Abstract
Background
Within 1 year after percutaneous coronary intervention, more than 20% of patients experience new adverse events. Physical activity confers a 25% reduction in mortality; however, physical activity is widely underused. Thus, there is a need for more powerful behavioral interventions to promote physical activity. Our objective was to motivate patients to achieve an increase in expenditure of 336 kcal/wk or more at 12 months as assessed by the Paffenbarger Physical Activity and Exercise Index.
Methods
Two hundred forty-two patients were recruited immediately after percutaneous coronary intervention between October 2004 and October 2006. Patients were randomized to 1 of 2 groups. The patient education (PE) control group (n=118) (1) received an educational workbook, (2) received a pedometer, and (3) set a behavioral contract for a physical activity goal. The positive affect/self-affirmation (PA) intervention group (n=124) received the 3 PE control components plus (1) a PA workbook chapter, (2) bimonthly induction of PA by telephone, and (3) small mailed gifts. All patients were contacted with standardized bimonthly telephone follow-up for 12 months.
Results
Attrition was 4.5%, and 2.1% of patients died. Significantly more patients in the PA intervention group increased expenditure by 336 kcal/wk or more at 12 months, our main outcome, compared with the PE control group (54.9% vs 37.4%, P=.007). The PA intervention patients were 1.7 times more likely to reach the goal of a 336-kcal/wk or more increase by 12 months, controlling for demographic and psychosocial measures. In multivariate analysis, the PA intervention patients had nearly double the improvement in kilocalories per week at 12 months compared with the PE control patients (602 vs 328, P=.03).
Conclusion
Patients who receive PA intervention after percutaneous coronary intervention are able to achieve a sustained and clinically significant increase in physical activity by 12 months.
Trial Registration
clinicaltrials.gov Identifier: NCT00248846
Between 20% and 28% of patients who undergo percutaneous coronary intervention (PCI) experience adverse events within 1 year.1,2 American Heart Association, American College of Sports Medicine, and the American College of Cardiology guidelines for care after PCI advise 30 to 60 minutes of aerobic activity on most preferably all days.3,4 Patients who undergo revascularization and then engage in physical activity have a 25% decrease in all-cause mortality by 12 to 24 months.5–8 Despite these compelling data, most patients who undergo PCI do not engage in regular physical activity.9,10 Therefore, more powerful behavioral interventions are needed to increase physical activity.
Our objective was to use a novel psychoeducational intervention combining positive affect and self-affirmation to motivate increased physical activity over the course of 12 months in a cohort of patients who recently underwent PCI. We hypothesized that positive affect and self-affirmation, implemented together, would have a synergistic effect and assist these patients in successfully overcoming the challenges of maintaining physical activity after PCI. Positive affect describes an improved mental state that follows small positive experiences, such as an unexpected compliment or small gift.11,12 Research13,14 indicates that positive affect increases the enjoyment of tasks and fosters flexible thinking, both contributing to enhanced problem solving and ability to appreciate long-and short-term costs and benefits. Self-affirmation draws on past accomplishments that make an individual feel proud. By recalling such events, experimental participants have shown increased self-confidence and resolve to overcome challenges.15–17
STUDY DESIGN
This randomized controlled trial assigned patients who recently underwent PCI to 1 of 2 groups: (1) patient education and behavioral contracting for physical activity (PE control) or (2) patient education and behavioral contracting plus combined positive affect/self-affirmation (PA intervention). The trial was conducted at an academic teaching hospital in New York City. Our primary objective was to motivate a within-patient increase in expenditure of 336 kcal/wk or more at 12 months (to convert energy expenditure to kilojoules per week, multiply by 4.186), assessed by the Paffenbarger Physical Activity and Exercise Index (hereinafter referred to as the Paffenbarger Index).18,19 Our secondary objectives were to examine patterns of kilocalorie expenditure and document the rate of cardiovascular and noncardiovascular events that could influence or confound physical activity outcomes.
PARTICIPANTS
Patients were recruited at the New York Presbyterian Hospital–Weill Cornell campus. Eligible patients were identified after PCI and enrolled when they provided informed written consent. Exclusion criteria included (1) inability to walk, (2) enrollment in other risk-reduction trials, (3) refusal, (4) inability to speak English, and (5) lack of medical clearance by physician to increase physical activity (for safety purposes). The methods are described elsewhere.20
STUDY GROUPS
PE Control Group
The PE control patients (1) received a culturally tailored educational workbook, Living With Heart Disease: Taking Control After Angioplasty.21 This workbook was developed from qualitative interviews with patients who had undergone PCI22 and from established sources23–25; (2) received a pedometer (Yamax Digiwalker; Yamax Corp) to provide feedback and reinforcement; and (3) set a behavioral contract for a self-selected physical activity.23
PA Intervention Group
Patients in the PA intervention group received the identical 3 components as the PE control group. In addition, PA intervention patients were taught how to self-induce positive affect and self-affirmation. The PA intervention components were (1) an additional workbook chapter focused on the constructs of positive affect and self-affirmation26; (2) bimonthly inducement of positive affect and self-affirmation by telephone, at the end of each call, following data collection; and (3) small, unexpected, bimonthly gifts mailed several weeks before follow-up calls (positive affect). Positive affect induction consisted of reminding patients to “think about things that make you feel good” and take a moment each day to enjoy positive thoughts. Self-affirmation induction consisted of asking patients to think about “proud moments” in their life when they find it difficult to exercise. Further details of the intervention are available.20
FOLLOW-UP
Patients in both groups received identical follow-up telephone calls at 2, 4, 6, 8, 10, and 12 months from a trained research assistant (K.A.B.). Follow-up calls reinforced workbook content and assessed clinical events and physical activity. Interviewers used a standardized script, and fidelity was monitored.
