Abstract
Efforts to translate efficacious interventions into long-term care practice have had limited success due to the lack of consideration of key translational intervention components. A multi-faceted intervention was implemented in two veteran affairs facilities to improve feeding assistance care. There were three study phases: baseline, intervention, and follow-up. During each phase, trained research staff conducted standardized observations of 12 meals/participant to assess feeding assistance care quality. The staff received three initial training sessions followed by six consecutive weeks of feedback sessions wherein the observation-based care process measures were shared with the staff. There were significant, but modest, improvements in mealtime feeding assistance care processes, and most of the improvements were maintained during follow-up. A multi-faceted intervention resulted in significant, but modest, improvements in mealtime feeding assistance care quality. Organizational (staff schedules, communication) and environmental (dining location) barriers were identified that interfered with improvement efforts.
Keywords: Long-term care, Community living centers, Unintentional weight loss, Feeding assistance, Nutrition, Staff training, Intervention, Quality improvement
INTRODUCTION
Numerous studies have demonstrated the effectiveness of interventions to improve care quality for long-term care (LTC) residents in many areas including incontinence, mobility decline, and unintentional weight loss [1, 2]. Unfortunately, despite this growing evidence base, efforts to translate efficacious clinical interventions into LTC practice have had limited success [3–6]. However, research has identified several key issues important to address to facilitate the successful translation of care quality improvements into LTC practice [1, 3, 7–9]. First, multiple levels of staff should be involved in training—namely, licensed nurses in a supervisory role and nurse aides responsible for direct care [4–6]. Second, medical record documentation has been shown to be erroneous for many aspects of daily care provision and in the direction of overestimating care quality; thus, standardized observations are recommended as an alternative information source to monitor quality improvement efforts [10–12]. Third, the implementation of care quality improvements is often time-intensive for the staff and likely to exceed usual care staffing resources; therefore, consideration of staff time requirements is critical [13–17]. Furthermore, given limited staffing resources, it is important to have clinically defensible criteria to target residents for care delivery [1, 2, 6, 7]. In short, while staff education about evidence-based practices may be a necessary prerequisite for LTC quality improvements, it is often not sufficient to effect real change in daily care provision. This is due, at least in part, to these other key translational issues.
Few studies have applied multi-faceted interventions to improve LTC quality that addressed one or more of these translational issues. A recent pilot intervention resulted in significant improvements in nutritional care quality [6]. Specifically, the intervention included core components to address each of the aforementioned translational research issues including training both licensed nurse supervisors and nurse aides and targeting at-risk residents. In addition, supervisory staff received training in how to monitor daily care quality based on standardized observations of care provision. These observational data were then used to provide feedback to nurse aide staff. This translational research effort resulted in significant improvements in daily feeding assistance care processes during a 12-week intervention period. However, this study was limited to only one community facility, and there was not a follow-up period to determine if intervention effects were maintained after study completion. In addition, it is likely that high nurse aide staffing levels (seven residents per nurse aide on both day and evening shifts) contributed to the success of this translational research effort beyond the core components of the intervention itself [6].
Several studies have established that higher staffing levels are associated with significantly better care quality and resident outcomes, including feeding assistance care and unintentional weight loss [13–16, 18]. In general, Veterans Affairs (VA) facilities tend to have higher total staffing levels relative to community facilities [19, 20]. Thus, VA facilities may be in a better position to translate efficacious interventions into daily care practice than many community facilities. A recent descriptive study showed that a sample of VA facilities provided better feeding assistance care relative to a sample of community facilities, but there were still multiple areas in need of improvement [21].
The primary aim of this translational study was to implement a multi-faceted staff training and management intervention in the VA to improve feeding assistance care processes for LTC veterans at risk for weight loss. A secondary aim was to identify barriers to translation in daily care practice. The following primary research questions were addressed:
What are the effects of a staff training and management intervention on daily feeding assistance care processes as measured by research staff observations of care delivery?
Are intervention effects maintained during a 12-week follow-up period without research staff involvement?
METHODS
Subjects and setting
Participants were recruited from two VA facilities (one federal and one state home) in one geographic region that housed a total of 282 residents. Nurse aide level staff-to-resident ratios, as reported by the Directors of Nursing, ranged from eight to nine residents per nurse aide during the day (7 a.m. to 3 p.m., breakfast and lunch meals), nine to ten in the evening (3 p.m. to 11 p.m., dinner meal), and 14 to 15 at night (11 p.m. to 7 a.m., breakfast meal). Licensed nurse (RN + LPN) staffing ratios at the two sites ranged from 6 to 15 residents per licensed staff during the day, 12 to 15 in the evening, and 13 to 15 at night. Nurse aide staffing levels were comparable, but licensed nurse staffing levels were higher for these two VA sites relative to community facilities in previous studies [2, 12, 13, 18]. The two VA study sites reported 3.82 and 5.0 total nursing hours per resident day, which placed both in the upper quartile of all homes in the nation for total staffing levels [14, 19, 20].
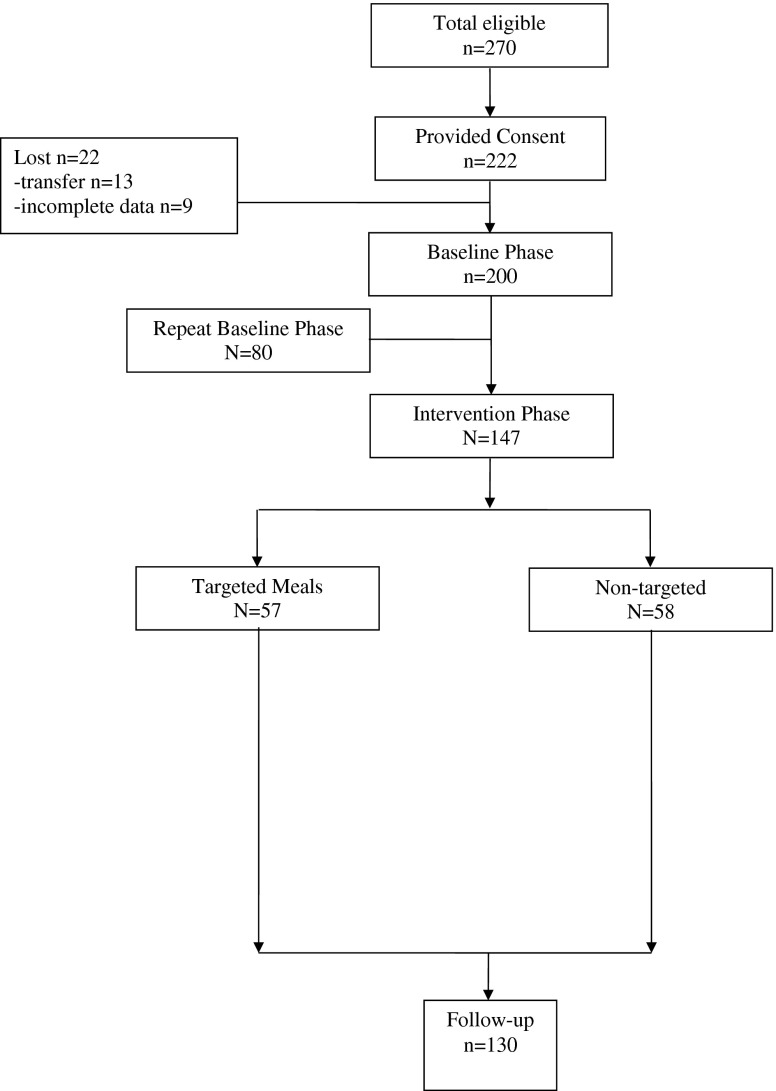
Figure 1 shows the flow of study participants through the intervention trial. A total of 270 (96 %) residents met study inclusion criteria, which required residents to be long-stay (non-Medicare), free of a feeding tube, not receiving hospice, and not on a planned weight loss diet. Consent was obtained for 222 (82 %) eligible residents. All study procedures were approved by the VA Institutional Review Board. Following consent, 22 participants were lost from the study (Fig. 1). A total of 200 participants completed the initial baseline study phase. Eighty of these participants were assigned to a delayed-intervention group and, thus, also completed a repeat baseline phase (see “Study design”). A total of 147 participants completed the intervention phase of the study. Of these, 57 participants were targeted for mealtime feeding assistance (see “Targeting criteria”). Overall, 130 participants completed all study phases, including follow-up (Fig. 1).
