Abstract
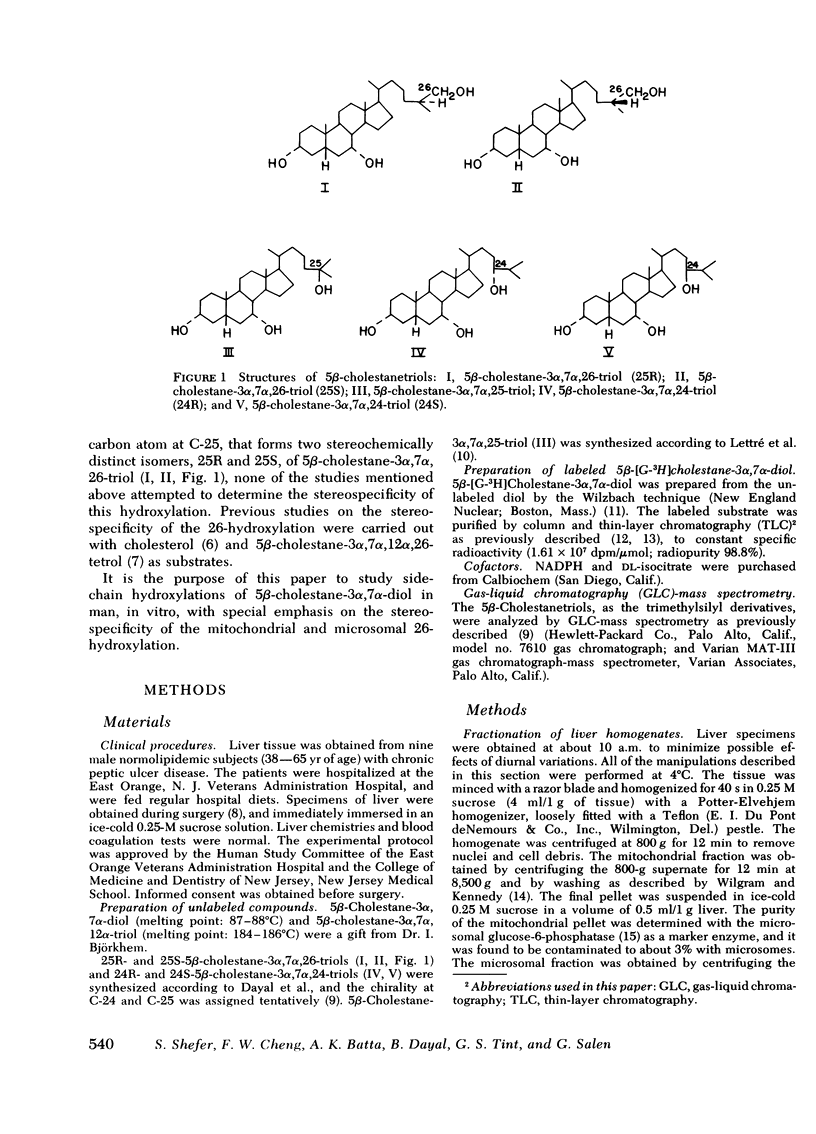
Stereospecific side-chain hydroxylations of 5β-cholestane-3α, 7α-diol were studied in mitochondrial and microsomal fractions of human liver. Incubation of 5β-cholestane-3α, 7α-diol resulted in hydroxylations at C-12, C-24, C-25, and C-26. Hydroxylations at C-24 and C-26 were accompanied by the introduction of additional asymmetric carbon atoms at C-24 and C-25 respectively, that led to the formation of two distinct pairs of diastereoisomers, namely 5β-cholestane-3α, 7α,24-triols (24R and 24S) and 5β-cholestane-3α, 7α,26-triols (25R and 25S). A sensitive and reproducible radioactive assay to measure the formation of the different biosynthetic 5β-cholestanetriols was developed. Optimal assay conditions for human mitochondrial and microsomal systems were tentatively established.