RANDOMIZATION
Patients were assigned to either the PE control or PA intervention group on the basis of a randomization schedule known only to the study biostatistician (M.T.W.). Research assistants who administered the standardized study protocols and scripted interviews were not blinded, which is common in behavioral intervention trials.27 However, the coinvestigators (J.C.P., M.E.C., and S.C.W.), the patient’s treating physician, the research coordinator, and the outcome assessors were all blinded.
PRIMARY OUTCOME
Physical Activity
The Paffenbarger Index18,28,29 is one of the most widely used self-report physical activity measures in studies of adults reporting longitudinal morbidity and mortality outcomes. It has demonstrated validity and reliability.30 Compared with activity diaries maintained for 1 month, the Paffenbarger Index has a correlation of 0.62 to 0.65.30,31 Test-retest reliability at 1 month was shown to be r=0.72.32
Demographic, Clinical, and Psychosocial Measures
Baseline demographic data (eg, age, sex, and marital status) were collected. Medical history (eg, angina and diabetes mellitus) was documented, including the Charlson Comorbidity Index33 and Seattle Angina Questionnaire.34 The following measures were administered at baseline and 12 months: Medical Outcomes Study Social Support Survey,35 Perceived Stress Scale,36 Center for Epidemiologic Studies Depression Scale,37–39 and the Positive and Negative Affect Schedule.40
Clinical Outcomes
Clinical outcomes were obtained by patient report during follow-up interviews. All information was corroborated by treating physicians and clinical records, whenever possible. Two blinded clinicians (J.C.P. and M.E.C.) reviewed all clinical events.
PROCEDURES
Enrollment and Baseline Data Collection
Patients were identified from the daily cardiac catheterization schedule. Participants completed baseline forms during the index hospitalization. Following completion of the questionnaires, all enrolled patients (PE and PA groups) were given the workbook Living With Heart Disease: Taking Control After Angioplasty and a pedometer. Physical activity goal setting was conducted by telephone approximately 3 weeks after PCI. Patients considered to be able to participate in exercise by their physician were then randomized. Each patient’s treating physician reviewed and approved the physical activity goal.
Sample Size
The primary outcome of the trial was within-patient change in physical activity from baseline to 12 months, measured in kilocalories per week by the Paffenbarger Index.18,19 According to the Multiple Risk Factor Intervention Trial,41 mean energy expenditure in kilocalories per week in men ranged from 200 to 350. Women who walked 4 to 9 blocks each day had a multivariate relative risk of 0.84 for cardiovascular disease (CVD) compared with women who walked less than 4 blocks each day. This difference corresponds to 336 kcal/wk.42 If we conservatively required a mean difference of 250 kcal/wk between groups and conservatively used an SD of 450 kcal/wk, we required 68 patients per arm, with e set at .05 and a power of 0.90. We estimated that 80% of the patients would be participating in physical activity at 12 months and that there would be 15% attrition. Therefore, 121 patients were allocated per arm, totaling 242 patients.
Statistical Analysis
The data were analyzed using commercial software (SAS version 9.1, SAS Institute, Inc, and Stata version 10, StataCorp, both for Windows, Microsoft Corp). For continuous variables, means (SDs) were calculated, and for categorical variables, counts and percentages were determined. For baseline comparison between the randomization groups, X2 tests were used to investigate the categorical data. For continuous variables, t tests were conducted. An intention-to-treat analysis, in which participants with valid data were included, was conducted. The closeout outcome measure used the final time point before closeout, dropout, or death. A linear mixed model approach was used to assess the intervention effect, controlling for baseline demographic and clinical (age, comorbidity, and sex) as well as psychosocial (affect, depression, perceived stress, and social support) measures, interval medical events, and the interaction between interval medical events and randomization group. A multivariate logistic regression was applied to test for differences in the proportions between the PA intervention and PE control groups while accounting for interval medical events, diabetes, and the interaction between interval medical events and randomization group. Of the variables that were unbalanced between the 2 groups at baseline, only diabetes had a relationship with our main outcome (P=.07) and was adjusted for in the final models. No data were imputed.
RESULTS
PARTICIPANT CHARACTERISTICS
A total of 2605 patients were screened between October 2004 and October 2006 (Figure 1).43 Of these, 242 patients were randomized; 95.2% of the PA intervention group and 91.5% of the PE control group completed 12 months of follow-up. Attrition was 4.5% and 2.1% of the patients died.
Figure 1.
