antagonist-deficient mice: impact on adipogenesis, food intake, and energy expenditure. Diabetes 2005;54:3503–3509 [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Ge A, Cheng M, et al. Increased hypothalamic inflammation associated with the susceptibility to obesity in rats exposed to high-fat diet. Exp Diabetes Res 2012;2012:847246 [DOI] [PMC free article] [PubMed]
- 49.Kocalis HE, Turney MK, Printz RL, et al. Neuron-specific deletion of peroxisome proliferator-activated receptor delta (PPARδ) in mice leads to increased susceptibility to diet-induced obesity. PLoS ONE 2012;7:e42981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. McGraw-Hill, Health Professions Division, New York, 2000 [Google Scholar]
- 51.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 2009;32:638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia 2005;50:427–434 [DOI] [PubMed] [Google Scholar]
- 53.Amantea D, Tassorelli C, Russo R, et al. Neuroprotection by leptin in a rat model of permanent cerebral ischemia: effects on STAT3 phosphorylation in discrete cells of the brain. Cell Death Dis 2011;2:e238 [DOI] [PMC free article] [PubMed]
- 54.Fuente-Martín E, García-Cáceres C, Granado M, et al. Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest 2012;122:3900–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011;333:1456–1458 [DOI] [PubMed]
- 56.Pellerin L, Bouzier-Sore AK, Aubert A, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 2007;55:1251–1262 [DOI] [PubMed] [Google Scholar]
- 57.Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science 2001;292:923–926 [DOI] [PubMed]
- 58.Neumann J, Gunzer M, Gutzeit HO, Ullrich O, Reymann KG, Dinkel K. Microglia provide neuroprotection after ischemia. FASEB J 2006;20:714–716 [DOI] [PubMed] [Google Scholar]
- 59.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011;121:2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yi CX, Al-Massadi O, Donelan E, et al. Exercise protects against high-fat diet-induced hypothalamic inflammation. Physiol Behav 2012;106:485–490 [DOI] [PubMed] [Google Scholar]
- 62.Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 2010;151:1622–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hidalgo J, Florit S, Giralt M, Ferrer B, Keller C, Pilegaard H. Transgenic mice with astrocyte-targeted production of interleukin-6 are resistant to high-fat diet-induced increases in body weight and body fat. Brain Behav Immun 2010;24:119–126 [DOI] [PubMed] [Google Scholar]
- 64.Tapia-González S, García-Segura LM, Tena-Sempere M, et al. Activation of microglia in specific hypothalamic nuclei and the cerebellum of adult rats exposed to neonatal overnutrition. J Neuroendocrinol 2011;23:365–370 [DOI] [PubMed] [Google Scholar]
- 65.Yi CX, Tschöp MH, Woods SC, Hofmann SM. High-fat-diet exposure induces IgG accumulation in hypothalamic microglia. Dis Model Mech 2012;5:686–690 [DOI] [PMC free article] [PubMed]
- 66.Moraes JC, Coope A, Morari J, et al. High-fat diet induces apoptosis of hypothalamic neurons. PLoS ONE 2009;4:e5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cazettes F, Cohen JI, Yau PL, Talbot H, Convit A. Obesity-mediated inflammation may damage the brain circuit that regulates food intake. Brain Res 2011;1373:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]