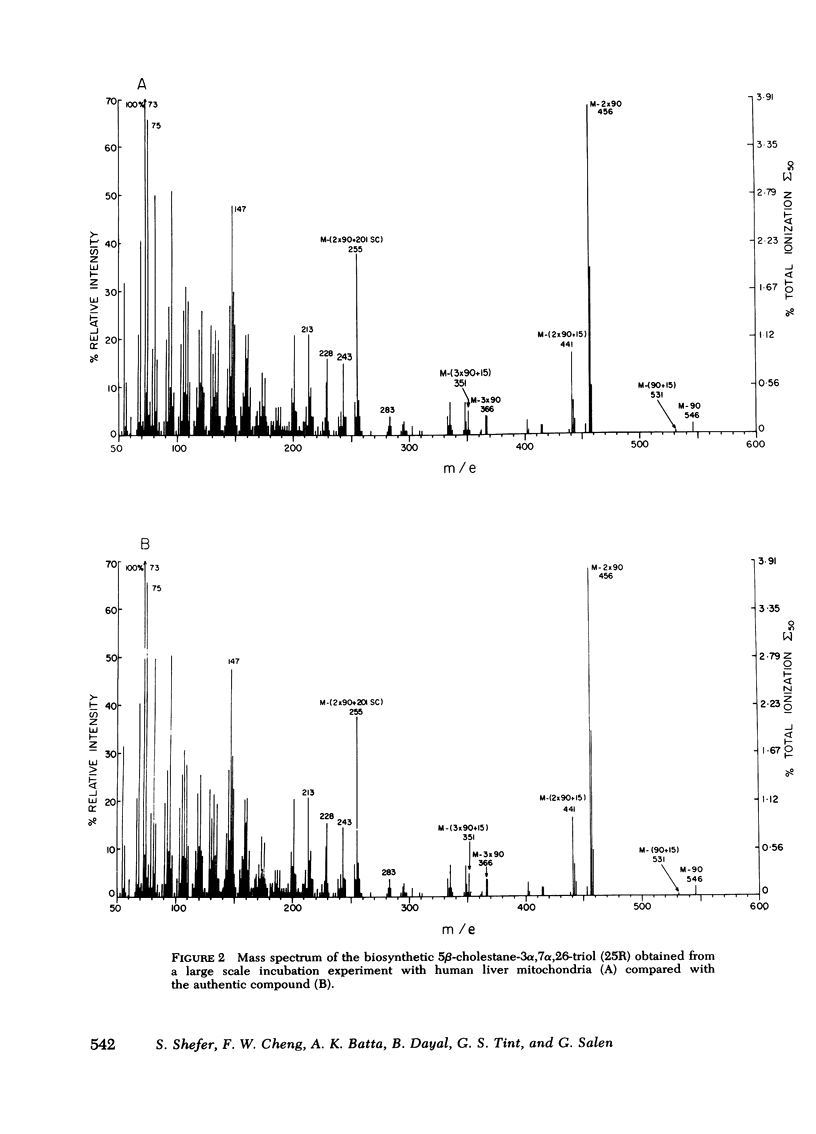
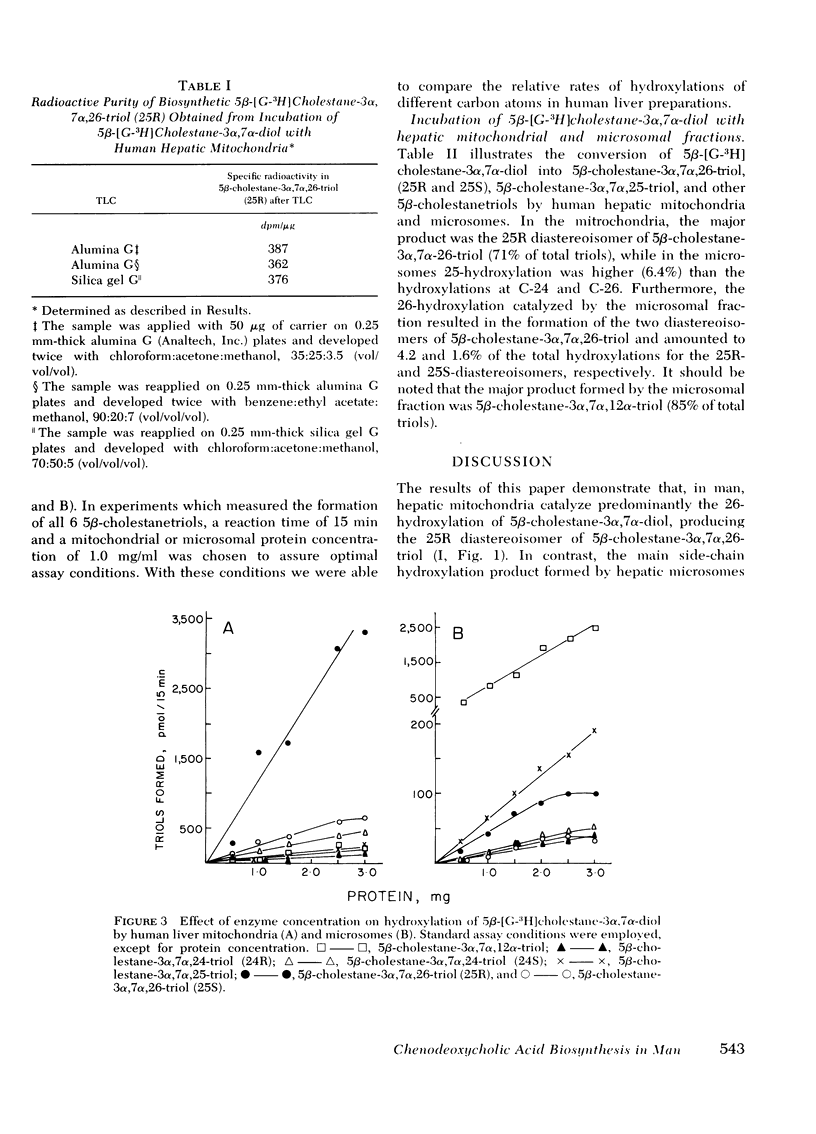
The mitochondrial fraction was found to predominantly catalyze the 26-hydroxylation of 5β-cholestane-3α, 7α-diol with the formation of the 25R-diastereoisomer of 5β-cholestane-3α, 7α,26-triol as the major product. In the microsomal fraction, on the other hand, 25-hydroxylation was more efficient than 26-hydroxylation and accounted for 6.4% of the total hydroxylations. The microsomes catalyzed the formation of both diastereoisomers of 5β-cholestane-3α, 7α,26-triol (25R and 25S, 4.2 and 1.6% respectively).
These experiments suggest that the initial step in the degradation of the steroid side chain during the biosynthesis of chenodeoxycholic acid in man is mediated by the mitochondria, and involves the formation of the 25R-diastereoisomer of 5β-cholestane-3α, 7α,26-triol. The role of the microsomal 25- and 26-hydroxylated intermediates requires further exploration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkheim I., Danielsson H., Einarsson K., Johansson G. Formation of bile acids in man: conversion of cholesterol into 5-beta-cholestane-3-alpha, 7-alpha, 12-alpha-triol in liver homogenates. J Clin Invest. 1968 Jul;47(7):1573–1582. doi: 10.1172/JCI105849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Gustafsson J., Johansson G., Persson B. Biosynthesis of bile acids in man. Hydroxylation of the C27-steroid side chain. J Clin Invest. 1975 Mar;55(3):478–486. doi: 10.1172/JCI107954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Gustafsson J. Mitochondrial omega-hydroxylation of cholesterol side chain. J Biol Chem. 1974 Apr 25;249(8):2528–2535. [PubMed] [Google Scholar]