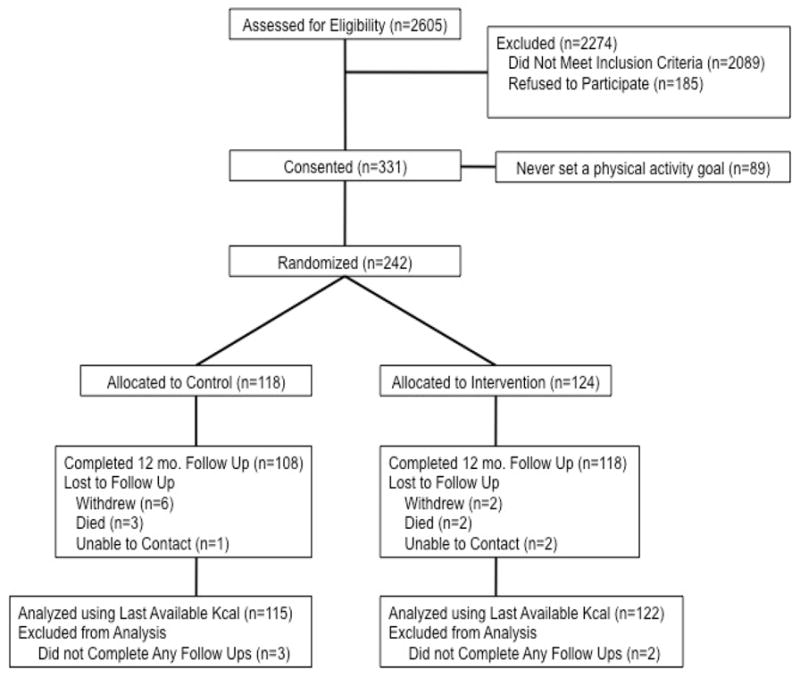
Flow of participants from screening to completion of the final follow-up assessment.
Baseline demographic and clinical characteristics are reported in Table 1. Our population was primarily older and male, with 10.7% African American and 12.8% Hispanic. Patients reported a high burden of comorbidity, with 23.1% scoring 4 or more on the Charlson Comorbidity Index.33 Approximately 75.2% of the patients were overweight or obese.
Table 1.
Baseline characteristics
| PE Control | PA Intervention | ||||
|---|---|---|---|---|---|
| Characteristic | (n=118) No. (%) | (n=124) No. (%) | P Value | ||
| Age, mean (SD), y | 64.4 | (11) | 62.1 | (11) | .12 |
| Women | 35 | (29.7) | 38 | (30.6) | .94 |
| Race | .07 | ||||
| White | 101 | (85.6) | 95 | (76.6) | |
| African American | 9 | (7.6) | 17 | (13.7) | |
| Asian | 4 | (3.4) | 6 | (4.8) | |
| Multiracial | 4 | (3.4) | 6 | (4.8) | |
| Hispanic ethnicity | 18 | (15.2) | 13 | (10.5) | .27 |
| Working | 64 | (54.2) | 72 | (58.1) | .55 |
| Married | 86 | (72.9) | 81 | (65.3) | .20 |
| Completed college | 62 | (52.5) | 71 | (57.2) | .46 |
| Clinical characteristics | |||||
| Previous PCI | 50 | (42.4) | 41 | (33.1) | .14 |
| Ejection fraction, mean (SD), % | 50 | (11) | 52 | (9) | .25 |
| Ever smoked | 86 | (72.9) | 80 | (64.5) | .16 |
| MI on this admission | 20 | (16.9) | 17 | (13.7) | .48 |
| Previous CABG | 17 | (14.4) | 16 | (12.9) | .73 |
| Previous MI | 32 | (27.1) | 38 | (30.6) | .55 |
| Previous CHF | 7 | (6.0) | 5 | (4.0) | .50 |
| Previous CVA | 8 | (6.8) | 8 | (6.4) | .92 |
| Cancer | 21 | (17.8) | 13 | (10.5) | .10 |
| Renal disease | 5 | (4.2) | 12 | (9.7) | .10 |
| Diabetes mellitus | 19 | (16.1) | 42 | (33.9) | .002 |
| End organ damage | 8 | (42.1) | 16 | (38.1) | .77 |
| Seattle Angina Questionnaire, mean (SD), score | |||||
| Physical limitation | 74 | (15) | 67 | (20) | .002 |
| Angina stability | 39 | (28) | 37 | (30) | .59 |
| Angina frequency | 69 | (23) | 72 | (23) | .38 |
| Treatment satisfaction | 91 | (9) | 90 | (9) | .71 |
| Disease perception | 54 | (23) | 46 | (27) | .02 |
| BMI (kg/m2) | |||||
| Normal, < 25 | 32 | (27.1) | 28 | (22.6) | .85 |
| Overweight, ≥ 25 to < 30 | 43 | (36.4) | 52 | (42.0) | |
| Obese, ≥ 30 | 43 | (36.4) | 44 | (35.5) | |
| Charlson Comorbidity Index | |||||
| 0–1 | 69 | (58.5) | 68 | (54.8) | .81 |
| 2–3 | 21 | (17.8) | 28 | (22.6) | |
| ≥4 | 28 | (23.7) | 28 | (22.6) | |
| Psychosocial characteristics, mean (SD), score | |||||
| Positive affect | 32.4 | (9.0) | 32.6 | (7.4) | .79 |
| Negative affect | 22.6 | (8.9) | 23.0 | (8.4) | .76 |
| Depressive symptoms | 9.1 | (6.4) | 9.2 | (6.8) | .88 |
| Stress | 13.7 | (6.8) | 14.2 | (8.4) | .62 |
| Support | 81.6 | (19.8) | 79.3 | (21.4) | .39 |
| Life events | |||||
| None | 63 | (53.4) | 72 | (58.1) | .47 |
| Negative | 23 | (19.5) | 29 | (23.4) | .46 |
| Positive | 32 | (27.1) | 23 | (18.5) | .11 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass graft; CHF, congestive heart failure; CVA, cerebrovascular accident; MI, myocardial infarction; PA, positive affect/self-affirmation; PCI, percutaneous coronary intervention; PE, patient education. Positive and negative affect schedule: possible score range for each subscale, 10 to 50; a higher score means more of an attribute. Depressive symptoms (Center for Epidemiologic Studies Depression Scale): possible score range, 0 to 30; a score of 10 or higher indicates clinically significant depressive symptoms. Stress (Perceived Stress Scale): possible score range, 0 to 40; a higher score means more stress. Support (Medical Outcomes Study Social Support Scale): possible score range, 0 to 100; a higher score means more support.