Fig 1.
Study participant flow chart
Study design
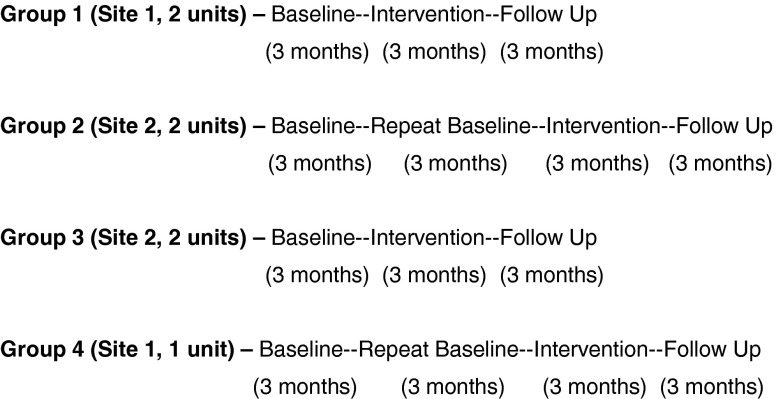
Figure 2 shows the study design and timeline for data collection. Across the two VA facilities, there were a total of seven LTC units (three units in site 1 and four units in site 2). Each unit consisted of 30 to 60 beds. This study used a randomized intervention design wherein participants were randomized by site and unit into either an immediate (groups 1 and 3) or delayed (groups 2 and 4) intervention group. Randomization was conducted at the site and unit level due to the staff training component of the intervention, which included all routine staff on the unit (see “Intervention implementation”). Units with shared staffing resources were grouped together for randomization (see Fig. 2, groups). There were three phases of data collection, and each phase consisted of 12 study weeks: baseline (usual care), intervention, and follow-up. The units randomized to the delayed-intervention groups (Fig. 2, groups 2 and 4) also had a 12-week repeat baseline (usual care) phase. This study design ensured that all eligible study participants would receive the intervention and avoided the possibility of contamination effects if randomization was conducted at the resident level within each site.
Fig 2.
Randomization and data collection timeline across all units in both sites
Measures
Descriptive information was retrieved from each participant’s medical record using a standardized form. Additionally, each participant’s most recent Minimum Data Set (MDS version 2.0) completed by LTC staff was retrieved to calculate MDS-derived scale scores for cognitive and physical functioning [22–24] and history of weight loss (section K, item 3a, ≥5 % in the last 30 days or 10 % in the last 180 days). The MDS-derived Cognitive Performance Scale (CPS) score ranges from 0 (cognitively intact) to 6 (severely impaired) [22]. The MDS-derived Activities of Daily Living (ADL) scale score ranges from 0 (rated by staff as independent in each of seven areas) to 28 (rated by staff as completely dependent in all areas) [23]. Staff ratings of eating dependency (section G, Physical functioning, item 1h) also were abstracted separately (score ranges from 0 = completely independent, to 4 = total dependence) [24].
Weighing procedures
Independent assessments of body weight were conducted monthly by the research staff during each study phase by using a standardized protocol. This protocol required research staff to weigh residents in the morning, prior to breakfast but following incontinence care, while the resident remained in bed clothes [2, 25]. Assessments of participants’ body weights were used to calculate body mass index (BMI, refer to Table 3 footnote for formula). A BMI value less than 21 was considered indicative of undernutrition [26]. Monthly weight values also were used to determine changes in weight from baseline to intervention to follow-up.
Table 3.
Participant characteristics overall and by VA study site (n = 130)
| Measure | Overall (n = 130) percent (n) or mean (±SD) | Site 1 (n = 45) percent (n) or mean (±SD) | Site 2 (n = 85) percent (n) or mean (±SD) |
|---|---|---|---|
| Percent male* | 89 % (115) | 71 % (32) | 98 % (83) |
| Percent white | 87 % (112) | 91 % (41) | 85 % (71) |
| Age in years* | 76.55 (±11.24) | 80.48 (±8.13) | 74.47 (±12.11) |
| Length of stay in years** | 3.24 (±2.56) | 2.34 (±1.94) | 3.68 (±2.73) |
| Percent with dementia diagnosis** | 64 % (82) | 80 % (36) | 55 % (46) |
| Percent with depression diagnosis | 45 % (58) | 56 % (25) | 40 % (33) |
| MDS-CPS (0–6)* | 2.29 (±1.67) | 2.87 (1.53) | 1.99 (1.67) |
| MDS-ADL (0–28)** | 12.54 (±8.91) | 15.02 (7.24) | 11.22 (9.46) |
| MDS eating dependency (0–4) | 1.28 (±1.28) | 1.56 (1.18) | 1.13 (1.31) |
| Percent with MDS eating dependency rating of required assistance | 33 % (42) | 36 % (16) | 31 % (26) |
| Percent with recent weight loss on MDS | 9 % (12) | 9 % (4) | 9 % (8) |
| Percent with prescribed diet order* | 78 % (101) | 58 % (26) | 88 % (75) |
| Percent with caloric supplementation order* | 69 % (89) | 38 % (17) | 86 % (72) |
| Percent with BMI <21 | 15 % (19) | 12 % (5) | 17 % (14) |
MDS eating dependency item (section G, physical functioning) rated as 2 (limited assistance), 3 (extensive assistance), or 4 (totally dependent). Prescribed diet order: any type of altered diet (no added salt, no concentrated sugars, mechanically altered, ground, puree). Caloric supplementation order: physician or dietitian order for oral liquid nutrition supplement or the provision of additional foods and fluids between meals. BMI formula = 0.454 × weight in pounds / (0.254 × height in inches)2
MDS-CPS Minimum Data Set derived Cognitive Performance Scale (total score ranges from 0 (cognitively intact) to 6 (severely impaired or comatose)); MDS-ADL Minimum Data Set derived Activities of Daily Living (total score range 0 (rated by staff as completely independent) to 28 (rated by staff as completely dependent in seven ADLs)); SD standard deviation
*p < 0.01; **p < 0.05
Mealtime observations and care process measures
Trained research staff used a standardized observation protocol shown to be reliable and valid in previous studies [12, 27, 28] to conduct observations during regularly scheduled meals for a total of 12 meals per person (six meals per month, or breakfast, lunch, and dinner on two consecutive weekdays) during the last 2 months of each study phase (Fig. 2). The week of the month and days of the week were randomly selected to ensure that resident-level observations varied by week and day throughout each study phase.
