ABSTRACT
Because diabetes is associated with increased colorectal cancer (CRC) risk, it is important that people with diabetes receive CRC screenings according to guidelines. In addition, many diabetes self-care recommendations are associated with a reduced risk of CRC. This study aims to identify potential opportunities for enhancing CRC prevention within the context of diabetes management. Using data from 1,730 adults with diabetes aged 50–75 years who responded to the 2010 National Health Interview Survey, we calculated population estimates of behaviors consistent with US Preventive Services Task Force guidelines for CRC screening and American Diabetes Association recommendations for diabetes care. We examined bivariate associations between CRC screening and selected diabetes self-care behaviors associated with CRC risk. Results were stratified by demographic characteristics. Thirty-nine percent of adults with diagnosed diabetes were not up-to-date with CRC screenings. Sixteen percent smoked and 2 % exceeded alcohol intake recommendations. Among those capable of exercise, 69 and 90 % did not meet aerobic exercise and resistance training recommendations, respectively. CRC screening was generally not associated with diabetes self-care behaviors. Among some demographic groups, CRC screening was associated with adequate aerobic activity, not smoking, and being overweight or obese. Many adults with diabetes do not follow guidelines for CRC screening or recommendations for diabetes care that may also reduce CRC risk. Thus, opportunities may exist to jointly promote CRC screening and prevention and diabetes self-management among adults with diabetes.
KEYWORDS: Colorectal cancer, Cancer screening, Diabetes mellitus, Self-management, Self-care, Health behaviors, Multiple health behavior change
INTRODUCTION
Colorectal cancer (CRC) is a major form of cancer in the USA, accounting for approximately 143,000 new cases and 53,000 deaths each year. Over half of all new CRC cases and over two thirds of all deaths from CRC occur among adults aged 65 years and older [1]. Diabetes is also more prevalent at older ages. While over 11 % of adults aged 20 years and older (25.6 million) have been diagnosed with or have undiagnosed diabetes, this figure is over 25 % for adults aged 65 years and older [2], and the prevalence of diabetes is increasing [3]. Diabetes has been linked with an increased risk of several cancers, including CRC [4–6]. Furthermore, diabetes has been associated with increased mortality and poorer clinical outcomes among persons with CRC [7, 8]. It is therefore important that persons with diabetes receive CRC screenings according to age-appropriate guidelines [4]. The US Preventive Services Task Force (USPSTF) currently recommends routine CRC screening for average-risk adults starting at age 50 years and continuing until age 75 years and has not issued separate guidelines for persons with diabetes [9].
The American Diabetes Association (ADA) publishes standards for diabetes care [10] which recommend health behaviors and other activities for the prevention and management of diabetes. These recommendations include at least 150 min weekly of moderate aerobic activity, resistance training at least three times weekly, weight loss for individuals who are overweight or obese, daily aspirin use for those with a history of cardiovascular disease (CVD), limited alcohol consumption, and not smoking or smoking cessation [10].
Many health behaviors addressed in the prevention and management of diabetes and its complications have also been associated with CRC risk. Behaviors such as high alcohol consumption and smoking have been linked to increased CRC risk, while aspirin and physical activity have been associated with reduced risk [11–13]. Previous studies have reported that people with diabetes are less likely to engage in behaviors such as regular physical activity than are members of the general population [14–16]. Synergistic effects from health promotion efforts might be possible by linking cancer prevention with diabetes management.
Some studies have found factors such as socioeconomic status, glycemic control, health insurance coverage, utilization of health services, and filling of diabetes prescriptions to be associated with CRC screening among adults with diabetes [17–21]. However, these studies have not examined CRC screening in relation to diabetes self-management. The aims of this study were to address this gap by identifying potential opportunities for enhancing CRC screening and prevention within the context of diabetes management. Using the 2010 National Health Interview Survey (NHIS), we produce population estimates of the prevalence of CRC screening and various diabetes self-care behaviors and risk factors that may also affect risk for CRC among adults aged 50–75 years with diabetes. We also evaluate whether those who meet recommendations for diabetes self-management are more, less, or equally likely to be up-to-date with CRC screening compared with those who do not meet these recommendations.