No significant differences in psychosocial characteristics were found between the groups at baseline.
TREATMENT FIDELITY
Ninety percent of the PA intervention patients vs 91% of the PE control patients received 5 to 6 follow-up telephone calls (P=.75). Eighty-four percent of the PA intervention patients received 5 to 6 of a total of 6 gifts.
Primary Outcome
Significantly more PA intervention patients achieved the main study outcome, an increase of 336 kcal/wk or more at 12 months (54.9% vs 37.4%, P=.007). Two-thirds of patients chose walking for their physical activity. When we analyzed the data with only patients who completed the 12-month follow-up, the results were the same. The PA intervention patients were 1.7 times more likely to reach our main outcome of increasing energy expenditure by 336 kcal/wk or more by 12 months. A decrease in perceived stress (P=.03) and not sustaining an interval medical event (P=.01) were predictive of achieving this goal, controlling for demographic and psychosocial measures (Table 2). Controlling for baseline affect, baseline stress, demographic and clinical variables, and interval medical events, a change in the Positive and Negative Affect Schedule score predicted a change in kilocalories expended at 12 months (P=.01).
Table 2.
Multivariate predictors of reaching 336 kcal/week
| Variable | odds ratio | p |
|---|---|---|
| PA intervention group | 1.74 | .025 |
| Stress | .96 | .034 |
| Interval Medical Event* | .30 | .009 |
|
| ||
| n = 237 | ||
Abbreviations: PA, positive affect/self-affirmation.
Controlling for demographic (age, comorbidity, and sex) and psychosocial (affect, depression, perceived stress, and social support) measures, interval medical events, and the interaction between interval medical events and randomization group.
Interval medical events included cardiovascular and noncardiovascular events that would impede the patient’s ability to engage in physical activity: myocardial infarction, congestive heart failure, percutaneous coronary intervention, cardiac surgical procedures, ischemic colitis, stroke, and major medical complications (eg, shock and metastatic disease).
Patients in the PA intervention group had nearly double the kilocalorie improvement when compared with the PE control group at 12 months. The within-patient increase in kilocalories per week was 602 vs 328, respectively (P=.14). When we controlled for interval medical events, diabetes mellitus, and the interaction between interval medical events and randomization group, PA intervention was a significant predictor (P=.03, Table 3). This expenditure is equivalent to the PA intervention group walking 7.5 miles each week vs the PE control group walking 4.1 miles each week.
Table 3.
Change in Physical Activity and Complications
| Within-Patient Change in Physical Activity From Baseline to 12 mo, kcal/wk | ||
|---|---|---|
| PE control | PA intervention | |
| Interval medical events | ||
| No (n=193) | 389 | 775 |
| 1 interval medical event (n=39) | 83 | 10 |
| 2 interval medical events (n=5) | 28 | −1239 |
| Total | 328 | 602 * |
| *p=0.027 | ||
| Controlling for interval medical events, diabetes and the interaction between interval medical events and randomization group | ||
Abbreviations: PA, positive affect/self-affirmation; PE, patient education.
Interval medical events included cardiovascular and noncardiovascular events that would impede the patient’s ability to engage in physical activity: myocardial infarction, congestive heart failure, percutaneous coronary intervention, cardiac surgical procedures, ischemic colitis, stroke, and major medical complications (eg, shock and metastatic disease).
Secondary Outcomes
Patterns of Kilocalorie Expenditure
As shown in Figure 2, PA intervention patients expended significantly more kilocalories per week over the course of 12 months when compared with the PE control patients (P=.04). Furthermore, the PA intervention group demonstrated a 3-fold within-patient increase in kilocalories per week between 6 and 12 months compared with the PE control group (216 vs 68, P<.001).
Figure 2.
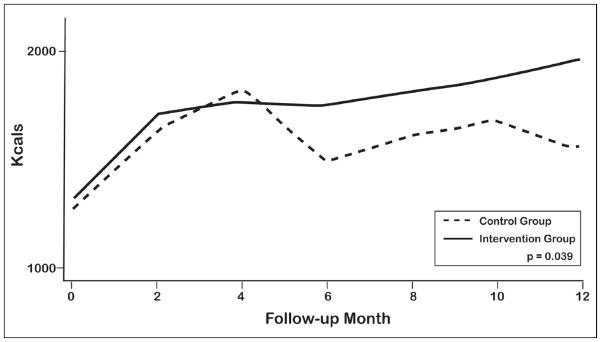
Kcal Expenditure for the PA intervention and PE control groups over 12 months
Interval Medical Events
Overall, 17.2% of the PA intervention group and 20.0% of the PE control group experienced interval medical events (P=.58). Not surprisingly, patients in both groups who experienced interval medical events reported decreases in kilocalorie expenditure (Table 3).
Cardiovascular Events
Cardiovascular events were slightly more frequent in the PE control group; however, the difference was not statistically different between the randomization groups. The rate of combined cardiovascular morbidity (repeat percutaneous coronary intervention, coronary bypass surgery, congestive heart failure, ischemia, and myocardial infarction) and all-cause mortality was 12.3% in the PA intervention group vs 19.1% in the PE control group (P=.15).