Continuous observations were conducted from the time of meal delivery until the time of meal retrieval (mean observation time = 80.55 ± 24.51 min per person per meal). The research staff documented the presence or absence of each type of staff assistance provided during the meal to encourage intake including setup (e.g., opening containers, cutting up meat), verbal reminders or encouragement, socialization, and physical help to eat. All episodes of any type of assistance were combined to yield a total assistance time (minutes/seconds) per person per meal timed with a stop watch. The research staff estimated total percent consumed (all foods and fluids combined) per person per meal based on observation because this is the method used by facility staff to document daily meal intake and identify residents who are eating poorly [12, 24, 27, 28]. In addition, digital photographs were taken for at least one meal per person per study phase and rated by a trained staff member different from the observer for reliability. Both observation and photography methods have been shown to be reliable methods for estimating residents’ meal intake [28]. The Pearson correlation coefficients between the observation and photo-based estimates for total percent intake of meals across all study phases was r = 0.96, p < 0.001 (n = 1,008 resident meals).
Inter-rater reliability among the research staff for each of the observation-based data elements across all study phases (n = 489 resident meals) were as follows: total observation time (r = 0.873, p < 0.001); meal delivery and retrieval (r = 0.956, p < 0.001); total assistance time (r = 0.964, p < 0.001); total percent eaten (r = 0.974, p < 0.001); percent eaten above or below 50 % (Kappa = 0.909, p < 0.001); physical assistance to eat (Kappa = 0.983, p < 0.001); verbal reminders or encouragement to eat (Kappa = 0.575, p < 0.001); social interaction during the meal (Kappa = 0.609, p < 0.001); and alternatives offered (Kappa = 0.788, p < 0.001). These data elements were used to calculate care process measures shown to be reliable, valid measures of feeding assistance care quality [12, 13, 27]. Each measure is scored per person per meal because, for example, the staff could provide assistance during breakfast but not lunch. Each measure is scored as a “pass” or “fail” to yield an overall “percent pass rate” across all resident-meal observations, where a higher score translates into better care quality. The scoring rule and rationale for each care process measure has been specifically described elsewhere [12, 13, 27]; thus, only a brief description is presented in Table 1.
Table 1.
Mealtime feeding assistance care process measures and scoring rules
| Feeding assistance care process measures | Scoring rule for a “pass” (scored per person per meal) |
|---|---|
| 1. Staff ability to accurately identify residents with clinically significant low intake of meals | If resident consumes <50 % of meal based on observation, staff documentation in resident’s chart also shows a value ≤60 % for the same meal. Only meals with intake <50 % are scored. |
| 2. Staff ability to offer an alternative to the served meal when a resident’s intake is low | If resident consumes <50 % of served meal, staff offer an alternative to encourage intake. Only meals with intake <50 % are scored. |
| 3. Staff ability to provide assistance to residents requiring assistance to eat | If resident is rated on their most recent MDS as requiring feeding assistance (section G, item 1h rated 2–4), staff provides more than 5 min of assistance during each meal. |
| 4. Staff ability to provide a verbal prompt to residents who receive physical help to eat | If staff provides physical assistance to eat, staff also provides at least one episode of verbal prompting to enhance independence. Only meals during which staff provides physical assistance are scored. |
| 5. Staff ability to provide social interaction to all residents during meals | All residents should receive at least one episode of social interaction during every meal. The duration of the interaction is not considered. All meals are scored. |
Targeting criteria: low intake and responsive to mealtime feeding assistance
The care process measures were scored for all resident-meal observations during each study phase with the rationale that some measures are applicable to all residents, irrespective of nutritional risk status (e.g., social interaction), while others are applicable to individual resident meals during which intake is low, even if the resident otherwise is not considered nutritionally at risk (e.g., availability of alternatives during any meal wherein intake is below 50 %). In addition, a subgroup of participants were targeted for improvements in mealtime feeding assistance based on nutritional risk as defined by low intake and responsiveness (i.e., showing a significant gain in intake) to assistance. The rationale for the targeting criteria was that, given limited staff time, the focus should be on ensuring feeding assistance care quality for this targeted at-risk group.
Low intake was based on research staff observations during meals at baseline and defined using the MDS criterion, “leaves 25 % or more of food uneaten at most meals” [24]. Thus, participants whose intake fell below 75 % for most observed meals at baseline were considered to have “low intake” and received a 2-day trial of mealtime assistance. The 2-day, or six-meal (i.e., breakfast, lunch, and dinner on two consecutive days), trial of mealtime feeding assistance was implemented by the trained research staff during baseline using the same protocol applied in previous research [2, 18]. Briefly, the research staff provided assistance individually or in small groups (one research staff member to two to three residents), ensured proper positioning for eating, and offered alternatives to the served meal. In addition, a graduated prompting protocol that enhanced the resident’s self-feeding ability was used during each episode of assistance. Food and fluid intake was estimated for each of the six evaluation meals by using the same observation protocol used at baseline. Participants were deemed responsive to mealtime assistance based on a gain of 15 % or more in meal intake based on a previous research [2, 18].