METHODS
Data source and study population
Data were obtained from the 2010 NHIS. The NHIS is a nationally representative, in-person household survey of the non-institutionalized civilian population in the USA conducted by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC). Sample adults were randomly selected by NHIS from each family interviewed. In 2010, 27,157 sample adults were interviewed. The conditional response rate for sample adults was 77.3 %, and the final response rate for sample adults was 60.8 % [22]. CRC screening data were obtained from the Cancer Control Supplement of the 2010 NHIS which was sponsored by the CDC’s Division of Cancer Prevention and Control and the National Cancer Institute’s Division of Cancer Control and Population Sciences.
The sample analyzed for this study included sample adults who reported having been diagnosed with diabetes and who did not report a diagnosis of colon or rectal cancers. Adults were included if they were within the age range for which the USPSTF recommends routine CRC screening (aged 50–75 years). This resulted in a final sample of 1,730 individuals.
CRC screening measures
Being up-to-date with CRC screening was defined using current USPSTF guidelines for CRC screening [9]. The recommendations for CRC screening are completion of a home fecal occult blood test (FOBT) within the past year, sigmoidoscopy within the past 5 years and home FOBT within the past 3 years, or colonoscopy within the past 10 years. We used the same NHIS screening recodes as had been employed previously [23, 24].
Diabetes self-care measures
Diabetes self-care behaviors and risk factors were identified using the ADA “Standards of Medical Care in Diabetes—2009” as described above [10]. We selected recommendations pertaining to behaviors that influence CRC risk and for which data were available in the NHIS 2010. We use the term “diabetes risk behaviors” to refer to behaviors inconsistent with ADA recommendations regarding physical activity, smoking, alcohol consumption, and aspirin therapy, as described below. We consider overweight and obesity to be risk factors.
Adequate aerobic activity was defined as at least 150 min/week of moderate aerobic activity. Following the US Department of Health and Human Services (DHHS) 2008 Physical Activity Guidelines for Americans [25, 26], at least 75 min/week of vigorous aerobic activity or an equivalent combination of moderate and vigorous activity were also classified as adequate aerobic activity. For this analysis, only reported leisure-time physical activity (LTPA) was used to estimate aerobic activity. NHIS questions combined light and moderate LTPA.
Although most studies on physical activity and CRC risk have not examined resistance training separately, resistance training, like aerobic activity, has been shown to improve insulin sensitivity which has been linked to CRC risk [27–29]. The ADA recommends that those with type 2 diabetes do resistance training three times per week [10]. Adequate resistance training was therefore measured as reporting leisure-time muscle-strengthening activities at least three times weekly. Physical activity variables were modified from NCHS recodes [30].
The ADA recommends weight loss for overweight or obese individuals to reduce insulin resistance. Moreover, obesity is a risk factor for CRC. We therefore included a measure of overweight and obesity. Overweight was defined as having a body mass index (BMI, in kilograms per square meter) at or over 25 and below 30 while obesity was defined as having a BMI of 30 or more [22].
NHIS questions about frequency of aspirin use only inquired about whether aspirin was taken regularly (at least three times per week); no measure of daily aspirin use was available. Therefore, we defined regular aspirin use as reporting use of aspirin products at least three times weekly. Because the ADA recommends aspirin therapy for those with a history of CVD, aspirin use analyses were restricted to those reporting a diagnosis of either myocardial infarction/heart attack, angina pectoris, coronary heart disease, or stroke (n = 486).
Alcohol consumption was dichotomized consistent with the ADA recommendation of an average of no more than two alcoholic drinks daily for men and no more than one alcoholic drink daily for women. Both intensity and duration of smoking have been shown to affect CRC risk. However, following ADA recommendations advising patients with diabetes not to smoke, we dichotomized smoking by whether or not respondents reported being current smokers.
Demographic and disease characteristics
Age, sex, race/ethnicity, education, health insurance coverage, and use of medications for diabetes were also examined to describe the demographics and disease characteristics of the sample (see Table 1). Age was categorized as 50 to 64 and 65 to 75 years because almost all adults aged 65 and over receive Medicare, and therefore potential differences may exist between these two age groups. Four racial/ethnic categories (non-Hispanic white, non-Hispanic black, non-Hispanic other, and Hispanic) were used; because of the small numbers of American Indians/Alaska Natives, those of multiple races or whose primary race was not available, and those of Asian race, these groups were combined into the “Non-Hispanic other” category. Those without private or public health insurance and those covered only by the Indian Health Service were classified as not covered by health insurance as defined in Health, United States, 2010 [31]. Use of diabetes medications was categorized as use of insulin, oral hypoglycemic agents, both types of medications, or neither type of medication. The NHIS did not provide data on specific classes of oral hypoglycemic agents.
