Obesity and weight gain are frequently associated with increases in blood pressure, driven in part by sympathoexcitation (1). Obesity also is linked with insulin resistance and is associated with ongoing public health concerns, including the so-called metabolic syndrome (2). Even small increases in plasma insulin concentration (as low as one-fifth of that achieved after a mixed meal) (3) have marked and potentially long-lasting sympathoexcitatory effects (4). Such findings have important implications for the fluctuations in circulating catecholamines and blood pressure that occur in the postprandial state, as well as for conditions with sustained hyperinsulinemia. Yet the question remains, what are the mechanisms driving sympathoexcitation with hyperinsulinemia?
In human studies, a systemic infusion of insulin has been shown to result in a gradual increase in muscle sympathetic nerve activity that remains elevated after plasma levels return to baseline (5). This finding supports the idea that insulin-mediated sympathoexcitation is the result of a central mechanism, requiring time for insulin to pass through the blood-brain barrier. Along these lines, recent data from animals suggest insulin acts within the arcuate nucleus to increase sympathetic activity via pathways including the paraventricular nucleus of the hypothalamus and the rostral ventrolateral medulla (6–8). These new ideas have important implications for the myriad of disorders related to sustained hyperinsulinemia and sympathoexcitation. But are there other sites important in the insulin-mediated sympathoexcitatory response?
More than 50 years ago, Pereda et al. (9) showed that insulin injected into the carotid artery of anesthetized dogs increases arterial blood pressure, and this increase could not be achieved when similar doses were infused systemically. In addition, the insulin-mediated increase in blood pressure was abolished by ganglionic blockade, suggesting the increase in pressure was neurally mediated (9). Such findings led investigators to propose that insulin-mediated sympathoexcitation was central in origin; however, could this sympathoexcitation be caused, in part, by stimulation of the carotid body?
The carotid body chemoreceptors are recognized as multimodal sensors capable of sensing and responding to a variety of peripheral stimuli (e.g., hypoxia, hypercapnia, inflammation). In addition to their well-known ability to sense and respond to changes in peripheral oxygen levels, there is emerging evidence that the carotid body chemoreceptors sense low blood glucose and play an important role in neuroendocrine responses during a hyperinsulinemic–hypoglycemic clamp (10). Consistent with possible insulin-sensing capabilities of the carotid chemoreceptors, Bin-Jaliah et al. (11) observed an increase in ventilation after insulin infusion in rats that was blunted after carotid sinus nerve resection. However, a direct role for the carotid body chemoreceptors in insulin-mediated sympathoexcitation was previously unknown.
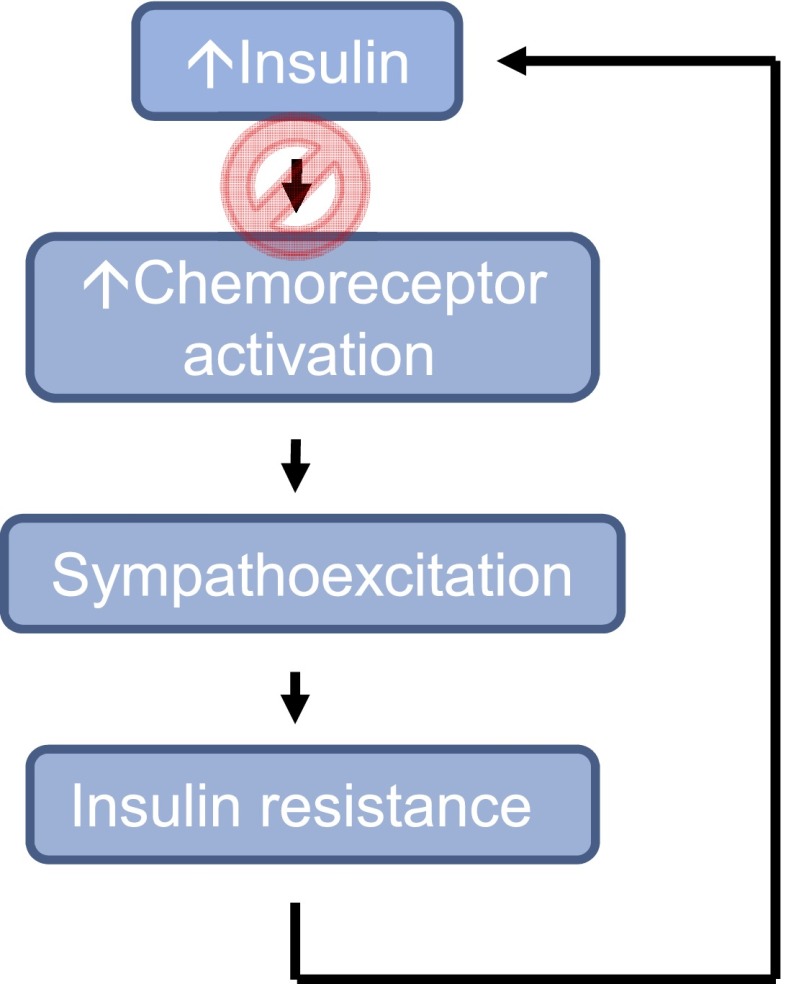