Staff training and management intervention implementation
Table 2 displays the primary intervention components, which were consistent with those necessary for an effective translational research effort (see “Introduction”) [1, 3, 7–9]. Staff training activities were conducted at the unit level and included all scheduled nurse aides and supervisory nurses (e.g., unit charge nurse) on each of the seven units for all three shifts (day, evening, and night). All training sessions were led by the study PI, and the staff earned continuing education credit for attendance. There were three initial training sessions, each of which lasted 45 min to 1 h and consisted of the following content:
Table 2.
Translation intervention components
| Key translational research components | Intervention components |
|---|---|
| 1. Inclusion of multiple levels of staff | Licensed nurses, nurse aides and dietary and upper-level administration (e.g., administrator, director of nursing, assistant director of nursing, supervisors) |
| 2. Accurate information about care delivery | Observation-based care process measures conducted weekly during meals |
| 3. Staff time-efficiency considerations | Mealtime assistance considered dining location, seating arrangements, and staff time required for efficiency |
| 4. Clinically meaningful targeting criteria | Observations to determine low intake and 2-day intervention trial to identify residents responsive to mealtime assistance |
Session 1
A pretest was conducted related to staff knowledge of nutritional care issues followed by a presentation of relevant research findings and practice implications. This session also included group discussion related to staff perceptions of nutritional care challenges.
Session 2
This session consisted of a review of an observation-based tool to assess feeding assistance care quality [12], which included the scoring rule and rationale for each feeding assistance care process measure and the percent pass rates for each unit based on the research staff baseline observations during meals. This session was used to highlight areas in need of improvement at the unit level and address staff questions about the observation-based care process measures. Designated supervisory staff on each unit (e.g., charge nurses and unit dietitians) were identified during this session to receive training from the research staff in the observational tool, which required a staff to conduct observations jointly with the research staff for one to three meals (with three to six residents selected for observation during each period) as part of the training.
Session 3
The research staff shared the results of the baseline observations to identify those with low oral intake and the 2-day mealtime feeding assistance trial to identify residents on each unit to be targeted for mealtime assistance. This session also included training on how to estimate residents’ food and fluid intake using the same standardized rules applied by the research staff and photographs of meal trays to improve the accuracy of LTC staff intake estimates [28].
Following these initial three training sessions, which occurred during the first month of the intervention phase, the research staff then conducted weekly observations for six consecutive weeks for the targeted group (two meals per person per week) and continued to conduct observations monthly for the entire group (12 meals per person during months 2 and 3 of the intervention phase). For the targeted group, the meal and day of the week were randomly selected each week to yield observations across all three scheduled meals and each weekday. These weekly observational data were translated into percent pass rates for each care process measure (see “Mealtime observations and care process measures” and Table 1), and the unit staff were given feedback each week based on the observed care during the previous week. Specifically, bar graphs were shown to display the percent pass rates at baseline followed by each consecutive week, so that the staff were aware of the amount of improvement for each specific aspect of feeding assistance care. These weekly feedback sessions were led by the study PI and included the unit charge nurse and all scheduled nurse aides on both the day and evening shifts. Dietary personnel also were encouraged to attend these sessions. Weekly feedback sessions required an average of 15 min per session (range 10–20 min) and were scheduled separately for each unit and shift at staff-preferred times.
Data analyses
Inter-rater reliability was established among all research personnel prior to baseline assessments as part of initial training and then monthly during each study phase to prevent observer drift using Pearson correlation coefficients for continuous measures and Kappa agreement statistics for binary measures. All characteristics shown in Table 3 were compared between study participants who completed all phases of data collection (n = 130) and those lost from the study (n = 92) by using independent samples t tests for continuous variables and chi-square analyses for categorical variables. A significantly larger proportion of participants who completed the study had a dementia diagnosis relative to those lost from the study (64 versus 48 %, chi-square = 5.359, p = 0.025). There were no other significant differences between these two groups. These same comparisons also were conducted between those who completed the study in each of the two VA sites (see Table 3 and “Results”).
Participants with six or more observations within each study phase were included in the analyses (median and mode = 12 observations per person). Because data collection was conducted as part of a repeated measures design, observations for individual participants may exhibit serial correlation. To account for this, odds ratio (OR) estimates and corresponding 95 % confidence intervals (CI) were constructed using a nonparametric cluster bootstrap technique, where each cluster corresponds to an individual participant [29]. In the cluster bootstrap, whole clusters are resampled independently and uniformly at random. Resampling in this fashion confers a sampling distribution over summary statistics that accounts for serial correlation among the observed data. The sampling distribution of odds ratios were computed by drawing 1,000 cluster bootstrap resamples from the baseline-to-intervention-to-follow-up meal observation data and summarized by the lower confidence limit (0.025), median (0.05), and upper confidence limit (0.975) sample quantiles. The odds associated with baseline care process measures are considered significantly different from intervention measures when the corresponding 95 % confidence limits are both greater than one or both less than one. These analyses were completed for the overall group of participants who completed all three study phases (n = 130) and separately for the targeted at-risk subgroup (n = 57).
Two models were constructed to analyze change in body weight over the course of the study: a “full” model and a “reduced” model. Both models expressed weight as a function of time, accounting for individual variability (a mixed effects model). The full model also permitted the weight trajectory (slope) to change from baseline to intervention and then again from intervention to follow-up. The reduced model did not permit this. Hence, if the full model had fit the data significantly better than the reduced model, this would suggest an intervention/follow-up effect. The average change in weight over the study period was expressed in pounds (lbs) lost per day. An estimate and 95 % confidence intervals for the rate of weight loss was computed for the overall group (n = 130) and targeted at-risk subgroup (n = 57).
RESULTS
Participants
Table 3 shows the participant characteristics overall for those who completed the study (n = 130) and for each of the VA study sites. Overall, participants were predominately male (89 %) and white (87 %), with an average age of 76 years and an average length of LTC stay of 3.2 years. Participants were moderately cognitively impaired as evidenced by dementia diagnosis (64 %) and MDS-CPS total score (2.3 ± 1.7). Forty-five percent had a diagnosis of depression. Participants were mildly to moderately physically impaired based on the MDS-ADL total score (12.5 ± 8.9), and 33 % were rated by the staff as requiring assistance to eat. The majority (78 %) had a prescribed diet order and/or an order to receive caloric supplementation daily (69 %). Nine percent had a recent weight loss episode (MDS, ≥5 % in 30 days or ≥10 % in 180 days), and 15 % had a body mass index below 21, which is indicative of undernutrition [26]. There were significant differences in participant characteristics between the two VA sites (denoted in Table 2 with asterisks), which was reflective of differences in resident populations between the state (site 1) versus federal (site 2) VA facilities in this study.