Subanalyses
Comorbidity
When we compared attainment of the primary outcome among patients with extremely high comorbid disease burden, there was a significant difference between the randomization groups. Among patients with a Charlson Comorbidity Index of 8 or higher, 87.5% in the PA intervention group reached an increase of 336 kcal/wk or more at 12 months compared with 20.0% in the PE control group (P=.03).
Depressive Symptoms
The change in depressive symptoms from baseline to 12 months differed significantly between the groups. The PA intervention patients were twice as likely to recover from high baseline depressive symptoms (Center for Epidemiologic Studies Depression Scale score ≥10) by 12 months when compared with the PE control patients (odds ratio, 2.58; 95% CI, 1.086.19).
Comment
To our knowledge, this is the first randomized controlled trial of induced positive affect in a clinical population. Our results demonstrate a clinically and statistically important improvement in physical activity in the PA intervention group sustained throughout 12 months (Figure 2). The PA intervention patients were significantly more likely to achieve the primary outcome, a 336 kcal/wk or more increase at 12 months (54.9% vs 37.4%, P=.007). The PA intervention group also had a significant and nearly doubled increase in weekly kilocalorie expenditure at 12 months (328 vs 602 kcal/wk, P=.03; Table 3), primarily through increased walking. This is the equivalent of the PA intervention group’s walking 7.5 miles each week, which is 3.4 miles per week more than the PE control group. There were no significant differences in rates of interval medical events or mortality by randomization group, demonstrating the safety of a telephone-based approach to motivate physical activity in this population, although the study was not powered to detect such differences at 12 months.
Participation in exercise-only cardiac rehabilitation is associated with a 20% to 27% reduction in all-cause mortality by 12 to 24 months.8,9 In the Harvard Alumni Study,18,28 men who exercised had 37% lower mortality over the course of 9 years. In the Nurses’ Health Study,44 women who exercised had a 41% reduction in CVD risk over the course of 10 years. In interventional studies7,10 that have randomized patients to daily exercise vs PCI, the exercise group demonstrated significantly greater event-free survival at 12 months (88% vs 70%, P=.02) and 24 months (78% vs 62%, P=.04).
Physical activity’s salutary effects go beyond those afforded by risk reduction. Physical activity leads to favorable changes in endothelial function.45 Shear stress (the force of blood on the vessel wall) has been shown to cause exercise-induced endothelial adaptations,46 along with increased expression, activity, and bioavailability of nitric oxide synthase.47,48 As a result of endothelial changes, patients with coronary artery disease who engage in physical activity have significantly less vasoconstrictive reactivity and improved flow-dependent vasodilation of the coronary vasculature.49,50
Biologically, it has been proposed that positive affect may attenuate physiologic stress reactivity.51 Positive affect might act through activation of the central nervous system on the neuroendocrine, inflammatory, and immune responses of the body, and these effects may influence long-term outcomes.52 Specifically, positive affect has been associated with lower levels of salivary cortisol,52,53 C-reactive protein,54 and interleukin-6 levels,53,54 as well as greater parasympathetic control, as measured by heart rate variability.55 These effects are independent of negative affect.56
Research has demonstrated that positive affect is associated with favorable biological profiles, including lower heart rate, decreased salivary cortisol level, and decreased fibrinogen stress response, and suggested that these profiles may be associated with less chronic illness as people age.56 We found that patients with very high comorbid burden were particularly responsive to the PA intervention. For example, among patients with a Charlson Comorbidity Index score of 8 or higher, 87.5% of PA intervention patients attained an increase of 336 kcal/wk or more compared with 20% of PE control patients (P =.03). One possible explanation for an increased effect of the PA intervention among patients with high comorbidity levels could be that these individuals may possess less of a happy, positive outlook on life, making them more susceptible to the positive affect induction in the PA intervention. The question remains as to whether chronic illness leads to less-positive affect vs less-positive affect leads to biological changes, which over time lead to chronic illness.
Depression is an important predictor of outcomes in patients with CVD.57–60 However, interventions to treat depression and improve outcomes in patients with coronary heart disease have been disappointing.61 Physical activity may play an important role in reducing the risk of cardiovascular events among depressed patients with CVD. In a recent study of more than 1000 patients with CVD,62 cardiovascular events were largely explained by physical inactivity, which was associated with a 44% higher rate of cardiovascular events. In the present study, PA intervention patients with significant baseline depressive symptoms were twice as likely to experience improvement in depressive symptoms by 12 months compared with PE control patients. We also found that PA intervention patients were significantly more likely to engage in sustained physical activity (Figure 2). We hypothesize that, among people who began the study with more depressive symptoms, increased physical activity among PA intervention patients was mediated by decreased rates of depression over the course of 12 months. The rate of combined cardiovascular morbidity and mortality was 12.3% in the PA intervention group vs 19.1% in the PE control group (P=.15). Longer follow-up is required to determine whether the rates of improved physical activity, decreased depressive symptoms, and slightly lower outcomes that were seen at 12 months in the PA intervention group translate into improved long-term clinical outcomes.