In this issue of Diabetes, Ribeiro et al. (12) directly examined insulin-sensing capabilities of the carotid chemoreceptors and their potential role in the development of insulin resistance and hypertension. The authors presented key findings showing both the presence of insulin receptors in the carotid bodies and an increase in carotid body activation (neurotransmitter release, hyperventilation) with insulin exposure (12). Additionally, animal models of insulin resistance and hypertension were generated using high-fat and high-sucrose diets (similar to typical diets of many humans today, i.e., 35% sucrose in drinking water or a 45% fat diet). As expected, these models exhibited increased carotid body activity (spontaneous ventilation and ventilatory responses to ischemic hypoxia) (12). However, bilateral carotid sinus nerve resection prevented diet-induced insulin resistance, hypertension, and carotid body overactivation (12). Taken together, it appears that hyperinsulinemia triggers sympathoadrenal overactivity via the carotid chemoreceptor and perhaps contributes to the development of insulin resistance, hypertension, and other physiological disturbances typically associated with the metabolic syndrome (Fig. 1).
FIG. 1.
Insulin triggers carotid chemoreceptor activation in animal models and subsequent increases in sympathetic outflow, which potentially contributes to insulin resistance, hyperinsulinemia, and further activation of the carotid chemoreceptors. Thus, inhibition of chemoreceptor activation may be a viable treatment option for diseases with sustained hyperinsulinemia and sympathoexcitation.
Consistent with these findings, the carotid body was recently identified as a therapeutic target for the treatment of a myriad of sympathetically mediated diseases (13). For example, chemoreceptor denervation can blunt the hypertensive response to 30 days of intermittent hypoxia (14). Additionally, in patients with chronic obstructive pulmonary disease, insulin resistance is reversed with acute supplemental oxygen (which should “turn-off” the carotid body chemoreceptors) (15), and continuous positive airway pressure treatment was shown to improve the increased chemoreflex sensitivity and insulin resistance observed in patients with metabolic syndrome (16). Despite these suggestive findings, Ribeiro et al. (12) were the first to systematically address insulin-sensing capabilities of the carotid body chemoreceptors and to present novel ideas and provocative data identifying the carotid chemoreceptors as a potential therapeutic target.
However, there are some challenges associated with translating these findings to humans. First, this invasive animal work must be conducted under anesthesia, which can effect ventilation and may result in hypoxemia, which could independently alter carotid body–mediated responses. Additionally, it is difficult to distinguish chemoreceptor from baroreceptor activity after carotid sinus denervation. Given the increased prevalence and observed clustering of obesity, inflammation, intermittent hypoxia, hypertension, hyperglycemia, and insulin resistance, studies in humans on this topic are critical. However, determining if the carotid chemoreceptors respond to insulin in humans will require innovative experimental approaches because of the limitations noted.