Table 1.
Characteristics of study population, adults aged 50–75 years with diagnosed diabetes and no diagnosis of colorectal cancer [22]
| Characteristics | Weighted percentage | Sample size | Estimated population size |
|---|---|---|---|
| wt.% (95 % CI) | n | N (SE) | |
| Total | 100 | 1,730 | 13,028,181 (383,612.3) |
| Age | |||
| 50–64 | 62.7 (59.8, 65.5) | 1,034 | 8,169,053 (297,901.1) |
| 65–75 | 37.3 (34.5, 40.2) | 696 | 4,859,128 (241,895.9) |
| Sex | |||
| Male | 53.5 (50.8, 56.2) | 819 | 6,972,688 (282,514.5) |
| Female | 46.5 (43.8 49.2) | 911 | 6,055,493 (246,038.4) |
| Race/ethnicity | |||
| Non-Hispanic white | 65.2 (62.6, 67.8) | 892 | 8,494,237 (327,168.8) |
| Non-Hispanic black | 14.8 (13.0, 16.7) | 394 | 1,925,299 (129,100.3) |
| Non-Hispanic other | 5.7 (4.5, 7.2) | 112 | 745,047 (90,148.0) |
| Hispanic | 14.3 (12.6, 16.2) | 332 | 1,863,598 (125,010.5) |
| Educationa | |||
| Less than high school | 22.3 (20.1, 24.7) | 453 | 2,892,774 (177,461.5) |
| High school/GED | 29.5 (26.9, 32.2) | 483 | 3,822,604 (202,178.1) |
| Some college | 28.3 (25.6, 31.1) | 482 | 3,666,825 (219,648.2) |
| College degree | 19.9 (17.5, 22.7) | 303 | 2,584,508 (184,722.0) |
| Health insurance | |||
| Not covered by health insurance | 8.7 (7.3, 10.4) | 155 | 1,139,051 (103,267.0) |
| Covered by health insurance | 91.3 (89.6, 92.7) | 1,575 | 11,889,130 (377,157.2) |
| Diabetes medicationsb | |||
| None | 11.7 (9.9, 13.7) | 202 | 1,520,871 (133,570.2) |
| Insulin only | 13.2 (11.4, 15.2) | 213 | 1,717,729 (134,890.7) |
| Oral hypoglycemic agents only | 60.4 (57.5, 63.2) | 1,036 | 7,861,225 (312,748.1) |
| Both | 14.8 (12.9, 16.8) | 278 | 1,925,907 (132,926.8) |
All percentages are weighted to the US civilian non-institutionalized population. Some percentages may not sum up to 100 because of rounding
CI confidence interval, SE standard error, GED General Education Development, n sample size, N estimated population size
aNine individuals had an unknown or no response for this item. The sample size for this outcome is 1,721
bOne individual had an unknown or no response for this item. The sample size for this outcome is 1,729. The category “None” refers to neither oral hypoglycemic agents nor insulin
Statistical analysis
We used SAS-callable SUDAAN release 10.0.1 to account for the complex sample design. We calculated weighted prevalence estimates and 95 % confidence intervals of CRC screening and selected diabetes risk behaviors and risk factors as well as cross-tabulations of CRC screening by diabetes risk behaviors and risk factors. Results were stratified by demographic characteristics. Small numbers of uninsured individuals prevented us from presenting cross-tabulations of CRC screening and diabetes risk behaviors and risk factors by health insurance status.
For each variable, those with unknown or missing responses were excluded (see Tables 1, 2, and 3; Fig. 1 for final sample sizes). Those with unknown or missing responses on any outcome measure did not differ significantly from those with complete information on all outcomes by sex, age, race/ethnicity, educational attainment, health insurance coverage, or use of insulin and oral hypoglycemic medications. Aerobic activity analyses excluded 86 people reporting an inability to perform light or moderate LTPA, and resistance training analyses excluded 92 individuals reporting an inability to do leisure-time muscle strengthening activities.
Table 2.