Both the PA intervention and PE control groups demonstrated similar increases in kilocalorie expenditure until 4 months, but their patterns of kilocalorie expenditure then diverged (Figure 2). Beginning at 6 months, the PA intervention group continued to increase kilocalorie expenditure, whereas the PE control group decreased expenditure. The PA intervention group had within-patient weekly expenditures that were 3 times higher than those of the PE control group between 6 and 12 months (P<.001). Many patients perceive PCI as life-threatening; intrinsic motivation to engage in physical activity is greatest during the initial few months following PCI and wanes at approximately 6 months.12 The PA intervention likely boosted their commitment at a time of natural decrease of activity and assisted patients in maintaining physical activity throughout 12 months.
Our study has several strengths, including detailed psychosocial assessments for behavioral mediators of physical activity, a scripted and standardized intervention delivered every 2 months, fidelity checks, and prospective evaluation for kilocalorie expenditure and interval clinical events. However, there also are several limitations. First, kilocalorie expenditure was obtained by self-report and may have been overreported; however, this bias would be present in both groups. Although objective measures of physical activity are available (eg, accelerometry), the use of such devices in longitudinal studies of patients with CVD has limits in community-based populations because of cost, patient inconvenience, and logistical/feasibility issues involving battery life and data retrieval. Accelerometer studies63–66 in this population have reported 3-to 10-day data in small cohorts. Furthermore, it is unclear how accurately accelerometers reflect physical activity during the majority of time, when patients are not wearing the device. Thus, self-reports of physical activity are more commonly used than accelerometry in studies of patients with CVD. Second, patients were enrolled immediately after PCI. Thus, our results may not be generalizable to other patient groups. Third, depressive symptoms were assessed with the Center for Epidemiologic Studies Depression Scale.37–39 Therefore, we can refer to only depressive symptoms and not a diagnosis of clinical depression. Fourth, our sample size was based on previous epidemiologic studies. However, a recent meta-analysis67 of physical activity interventions in patients with CVD supports the assumption of a 336-kcal/wk increase. This meta-analysis (79 studies, 11 877 patients) reported a mean kilocalorie increase of 369 kcal/wk between intervention and control groups. This finding provides additional evidence of weekly kilocalorie improvements in CVD that are consistent with our goal of 336 kcal/wk or more.
In conclusion, to our knowledge, this is the first randomized controlled trial to demonstrate the efficacy of induced positive affect in a clinical population. These results demonstrate sustained and clinically significant improvements in physical activity at 12 months. It has been posited that behavioral interventions might be an effective strategy to improve depressive symptoms and decrease long-term cardiovascular events among patients with CVD.62 This study presents an intervention that successfully motivated patients who received PCI, resulting in increased physical activity and decreased depressive symptoms at 12 months. The long-term goal is to implement the PA intervention over an even longer time frame to document maintenance patterns of physical activity and the occurrence of improved longer-term clinical and psychosocial outcomes in patients who receive PCI.
Acknowledgments
Funding/Support: The study was supported by grant 1N01-HC-25196 from the National Heart, Lung and Blood Institute.
Footnotes
Financial Disclosure: None reported.
Author Contributions: Drs Peterson had full access to all the data in the study, and Drs Peterson, Charlson, and Wells take full responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Peterson, Charlson, Hollenberg, Jobe, Isen, and Allegrante. Acquisition of data: Peterson, Charlson, Hollenberg, and Boschert. Analysis and interpretation of data: Peterson, Charlson, Hoffman, Wells, Wong, Hollenberg, and Isen. Drafting of the manuscript: Peterson, Hoffman, Wells, Hollenberg, and Boschert. Critical revision of the manuscript for important intellectual content: Peterson, Charlson, Hoffman, Wells, Wong, Jobe, and Allegrante. Statistical analysis: Peterson, Wells, Hollenberg, and Isen. Obtained funding: Peterson, Charlson, and Allegrante. Administrative, technical, and material support: Charlson, Hoffman, Hollenberg, Boschert, and Allegrante. Study supervision: Peterson and Jobe.
Additional Contributions: We thank E. Marina Klimasiewfski, BA, BS, Jennifer L. Prokop, BA, and K. Patrick Lane, BA for their assistance in the conduct of the study. All were Weill Cornell Medical College employees and supported by the study grant. We also thank Alissa R. Link, BS, for her assistance in the preparation and editing of this manuscript.