Facility staff participation in training
Overall, staff participation in training was high at both sites and across all units. The initial three sessions were attended by a total of 134 staff (43 % licensed nurses, 37 % nurse aides, 7 % dietary, 7 % activities, and 6 % upper-level administrative personnel) across the two sites. A total of 57 staff members (mean = 8.14 total staff per unit) were trained in the quality monitoring tool (51 % licensed nurses, 21 % nurse aides, 5 % dietary, 9 % activities, and 14 % upper-level administrative personnel). For the weekly feedback sessions, the total number of staff in attendance across all six sessions was 163 (mean = 23.29 staff per unit), with an average attendance per session ranging from four to eight staff per week. Staff attendees were comprised mostly of licensed nurses (46 %) and nurse aides (42 %) with intermittent attendance by dietary (3 %) and other miscellaneous staff (e.g., activities, administrative).
Observation-based care process measures during meals
Table 4 shows the percent “pass” rates for the observation-based feeding assistance care process measures during meals for the group of 130 VA participants who completed all three study phases. The number of participant observations scored for each measure (denoted by the denominators) differed based on the scoring rule (see “Mealtime observations and care process measures” and Table 1) and the number of missed observations during each study phase. There was comparable missing data for each study phase (Table 4, row 1, range 3–4 %).
Table 4.
Mealtime care process measures: percent pass rates overall by study phase (n = 130)
| Care process measures | Baseline (NM = 1,510) | Intervention (NM = 1,504) | Follow-up (NM = 1,498) |
|---|---|---|---|
| % Pass (n/d) OR (95 % CI) | % Pass (n/d) OR (95 % CI) | % Pass (n/d) OR (95 % CI) | |
| Missed observations | 3.2 % (50 of 1,560) | 3.6 % (56 of 1,560) | 4.0 % (62 of 1,560) |
| Ate <50 % | 33.3 % (503/1,510) | 33.3 % (502/1,504) | 35.6 % (533/1,498) |
| Ate <50 % and chart ≤60 %a | 56.7 % (285/503) | 66.7 % (335/502) 0.71 (0.55, 0.89) | 61.9 % (330/533) 0.85 (0.64, 1.13) |
| Ate <50 % and alternativea | 3.2 % (16/503) | 7.6 % (38/502) 0.42 (0.24, 0.74) | 7.5 % (40/533) 0.40 (0.24, 0.68) |
| Assistance required | 34.7 % (524/1,510) | 33.6 % (505/1,504) | 34.7 % (519/1,498) |
| Assistance required and >5 min receiveda | 54.2 % (284/524) | 61.4 % (310/505) 0.76 (0.59, 0.96) | 61.5 % (319/519) 0.81 (0.51, 1.32) |
| If physical, then also verbal | 94.6 % (300/317) | 97.4 % (341/350) 0.42 (0.13, 1.16) | 96.3 % (341/354) 0.63 (0.24, 1.42) |
| Social interaction presenta | 42.7 % (645/1,510) | 47.2 % (710/1,504) 0.83 (0.73, 0.95) | 45.4 % (680/1,498) 0.92 (0.79, 1.05) |
Missed observations: Research staff attempted to observe each participant for a total of 12 meals per person per phase. The most common reason for a missed meal observation was due to the resident being “out of the facility” (e.g., medical appointment, hospital, family visit) for the entirety of the meal period.
NM total number of resident-meal observations completed across all study participants; n/d numerator/denominator for total number of resident meals scored for each measure; OR (95 % CI) odds ratio and 95 % confidence intervals (upper, lower)
aSignificant difference from baseline to intervention/follow-up
Overall, participants consumed less than half of the served meal during 33 % (503 of 1,510 observations) of baseline meals, and this proportion remained comparable during intervention and follow-up phases (Table 4, row 2. 33 and 36 %, respectively). Chart documentation showed a value ≤60 % for approximately half, or 56.7 % (285 of 503) of these meals at baseline (Table 4, row 3). The percent pass rate for this care process measure improved significantly during intervention to 66.7 % (OR = 0.71; 95 % CI = 0.55, 0.89) and remained higher than the baseline rate at follow-up (61.9 %), although not significantly so. For these same low-intake meals, the staff offered an alternative to the served meal during only 3.2 % (16 of 503) of meals at baseline (Table 4, row 4) with, again, a significant improvement during intervention (7.6 %, OR = 0.42; 95 % CI = 0.24, 0.74), and this improvement was maintained at follow-up relative to baseline (7.5 %, OR = 0.40; 95 % CI = 0.24, 0.68).
Approximately one-third of the participants were rated by LTC staff as requiring feeding assistance (Table 4, row 5). The staff provided more than 5 min of assistance during 54.2 % (284 of 524) of these meals (Table 4, row 5), with an average total assistance time of 11.0 (±11.6) minutes per person per meal during baseline. The percent pass rate for this care process measure improved significantly during intervention to 61.4 % (OR = 0.76; 95 % CI = 0.59, 0.96), and this improvement was maintained during follow-up (61.5 %). Average total assistance time for participants rated by the staff as requiring assistance to eat increased slightly, but not significantly, during the intervention (12.6 ± 11.9) and follow-up phases (12.2 ± 11.3).
For those who received physical assistance to eat, the staff also provided at least one episode of verbal cueing during almost all (94.6 %, or 300 of 317) baseline meals (Table 4, row 7). The percent pass rate did not change significantly but did remain high for this care process measure during both intervention and follow-up phases (97.4 and 96.3 %, respectively). Finally, social interaction between LTC staff and participants (Table 4, row 8) was present during 42.7 % of the baseline meals (645 of 1510), and this care process measure improved significantly during intervention (47.2 %, OR = 0.83; 95 % CI = 0.73, 0.95), and remained higher than baseline at follow-up (45.4 %), although not significantly so. The care process measure results for the subgroup of participants targeted for mealtime feeding assistance care quality improvements (Fig. 1, n = 57) were comparable to the results for the overall group (n = 130) shown in Table 4.
Change in body weight
For the overall group of participants (n = 130), the average change in weight per day prior to intervention was −0.0029 lbs/day (95 % CI, −0.0051, −0.00067). During the intervention phase, the average change in weight loss per day was improved by 0.0028 lbs/day (95 % CI, −0.012, 0.017). Hence, in these data, the trajectory of weight loss was near zero during the intervention period. While promising, this mitigating effect was not statistically significant. In addition, no significant change in the rate of weight loss was detected at the transition from intervention to follow-up. For the targeted subgroup (n = 57), the average change in weight per day prior to intervention was −0.0033 lbs/day (95 % CI, −0.0062, −0.00046). During the intervention phase, the average weight loss per day was improved by 0.0011 lbs/day (95 % CI, −0.016, 0.019). Again, this improvement was not statistically significant nor was there a significant change in the rate of weight loss at the transition from intervention to follow-up. The staff-reported rates of unintentional weight loss according to MDS data during intervention and follow-up phases (8 versus 9.2 %, respectively) remained comparable to the baseline rate (Table 3, 9 %).