- Björkhem I., Gustafsson J. Omega-hydroxylation of steriod side-chain in biosynthesis of bile acids. Eur J Biochem. 1973 Jul 2;36(1):201–212. doi: 10.1111/j.1432-1033.1973.tb02902.x. [DOI] [PubMed] [Google Scholar]
- Briggs T. Partial synthesis of 25D- and 25L-cholestanoic acids from some common bile acids. J Org Chem. 1970 May;35(5):1431–1434. doi: 10.1021/jo00830a037. [DOI] [PubMed] [Google Scholar]
- CAREY J. B., Jr, HASLEWOOD G. A. Crystallization of trihydroxycoprostani acid from human bile. J Biol Chem. 1963 Feb;238:855–856. [PubMed] [Google Scholar]
- Cheng F. W., Shefer S., Dayal B., Tint G. S., Setoguchi T., Salen G., Mosbach E. H. Cholic acid biosynthesis: conversion of 5beta-cholestane-3alpha,7alpha,12alpha,25-tetrol into 5beta-cholestane-3alpha,7alpha, 12alpha,24beta,25-pentol by human and rat liver microsomes. J Lipid Res. 1977 Jan;18(1):6–13. [PubMed] [Google Scholar]
- Danielsson H., Einarsson K. On the conversion of cholesterol to 7-alpha,12-alpha-dihydroxycholest-4-en-3-one. Bile acids and steroids 168. J Biol Chem. 1966 Apr 10;241(7):1449–1454. [PubMed] [Google Scholar]
- Dayal B., Batta A. K., Shefer S., Tint G. S., Salen G., Mosbach E. H. Preparation of 24(R)- and 24(S)-5beta-cholestane-3alpha,7alpha,24-triols and 25(R)- and 25(S)-5beta-cholestane-3alpha,7alpha,26-triols by a hydroboration procedure. J Lipid Res. 1978 Feb;19(2):191–196. [PubMed] [Google Scholar]