References
- 1.Health, 2009: With Special Feature on Medical Technology. Hyattsville, MD: National Center for Health Statistics; 2010. [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics— 2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King SB, III, Smith SC, Jr, Hirshfeld JW, Jr, et al. 2005 Writing Committee Members. 2007 Focused update of the ACC/AHA/SCAI 2005 guideline update for per-cutaneous coronary intervention: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to review new evidence and update the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention, writing on behalf of the 2005 Writing Committee. Circulation. 2008;117(2):261–295. doi: 10.1161/CIRCULATIONAHA.107.188208. [DOI] [PubMed] [Google Scholar]
- 4.Haskell WL, Lee IM, Pate RR, et al. American College of Sports Medicine; American Heart Association. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 5.Walther C, Möbius-Winkler S, Linke A, et al. Regular exercise training compared with percutaneous intervention leads to a reduction of inflammatory markers and cardiovascular events in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2008;15(1):107–112. doi: 10.1097/HJR.0b013e3282f29aa6. [DOI] [PubMed] [Google Scholar]
- 6.Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2001;(1):CD001800. doi: 10.1002/14651858.CD001800. [DOI] [PubMed] [Google Scholar]
- 7.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Hambrecht R, Walther C, Möbius-Winkler S, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation. 2004;109(11):1371–1378. doi: 10.1161/01.CIR.0000121360.31954.1F. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez RS, Griffiths R, Juergens C, Davidson P, Salamonson Y. Persistence of coronary risk factor status in participants 12 to 18 months after percutaneous coronary intervention. J Cardiovasc Nurs. 2006;21(5):379–387. doi: 10.1097/00005082-200609000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Reid RD, Morrin LI, Pipe AL, et al. Determinants of physical activity after hospitalization for coronary artery disease: the Tracking Exercise After Cardiac Hospitalization (TEACH) Study [published correction appears in Eur J Cardiovasc Prev Rehabil. 2008;15(6):747] Eur J Cardiovasc Prev Rehabil. 2006;13(4):529537. doi: 10.1097/01.hjr.0000201513.13343.97. [DOI] [PubMed] [Google Scholar]
- 11.Isen AM, Daubman KA, Nowicki GP. Positive affect facilitates creative problem solving. J Pers Soc Psychol. 1987;52(6):1122–1131. doi: 10.1037//0022-3514.52.6.1122. [DOI] [PubMed] [Google Scholar]
- 12.Estradaa CA, Isen AM, Young MJ. Positive affect facilitates integration of information and decreases anchoring in reasoning among physicians. Organ Behav Hum Decis Process. 1997;72(1):117–135. doi: 10.1006/obhd.1997.2734. [DOI] [Google Scholar]
- 13.Aspinwall LG, MacNamara A. Taking positive changes seriously. Cancer. 2005;104(11 suppl):2549–2556. doi: 10.1002/cncr.21244. [DOI] [PubMed] [Google Scholar]
- 14.Isen A, Reeve J. The influence of positive affect on intrinsic and extrinsic motivation: facilitating enjoyment of play, responsible work behavior, and self-control. Motiv Emot. 2005;29(4):297–326. doi: 10.1007/s11031-006-9019-8. [DOI] [Google Scholar]
- 15.Steele CM. A threat in the air: how stereotypes shape intellectual identity and performance. Am Psychol. 1997;52(6):613–629. doi: 10.1037//0003-066x.52.6.613. [DOI] [PubMed] [Google Scholar]
- 16.Steele CM, Liu TJ. Dissonance processes as self-affirmation. J Pers Soc Psychol. 1983;45(1):5–19. doi: 10.1037/0022-3514.45.1.5. [DOI] [Google Scholar]
- 17.Reed MB, Aspinwall L. Self-affirmation reduces biased processing of health-risk information. Motiv Emot. 1998;22(2):99–132. doi: 10.1023/A:1021463221281. [DOI] [Google Scholar]
- 18.Paffenbarger RS, Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25(1):60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108(3):161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Boutin-Foster C, Mancuso CA, et al. Translational Behavioral Science Research Consortium. Randomized controlled trials of positive affect and self-affirmation to facilitate healthy behaviors in patients with cardiopulmonary diseases: rationale, trial design, and methods. Contemp Clin Trials. 2007;28 (6):748–762. doi: 10.1016/j.cct.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Allegrante JP, Charlson ME, Isen AM, Peterson JC, Ravenell KL, Robbins L. Living With Heart Disease: Taking Control After Angioplasty. New York, NY: Joan and Sanford I Weill Medical College of Cornell University; 2004. [Google Scholar]
- 22.Peterson JC, Allegrante JP, Pirraglia PA, et al. Living with heart disease after angioplasty: a qualitative study of patients who have been successful or unsuccessful in multiple behavior change. Heart Lung. 2010;39(2):105–115. doi: 10.1016/j.hrtlng.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorig K, Fries JF. The Arthritis Helpbook: A Tested Self-management Program for Coping With Your Arthritis. Reading, MA: Addison-Wesley; 1990. [Google Scholar]
- 24.Rosamond W, Flegal K, Furie K, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics— 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee [published correction appears in Circulation. 2010;122(1):e10] Circulation. 2008;117(4):e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 25.Smith SC, Jr, Dove JT, Jacobs AK, et al. American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1993 Guidelines for Percutaneous Transluminal Coronary Angioplasty); Society for Cardiac Angiography and Interventions. ACC/AHA Guidelines for Per-cutaneous Coronary Intervention (revision of the 1993 PTCA guidelines)— executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1993 Guidelines for Percutaneous Transluminal Coronary Angioplasty) endorsed by the Society for Cardiac Angiography and Interventions. Circulation. 2001;103(24):3019–3041. doi: 10.1161/01.cir.103.24.3019. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Allegrante JP, Peterson JC. Staying positive. In: Allegrante JP, Charlson ME, Isen AM, Peterson JC, Ravenell KL, Robbins L, editors. Living With Heart Disease: Taking Control After Angioplasty. New York, NY: Joan and Sanford I Weill Medical College of Cornell University; 2004. pp. 29–32. [Google Scholar]
- 27.Kamath CC, Vickers KS, Ehrlich A, et al. Behavioral interventions to prevent childhood obesity: a systematic review and metaanalyses of randomized trials. J Clin Endocrinol Metab. 2008;93(12):4606–4615. doi: 10.1210/jc.2006-2411. [DOI] [PubMed] [Google Scholar]