DISCUSSION
The results of this translational study in two VA facilities showed that a multi-faceted staff training and management intervention produced significant, but modest, improvements on several measures of mealtime feeding assistance care quality. Furthermore, most of these improvements were maintained by LTC staff during a 3-month follow-up period without research staff involvement. However, the relatively modest intervention effects were somewhat surprising given the intensity (i.e., weekly) and content of the intervention, which included multiple components identified as key to a successful translational research effort [1, 2, 7, 10–12], and the results of a previous study which resulted in larger effects [6]. For example, although LTC staff provided more assistance to those requiring assistance to eat (Table 4, Assistance required and >5 min received: 54–61 %), a previous translational research effort in a community facility showed a much larger improvement in this same care process measure (37–99 %) [6].
There are several potential reasons for the relatively modest intervention effects in this study. First, unlike the previous study [6], VA LTC staff in this study did not utilize the targeting criteria (i.e., low intake and responsive to mealtime assistance) to focus their improvement efforts on a subgroup of participants. Instead, the staff applied the training intervention equally to all participants. While this approach resulted in modest benefits to all participants (e.g., offering alternatives, socialization during mealtimes), it may have muted the benefits for those residents most in need (e.g., providing >5 min of assistance to those rated by the staff as requiring assistance to eat).
Second, although total staffing levels were high in these two VA facilities, nurse aide staffing levels remained below those levels necessary for optimal feeding assistance care (i.e., eight to ten residents as opposed to seven or fewer residents per aide) [13, 14, 18]. In contrast, nurse aide staffing levels in the previous study were consistent with recommended staffing ratios for optimal feeding assistance care [6]. Thus, the higher total staffing levels in these two VA facilities were predominately due to higher licensed nurse staffing levels. While licensed nurses were observed to intermittently assist with feeding (28–33 % of meals), feeding assistance remained the primary responsibility of nurse aide staff, who reported during weekly feedback sessions that they often worked short staffed (41 % of nurse aide attendees).
Third, only 30–40 % of participants ate one or more meals outside of their rooms, even though each unit had sufficient dining room space to accommodate most residents. A separate study showed that residents who dine in their rooms have lower meal intake and receive less staff attention during the meal to promote consumption relative to those who dine outside of their rooms [30]. In addition, staffing needs during mealtime are higher when a greater proportion of residents dine in their rooms because it requires a staff to feed residents individually [14, 31]. While the research staff made suggestions during weekly feedback sessions about how to provide feeding assistance in a more time-efficient manner (e.g., group dining), it was difficult for nurse aides to adjust other related care routines (e.g., morning care and transport to the dining room for breakfast) to make this possible on a routine basis.
Fourth, supervisory staff who attended the training sessions seemed to rely heavily on charted weight data as their primary information source for nutritional care planning. Independent weights collected by the research staff monthly during this study often identified gradual weight loss that was not detected by LTC staff according to charted weights. This discrepancy between the research staff weights based on a standardized weighing procedure and LTC staff-charted weights has been demonstrated in a previous study and shown to result in a delayed identification of weight loss episodes [25]. Despite the weekly feedback sessions, there was a tendency among supervisory staff to view inconsistent feeding assistance care as less problematic unless there was an associated weight loss event that met MDS criteria (i.e., ≥5 % in 30 days or 10 % in 180 days). In short, the emphasis on “prevalence of unintentional weight loss” as an MDS quality indicator was viewed by the staff as the most important nutritional outcome measure rather than the quality of daily feeding assistance care processes [27].
Although there were promising trends, the lack of an intervention effect on resident weight status was not surprising due to the modest and inconsistent improvements in daily feeding assistance care delivery provided by LTC staff in this study. A separate randomized, controlled trial demonstrated that the consistent delivery of optimal mealtime feeding assistance provided by the research staff twice per day, 5 days per week for 24 weeks resulted in significant effects on both weight status and body mass index [2]. Thus, weight status effects can be achieved if feeding assistance care quality improvements are consistent and maintained over time.
Finally, there were coordination and communication challenges between direct care staff on the units and dietary personnel that impeded some improvements. For example, although LTC staff increased their offers of alternatives to the served meal when resident intake was low from baseline to post-intervention (3.2–7.6 %, respectively), the majority of residents with low intake were not offered an alternative at either time point. This finding was particularly surprising in light of current federal and VA initiatives which require that alternatives to the served meal be made available routinely to all residents [32, 34]. Anecdotally, nurse aides shared during weekly feedback sessions that the process of requesting an alternative to the served meal required too much of their time (e.g., make the request, retrieve it from the kitchen, delay in serving, and feeding the resident), which prolonged their mealtime care and, thus, interfered with their ability to complete other job tasks. In addition, dietary menus were set days in advance such that options for alternatives were limited and not easily accommodated by the kitchen. Interestingly, most nurse aides (50–70 % of session attendees) reported that retrieval of alternatives was “not part of their job”, whereas dietary personnel reported that they relied completely on the unit staff for both requests and retrievals. Again, these challenges were discussed in weekly feedback sessions, and some adjustments were made but with limited success as evidenced by the results for this care process measure.
There are a few notable limitations of this study. First, this study was limited to only two VA facilities in one geographic region. Second, the intervention did not address other aspects of nutritional care that are the responsibility of licensed nurses, registered dietitians, and primary care physicians (e.g., supplement orders, dietary restrictions, depression treatment, adjustments in medications with appetite suppressant side effects), which may have been more amenable to intervention given the higher licensed nurse staffing levels in these two VA facilities.
Implications: practice, policy, and research
The results of this translational research effort underscore the importance of both adequate staffing levels during mealtimes and accurate information about the quality of daily care processes for ongoing quality improvement efforts. Nurse aide staffing levels in these two facilities, while typical of most facilities, remained insufficient to consistently provide optimal feeding assistance care to all residents in need. Recent federal regulations allow both community and VA facilities to train non-nursing staff to assist with feeding in an effort to augment existing, often limited, nurse aide staffing levels. Several studies have highlighted the potential benefits of these types of training programs for improving nutritional care quality [35–39].