Prevalence of colorectal cancer screening and diabetes risk behaviors and risk factors, adults aged 50–75 years with diabetes and no diagnosis of colorectal cancer [22]
| Up-to-date with CRC screening (n = 1,587) | Inadequate aerobic activity (n = 1,610) | Inadequate resistance training (n = 1,618) | Current smoker (n = 1,710) | Does not take aspirin regularly if CVD history (n = 449) | Exceeds alcohol intake recommendations (n = 1,698) | Overweight (n = 1,662) | Obese (n = 1,662) | |
|---|---|---|---|---|---|---|---|---|
| wt.% (95 % CI) | wt.% (95 % CI) | wt.% (95 % CI) | wt.% (95 % CI) | wt.% (95 % CI) | wt.% (95 % CI) | wt.% (95 % CI) | wt.% (95 % CI) | |
| Overall | 61.0 (58.0, 63.9) | 68.5 (65.4, 71.4) | 89.8 (87.7, 91.5) | 16.1 (13.9, 18.5) | 31.1 (26.3, 36.4) | 2.3 (1.6, 3.4) | 28.8 (26.2, 31.5) | 58.4 (55.6, 61.1) |
| Age | –*** | –*** | –* | –* | ||||
| 50–64 years old | 56.0 (52.0, 59.9) | 66.7 (62.6, 70.6) | 89.8 (87.3, 91.9) | 20.1 (17.1, 23.5) | 29.7 (23.4, 36.7) | 3.0 (1.9, 4.6) | 26.5 (23.2, 30.1) | 61.6 (57.8, 65.2) |
| 65–75 years old | 69.1 (64.7, 73.2) | 71.5 (67.2, 75.5) | 89.6 (86.5, 92.1) | 9.2 (6.8, 12.3) | 33.0 (25.7, 41.3) | 1.3 (0.7, 2.5) | 32.6 (28.6, 36.9) | 53.0 (48.4, 57.5) |
| Sex | –*** | –* | –* | –** | –*** | |||
| Male | 63.3 (58.8, 67.5) | 62.7 (58.4, 66.8) | 87.7 (84.7, 90.2) | 16.7 (13.7, 20.1) | 25.7 (19.7, 32.7) | 3.6 (2.4, 5.5) | 34.8 (31.0, 38.8) | 52.7 (48.5, 56.9) |
| Female | 58.4 (54.3, 62.3) | 75.3 (71.5, 78.8) | 92.2 (89.4, 94.2) | 15.3 (12.6, 18.6) | 39.0 (31.5, 47.1) | 0.9 (0.4, 1.8) | 21.4 (18.3, 24.9) | 65.3 (61.7, 68.7) |
| Race/ethnicity | –*** | –* | –c | –c | –*** | |||
| Non-Hispanic white | 63.1 (59.2, 66.7) | 66.4 (62.4, 70.2) | 89.2 (86.5, 91.4) | 17.4 (14.4, 20.9) | 28.4 (22.7, 34.8) | 3.1 (2.0, 4.7) | 27.3 (23.9, 31.0) | 60.4 (56.7, 64.0) |
| Non-Hispanic black | 66.6 (60.1, 72.6) | 69.8 (63.6, 75.4) | 92.8 (88.9, 95.4) | 17.4 (13.4, 22.2) | 37.7 (27.1, 49.7) | 1.4 (0.6, 3.2) | 31.7 (26.5, 37.3) | 58.6 (52.3, 64.7) |
| Non-Hispanic other | 46.9 (35.3, 58.7) | 67.5 (56.6, 76.7) | 87.7 (79.3, 93.0) | 11.7 (6.8, 19.4) | –c | –c | 37.1 (26.9, 48.7) | 33.7 (23.3, 46.1) |
| Hispanic | 51.1 (44.0, 58.1) | 76.4 (69.3, 82.3) | 90.0 (83.9, 93.9) | 10.3 (7.2, 14.7) | 39.5 (24.0, 57.5) | 0.9 (0.4, 2.3) | 29.0 (23.6, 35.2) | 58.8 (52.6, 64.8) |
| Health insurance | –*** | –c | ||||||
| Not covered | 28.5 (20.9, 37.7) | 71.9 (61.8, 80.3) | 93.6 (87.7, 96.8) | 21.4 (13.7, 32.0) | 38.2 (23.0, 56.1) | –c | 30.0 (21.8, 39.7) | 56.4 (46.7, 65.7) |
| Covered | 64.0 (61.0, 66.9) | 68.1 (65.0, 71.1) | 89.4 (87.1, 91.3) | 15.5 (13.4, 18.0) | 30.5 (25.6, 35.9) | 2.5 (1.7, 3.6) | 28.6 (26.0, 31.5) | 58.6 (55.7, 61.4) |
| Educationa | –** | –*** | –** | –* | –*** | –*** | ||