- HASLEWOOD G. A. D. Comparative studies of bile salts. V. Bile salts of Crocodylidae. Biochem J. 1952 Dec;52(4):583–587. doi: 10.1042/bj0520583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lettré H., Greiner J., Rutz K., Hofmann L., Egle A., Bieger W. Mehrwertige Alkohole aus Sterinen und Sterinderivaten. VI. Steroide mit Strukturmerkmalen des Ecdysons und der Elatericine. Justus Liebigs Ann Chem. 1972 Apr;758:89–110. doi: 10.1002/jlac.19727580109. [DOI] [PubMed] [Google Scholar]
- Nicolau G., Cohen B. I., Salen G., Mosbach E. H. Studies on the 12alpha and 26-hydroxylation of bile alcohols by rabbit liver microsomes. Lipids. 1976 Feb;11(2):148–152. doi: 10.1007/BF02532665. [DOI] [PubMed] [Google Scholar]
- Panveliwalla D. K., Pertsemlidis D., Ahrens E. H., Jr Tritiated bile acids: problems and recommendations. J Lipid Res. 1974 Sep;15(5):530–532. [PubMed] [Google Scholar]
- SWANSON M. A. Phosphatases of liver. I. Glucose-6-phosphatase. J Biol Chem. 1950 Jun;184(2):647–659. [PubMed] [Google Scholar]
- Shefer S., Cheng F. W., Dayal B., Hauser S., Tint G. S., Salen G., Mosbach E. H. A 25-hydroxylation pathway of cholic acid biosynthesis in man and rat. J Clin Invest. 1976 Apr;57(4):897–903. doi: 10.1172/JCI108366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi S., Hoshita N., Okuda K. Enzymatic characteristics of CO-sensitive 26-hydroxylase system for 5beta-cholestane-3 alpha, 7 alpha, 12 alpha-triol in rat-liver mitochondria and its intramitochondrial localization. Eur J Biochem. 1973 Dec 17;40(2):607–617. doi: 10.1111/j.1432-1033.1973.tb03233.x. [DOI] [PubMed] [Google Scholar]
- WILGRAM G. F., KENNEDY E. P. INTRACELLULAR DISTRIBUTION OF SOME ENZYMES CATALYZING REACTIONS IN THE BIOSYNTHESIS OF COMPLEX LIPIDS. J Biol Chem. 1963 Aug;238:2615–2619. [PubMed] [Google Scholar]