Finally, as mentioned previously, supervisory LTC staff rely too heavily on weight loss outcomes as opposed to a more preventative focus on the quality of daily feeding assistance care processes. Genuine quality improvement efforts require accurate information about daily care processes under the direct control of the staff [10, 11]. Numerous studies have revealed that LTC documentation is erroneous for many aspects of care including feeding assistance, supplement delivery, and monthly weight values [12, 25, 40, 41]. Thus, the type of standardized observations conducted weekly as part of this study represent a critical information source for supervisory staff to effectively monitor and manage daily care quality. It is also noteworthy that offering residents alternatives to the served meal has important quality of life implications beyond weight loss outcomes, as indicated by recent changes in regulatory guidelines [32, 34], and standardized observations is the only source of accurate information about this aspect of care. Consequently, new survey guidelines have incorporated similar mealtime observations to assess both feeding assistance care quality and the availability of alternatives to the served meal [32, 42]. This new emphasis in the survey process should motivate LTC staff to use similar observation-based protocols to routinely monitor care quality and identify areas in need of improvement. Finally, it is noteworthy that many of the barriers to improvement identified in this study could be mitigated with organizational and/or environmental changes that do not require significant financial investment or an increase in total staffing levels. For example, reorganization of existing staff schedules and task assignments and utilization of non-nursing staff for some mealtime tasks could greatly increase the magnitude of care quality improvements in this and other daily care areas [6, 37].
Acknowledgments
This research was supported by VA Health Services Research and Development Merit Grant IRR 07–250, “Prevention of Weight Loss in Long-Term Care Veterans” awarded to Dr. Sandra F. Simmons, PhD (principal investigator). The views expressed in this paper are those of the authors and may not reflect those of the funding agency. The authors thank the participating VA nursing homes, staff, and residents for their cooperation with this project. The authors also thank the members of the Center for Quality Aging research team involved in the data collection.
Footnotes
Implications
Practice: Long-term care staff should focus their efforts on ensuring daily care quality (e.g., feeding assistance) through the use of routine, standardized observations for ongoing quality improvement efforts, with less emphasis on related clinical outcomes (e.g., weight loss).
Policy: Federal regulators and surveyors should recognize the importance of utilizing standardized observations to evaluate care quality and also commend long-term care facilities for training non-nursing personnel to assist with feeding, or other related mealtime tasks, as a way to augment limited staffing resources to improve care quality.
Research: Those conducting translational research efforts in the long-term care setting should consider key translational components important to address and data sources independent of staff self-report for evaluating outcomes.
References
- 1.Schnelle JF, Alessi CA, Simmons SF, Al-Samarrai NR, Beck JC, Ouslander JG. Translating clinical research into practice: a randomized controlled trial of exercise and incontinence care with nursing home residents. J Am Geriatr Soc. 2002;50(9):1476–1483. doi: 10.1046/j.1532-5415.2002.50401.x. [DOI] [PubMed] [Google Scholar]
- 2.Simmons SF, Keeler E, Zhuo X, Hickey KA, Sato HW, Schnelle JF. Prevention of unintentional weight loss in nursing home residents: a controlled trial of feeding assistance. J Am Geriatr Soc. 2008;56(8):1466–1473. doi: 10.1111/j.1532-5415.2008.01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouslander JG. Quality improvement initiatives for urinary incontinence in nursing homes. J Am Med Dir Assoc. 2007;8:S6–S11. doi: 10.1016/j.jamda.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Rahman AN, Simmons SF, Applebaum R, Lindabury K, Schnelle JF. The coach is in: improving nutritional care in nursing homes. Gerontologist. 2012;52(4):571–580. doi: 10.1093/geront/gnr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman AN, Schnelle JF, Applebaum R, Lindabury K, Simmons SF. Distance coursework and coaching to improve nursing home incontinence care: lessons learned. J Am Geriatr Soc. 2012;60(6):1157–1164. doi: 10.1111/j.1532-5415.2012.03939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons SF, Schnelle JF. A continuous quality improvement pilot study: impact on nutritional care quality. J Am Med Dir Assoc. 2006;7(October):480–485. doi: 10.1016/j.jamda.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Rahman AN, Schnelle JF, Applebaum R, Lindabury K & Simmons SF. Translating research into practice: can we close the gap? Gerontologist. 2012 doi:10.1093/geront/gnr157. [DOI] [PMC free article] [PubMed]
- 8.Rantz MJ, Zwygart-Stauffacher M, Hicks L, Mehr D, Flesher M, Petroski GF, et al. Randomized multilevel intervention to improve outcomes of residents in nursing homes in need of improvement. J Am Med Dir Assoc. 2012;13(1):60–68. doi: 10.1016/j.jamda.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiener JM. An assessment of strategies for improving quality of care in nursing homes. Gerontologist. 2003;43:19–27. doi: 10.1093/geront/43.suppl_2.19. [DOI] [PubMed] [Google Scholar]
- 10.Schnelle JF, Bates-Jensen BM, Chu L, Simmons SF. Accuracy of nursing home medical record information about care process delivery: implications for staff management and improvement. J Am Geriatr Soc. 2004;52(8):1378–1383. doi: 10.1111/j.1532-5415.2004.52372.x. [DOI] [PubMed] [Google Scholar]
- 11.Schnelle JF, Osterweil D, Simmons SF. Improving the quality of nursing home care and medical-record accuracy with direct observational technologies. Gerontologist. 2005;45(5):576–582. doi: 10.1093/geront/45.5.576. [DOI] [PubMed] [Google Scholar]
- 12.Simmons SF, Babineau S, Garcia E, Schnelle JF. Quality assessment in nursing homes by systematic direct observations: feeding assistance. J Gerontol Med Sci. 2002;57A(10):M665–M671. doi: 10.1093/gerona/57.10.M665. [DOI] [PubMed] [Google Scholar]
- 13.Schnelle JF, Simmons SF, Harrington C, Cadogan M, Garcia E, Bates-Jensen BM. Relationship of nursing home staffing to quality of care. Heal Serv Res. 2004;39(2):225–250. doi: 10.1111/j.1475-6773.2004.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnelle JF, Simmons SF, Cretin S. Minimum nurse aide staffing required to implement best practice care in nursing homes. In Report to Congress: Phase II final. Appropriateness of Minimum Nurse Staffing Ratios in Nursing Homes. Cambridge, MA: Abt Associates; 2001:3.1–3.67.
- 15.Bostick JE, Rantz M, Flesner MK, Riggs CJ. Systematic review of studies of staffing and quality in nursing homes. J Am Med Dir Assoc, 2006; July:366–376. [DOI] [PubMed]
- 16.Dyck MJ. Nursing staffing and resident outcomes in nursing homes: weight loss and dehydration. J Nurs Care Qual. 2006;22(1):59–65. doi: 10.1097/00001786-200701000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Harrington C, Kovner C, Mezey M, Kayser-Jones J, Burger S, Mohler M, et al. Experts recommend minimum nurse staffing standards for nursing facilities in the United States. Gerontologist. 2000;40:5–16. doi: 10.1093/geront/40.6.673. [DOI] [PubMed] [Google Scholar]
- 18.Simmons SF, Osterweil D, Schnelle JF. Improving food intake in nursing home residents with feeding assistance: a staffing analysis. J Gerontol Med Sci. 2001;56A(12):M790–M794. doi: 10.1093/gerona/56.12.M790. [DOI] [PubMed] [Google Scholar]
- 19.Department of Veterans Affairs. 2009 VHA Nursing Hours per Patient Day (NHPPD) Inventory Report. Washington, DC: Veteran Health Administration, Healthcare Analysis and Information Group: 2010.