Altogether, recent evidence from animal models supports a direct effect of insulin on carotid body chemoreceptor–mediated activation of the sympathetic nervous system and a possible role for the carotid chemoreceptors in the development of hypertension and insulin resistance. Additionally, indirect evidence from a variety of human studies suggests the carotid bodies are sensitive to insulin, although definitive data are lacking. Finally, there is also some evidence that the carotid bodies are stimulated by circulating inflammatory mediators that also are elevated in the metabolic syndrome (17). In this context, studies are needed that examine the effects of insulin on the carotid bodies as well as the interactive effects of insulin, glucose, inflammation, and oxygen on carotid chemoreceptor–mediated responses. Understanding these interactions may be a key step in developing novel prevention or treatment interventions for the many diseases of the modern world related to sustained hyperinsulinemia.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 2905.
REFERENCES
- 1.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 2006;48:787–796 [DOI] [PubMed] [Google Scholar]
- 2.Kahn R. Metabolic syndrome: is it a syndrome? Does it matter? Circulation 2007;115:1806–1810; discussion 1811 [DOI] [PubMed]
- 3.Vollenweider P, Randin D, Tappy L, Jéquier E, Nicod P, Scherrer U. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. J Clin Invest 1994;93:2365–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherrer U, Sartori C. Insulin as a vascular and sympathoexcitatory hormone: implications for blood pressure regulation, insulin sensitivity, and cardiovascular morbidity. Circulation 1997;96:4104–4113 [DOI] [PubMed] [Google Scholar]
- 5.Anderson EA, Balon TW, Hoffman RP, Sinkey CA, Mark AL. Insulin increases sympathetic activity but not blood pressure in borderline hypertensive humans. Hypertension 1992;19:621–627 [DOI] [PubMed] [Google Scholar]
- 6.Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol 2011;589:1643–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luckett BS, Frielle JL, Wolfgang L, Stocker SD. Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin. Am J Physiol Heart Circ Physiol 2013;304:H1538–H1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 2011;57:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereda SA, Eckstein JW, Abboud FM. Cardiovascular responses to insulin in the absence of hypoglycemia. Am J Physiol 1962;202:249–25214485087 [Google Scholar]
- 10.Wehrwein EA, Basu R, Basu A, Curry TB, Rizza RA, Joyner MJ. Hyperoxia blunts counterregulation during hypoglycaemia in humans: possible role for the carotid bodies? J Physiol 2010;588:4593–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bin-Jaliah I, Maskell PD, Kumar P. Indirect sensing of insulin-induced hypoglycaemia by the carotid body in the rat. J Physiol 2004;556:255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC, Conde SV. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 2013;62:2905–2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paton JF, Sobotka PA, Fudim M, et al. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 2013;61:5–13 [DOI] [PubMed] [Google Scholar]
- 14.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia—influence of chemoreceptors and sympathetic nervous system. J Hypertens 1997;15:1593–1603 [DOI] [PubMed] [Google Scholar]
- 15.Jakobsson P, Jorfeldt L. Oxygen supplementation increases glucose tolerance during euglycaemic hyperinsulinaemic glucose clamp procedure in patients with severe COPD and chronic hypoxaemia. Clin Physiol Funct Imaging 2006;26:271–274 [DOI] [PubMed] [Google Scholar]
- 16.Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 2008;134:686–692 [DOI] [PubMed] [Google Scholar]
- 17.Fan J, Zhang B, Shu HF, et al. Interleukin-6 increases intracellular Ca2+ concentration and induces catecholamine secretion in rat carotid body glomus cells. J Neurosci Res 2009;87:2757–2762 [DOI] [PubMed] [Google Scholar]