- 28.Paffenbarger RS, Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314(10):605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 29.Lee IM, Paffenbarger RS., Jr Associations of light, moderate, and vigorous intensity physical activity with longevity: the Harvard Alumni Health Study. Am J Epidemiol. 2000;151(3):293–299. doi: 10.1093/oxfordjournals.aje.a010205. [DOI] [PubMed] [Google Scholar]
- 30.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 31.Ainsworth BE, Leon AS, Richardson MT, Jacobs DR, Paffenbarger RS., Jr Accuracy of the College Alumnus Physical Activity Questionnaire. J Clin Epidemiol. 1993;46(12):1403–1411. doi: 10.1016/0895-4356(93)90140-v. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25(1):81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 34.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 35.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 36.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 37.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5(2):179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 38.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 39.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) AmJPrevMed. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 40.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 41.Leon AS, Connett J, Jacobs DR, Jr, Rauramaa R. Leisure-time physical activity levels and risk of coronary heart disease and death: the Multiple Risk Factor Intervention Trial. JAMA. 1987;258(17):2388–2395. [PubMed] [Google Scholar]
- 42.Sesso HD, Paffenbarger RS, Ha T, Lee IM. Physical activity and cardiovascular disease risk in middle-aged and older women. Am J Epidemiol. 1999;150(4):408–416. doi: 10.1093/oxfordjournals.aje.a010020. [DOI] [PubMed] [Google Scholar]
- 43.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148(4):295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- 44.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116(19):2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green DJ, O’Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol. 2008;105 (2):766–768. doi: 10.1152/japplphysiol.01028.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55(2):312–318. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 47.Hambrecht R, Adams V, Erbs S, et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107(25):31523158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 48.Adams V, Linke A, Kränkel N, et al. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111(5):555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- 49.Hambrecht R, Wolf A, Gielen S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342(7):454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 50.Gielen S, Hambrecht R, Schuler GC. Exercise and cardiovascular risk reduction: time to update the rationale for exercise [comment]? J Appl Physiol. 2008;105(2):771. doi: 10.1152/japplphysiol.90348.2008. [DOI] [PubMed] [Google Scholar]
- 51.Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131(6):925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 52.Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc Natl Acad SciUSA. 2005;102(18):6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steptoe A, O’Donnell K, Badrick E, Kumari M, Marmot M. Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: the Whitehall II study. Am J Epidemiol. 2008;167(1):96–102. doi: 10.1093/aje/kwm252. [DOI] [PubMed] [Google Scholar]
- 54.Prather AA, Marsland AL, Muldoon MF, Manuck SB. Positive affective style covaries with stimulated IL-6 and IL-10 production in a middle-aged community sample. Brain Behav Immun. 2007;21(8):1033–1037. doi: 10.1016/j.bbi.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhattacharyya MR, Whitehead DL, Rakhit R, Steptoe A. Depressed mood, positive affect, and heart rate variability in patients with suspected coronary artery disease. Psychosom Med. 2008;70(9):1020–1027. doi: 10.1097/PSY.0b013e318189afcc. [DOI] [PubMed] [Google Scholar]
- 56.Dockray S, Steptoe A. Positive affect and psychobiological processes. Neurosci Biobehav Rev. 2010;35(1):69–75. doi: 10.1016/j.neubiorev.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barefoot JC, Helms MJ, Mark DB, et al. Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol. 1996;78(6):613–617. doi: 10.1016/s0002-9149(96)00380-3. [DOI] [PubMed] [Google Scholar]
- 58.Carney RM, Rich MW, Freedland KE, et al. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med. 1988;50(6):627–633. doi: 10.1097/00006842-198811000-00009. [DOI] [PubMed] [Google Scholar]
- 59.Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91(4):999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 60.Peterson JC, Charlson ME, Williams-Russo P, et al. New postoperative depressive symptoms and long-term cardiac outcomes after coronary artery bypass surgery. Am J Geriatr Psychiatry. 2002;10(2):192–198. [PubMed] [Google Scholar]
- 61.Berkman LF, Blumenthal J, Burg M, et al. Enhancing Recovery in Coronary Heart Disease Patients Investigators (ENRICHD) Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 62.Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ayabe M, Brubaker PH, Dobrosielski D, et al. The physical activity patterns of cardiac rehabilitation program participants. J Cardiopulm Rehabil. 2004;24 (2):80–86. doi: 10.1097/00008483-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Jones NL, Schneider PL, Kaminsky LA, Riggin K, Taylor AM. An assessment of the total amount of physical activity of patients participating in a phase III cardiac rehabilitation program. J Cardiopulm Rehabil Prev. 2007;27(2):81–85. doi: 10.1097/01.HCR.0000265034.39404.07. [DOI] [PubMed] [Google Scholar]
- 65.Stevenson TG, Riggin K, Nagelkirk PR, Hargens TA, Strath SJ, Kaminsky LA. Physical activity habits of cardiac patients participating in an early outpatient rehabilitation program. J Cardiopulm Rehabil Prev. 2009;29(5):299–303. doi: 10.1097/HCR.0b013e3181b4ca61. [DOI] [PubMed] [Google Scholar]
- 66.Oliveira J, Ribeiro F, Gomes H. Effects of a home-based cardiac rehabilitation program on the physical activity levels of patients with coronary artery disease. J Cardiopulm Rehabil Prev. 2008;28(6):392–396. doi: 10.1097/HCR.0b013e31818c3b83. [DOI] [PubMed] [Google Scholar]
- 67.Conn VS, Hafdahl AR, Moore SM, Nielsen PJ, Brown LM. Meta-analysis of interventions to increase physical activity among cardiac subjects. Int J Cardiol. 2009;133(3):307–320. doi: 10.1016/j.ijcard.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]