- 20.Harrington C, Carillo H, Blank BW, O’Brian T. Nursing facilities, staffing, residents and facility deficiencies, 2004 through 2009. Retrieved from: http://www.pascenter.org//nursing_homes/nursing_trends_2009.php. 2010
- 21.Simmons SF, Sims N, Durkin DW, Shotwell M, Erwin S, Schnelle JF. The quality of feeding assistance care practices for long term care veterans: implications for quality improvement efforts. J Appl Gerontol, 2012 (in press). doi: 10.1177/0733464811433487. [DOI] [PubMed]
- 22.Hartmaier SL, Sloane PD, Guess HA, Koch GG, Mitchell CM, Phillips CD. Validation of the Minimum Data Set cognitive performance scale: agreement with the Mini-Mental State Examination. J Gerontol. 1995;50A:M128–M133. doi: 10.1093/gerona/50A.2.M128. [DOI] [PubMed] [Google Scholar]
- 23.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol. 1991;54A(11):M546–M553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 24.Health Care Financing Administration. Long-Term Care Facility Resident Assessment Instrument (RAI) user's Manual, Minimum Data Set version 2.0. Natick, MA: Eliot. (1999, April)
- 25.Simmons SF, Peterson E, You C. The accuracy of monthly weight assessments in nursing homes: implications for the identification of weight loss. J Nutr Health Aging. 2009;13(3):284–288. doi: 10.1007/s12603-009-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiatarone Singh MA, Rosenberg IH. Nutrition and aging. In: Hazzard WR, Blass JP, Ettinger WH, Halter JB, Ouslander JG, editors. Principles of Geriatric Medicine and Gerontology. 4. New York: McGraw-Hill; 1999. pp. 81–96. [Google Scholar]
- 27.Simmons SF, Garcia ET, Cadogan MP, Al-Samarrai NR, Levy-Storms LF, Osterweil D, Schnelle JF. The Minimum Data Set weight loss quality indicator: does it reflect differences in care processes related to weight loss? J Am Geriatr Soc. 2003;51(10):1410–1418. doi: 10.1046/j.1532-5415.2003.51459.x. [DOI] [PubMed] [Google Scholar]
- 28.Simmons SF, Reuben D. Nutritional intake monitoring for nursing home residents: a comparison of staff documentation, direct observation, and photography methods. J Am Geriatr Soc. 2000;48(2):209–213. doi: 10.1111/j.1532-5415.2000.tb03914.x. [DOI] [PubMed] [Google Scholar]
- 29.Van der Leeden R, Meijer E, Busing FMTA. Resampling multilevel models. In: de Leeuw J, Meijer E, editors. Handbook of Multilevel Analysis. New York: Springer; 2008. pp. 401–433. [Google Scholar]
- 30.Simmons SF, Levy-Storms L. The effect of dining location on nutritional care quality in nursing homes. J Nutr Health Aging. 2005;9(6):434–439. [PubMed] [Google Scholar]
- 31.Kayser-Jones J, Schell E. The effect of staffing on the quality of care at mealtime. Nurs Outlook. 1997;45:64–72. doi: 10.1016/S0029-6554(97)90081-6. [DOI] [PubMed] [Google Scholar]
- 32.Center for Medicare and Medicaid Services (2008). Interpretive Guidelines State Operations Manual Appendix P- Survey Protocol for Long Term Care Facilities. Part 1. Retrieved from: http://www.cms.hhs.gov/manuals/
- 33.Veterans Healthcare Administration Handbook 1142.01 (2008). Criteria and Standards for VA Community Living Centers. Retrieved from: http://www1.va.gov/VHApublications/VHAHK1142.01
- 34.Kheirbek, R. (n.d.). Innovations in nursing home care: Culture transformation in VA. Retrieved from: http://www1.va.gov/geriatricsshg/docs/CultureTransformationProgressReport.doc
- 35.Musson ND, Kincaid J, Ryan P, et al. Nature, nurture, nutrition: Interdisciplinary programs to address the prevention of malnutrition and dehydration. Dysphagia. 1990;5:96–101. doi: 10.1007/BF02412651. [DOI] [PubMed] [Google Scholar]
- 36.Carrier N, West GE, Ouellet D. Dining experience, foodservices and staffing are associated with quality of life in elderly nursing home residents. J Nutr Health Aging. 2009;13(6):565–570. doi: 10.1007/s12603-009-0108-8. [DOI] [PubMed] [Google Scholar]
- 37.Dyck MJ. Weight loss prevention in nursing home residents. A pilot study to determine administrative strategies. J Gerontol Nurs. 2008;34(1):28–35. doi: 10.3928/00989134-20080101-05. [DOI] [PubMed] [Google Scholar]
- 38.Bertrand B, Porchak T, Moore T, Hurd D, Shier V, Sweetland R, Simmons SF. The nursing home Dining Assistant program: a demonstration project. J Gerontol Nurs. 2010;23:1–10. doi: 10.3928/00989134-20100730-04. [DOI] [PubMed] [Google Scholar]
- 39.Simmons SF, Bertrand R, Shier V, Sweetland R, Moore TJ, Hurd DT, Schnelle JF. A preliminary evaluation of the Paid Feeding Assistant regulation: impact on feeding assistance care process quality in nursing homes. Gerontologist. 2007;47(2):184–192. doi: 10.1093/geront/47.2.184. [DOI] [PubMed] [Google Scholar]
- 40.Kayser-Jones J, Schell ES, Porter C, et al. A prospective study of the use of liquid oral dietary supplements in nursing homes. J Am Geriatr Soc. 1998;46:1378–1386. doi: 10.1111/j.1532-5415.1998.tb06004.x. [DOI] [PubMed] [Google Scholar]
- 41.Simmons SF, Patel AV. Nursing home staff delivery of oral liquid nutritional supplements to residents at risk for unintentional weight loss. J Am Geriatr Soc. 2006;54(9):1372–1376. doi: 10.1111/j.1532-5415.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 42.Schnelle JF, Bertrand R, Hurd D, White A, Squires D, Feuerberg M, Hickey K, Simmons SF. The importance of standardized observations to evaluate nutritional care quality in the survey process. J Am Med Dir Assoc. 2009;10(8):568–74. doi: 10.1016/j.jamda.2009.05.004. [DOI] [PubMed] [Google Scholar]