| <High school | 51.8 (46.0, 57.6) | 78.6 (72.5, 83.6) | 94.1 (89.8, 96.6) | 17.4 (13.2, 22.5) | 38.7 (29.8, 48.4) | 1.7 (0.7, 4.1) | 30.8 (25.6, 36.4) | 55.9 (50.1, 61.5) |
| High school/GED | 59.5 (54.2, 64.7) | 77.5 (72.7, 81.7) | 91.7 (88.1, 94.3) | 19.0 (14.8, 23.9) | 40.1 (30.2, 50.8) | 3.6 (1.9, 6.5) | 25.8 (21.5, 30.6) | 59.5 (54.4, 64.3) |
| Some college | 66.4 (60.8, 71.6) | 64.6 (58.4, 70.3) | 87.7 (83.4, 91.0) | 15.8 (12.0, 20.5) | 22.9 (16.3, 31.2) | 1.5 (0.6, 3.4) | 26.4 (21.8, 31.6) | 66.0 (60.6, 70.9) |
| College | 65.3 (58.7, 71.4) | 49.7 (42.4, 57.1) | 85.2 (79.8, 89.4) | 10.3 (6.7, 15.5) | 18.3 (11.3, 28.3) | 2.5 (1.2, 5.1) | 34.4 (28.3, 41.0) | 48.8 (42.0, 55.7) |
| Diabetes medicationsb | –*** | –c | –* | |||||
| None | 65.6 (57.2, 73.1) | 62.7 (54.2, 70.5) | 88.0 (81.8, 92.2) | 15.2 (10.0, 22.3) | 24.5 (13.7, 39.9) | –c | 30.7 (23.6, 38.8) | 49.7 (41.6, 57.7) |
| Insulin only | 59.5 (50.8, 67.6) | 74.1 (65.7, 81.1) | 85.6 (77.4, 91.2) | 16.6 (11.3, 23.8) | 27.9 (18.4, 40.0) | –c | 24.0 (17.8, 31.7) | 59.3 (51.5, 66.7) |
| Oral hypoglycemic agents only | 59.6 (55.7, 63.5) | 66.1 (62.3, 69.8) | 90.4 (87.9, 92.4) | 16.1 (13.6, 19.1) | 33.7 (27.2, 40.8) | 2.7 (1.7, 4.2) | 30.2 (26.8, 33.9) | 58.2 (54.5, 61.7) |
| Both | 64.1 (57.1, 70.6) | 77.9 (71.3, 83.3) | 92.0 (85.7, 95.7) | 16.0 (11.1, 22.4) | 31.5 (21.3, 43.7) | 3.1 (1.4, 6.6) | 25.5 (19.7, 32.3) | 65.4 (58.3, 71.9) |
All percentages are weighted to the US civilian non-institutionalized population
CI confidence interval, CRC colorectal cancer, CVD cardiovascular disease, GED General Education Development, n sample size
*p < 0.05; **p < 0.01; ***p < 0.001 for tests of independence of demographic/diabetes characteristics and adherence to guidelines and recommendations
aFor those with known education information, the sample sizes are 1,581 for the CRC screening item, 1,603 for the aerobic activity item, 1,611 for the resistance training item, 1,690 for the alcohol item, 1,702 for the smoking item, 447 for the aspirin item, and 1,655 for the overweight/obesity item
bFor the diabetes medication item, one person had unknown or no response for insulin use. This affected the sample size for smoking only for this item, where the sample size was 1,709. “None” refers to those taking neither insulin nor oral hypoglycemic agents
cEstimates in which the relative standard error is >50 % or in which denominator is <30 are considered unstable and have been suppressed. p values are not reported in those cases. Estimates in which the relative standard error is >30 % but <50 % or in which the denominator is <50 but >30 are set in italics. Caution should be taken when interpreting these results
Table 3.
Percentage up-to-date with colorectal cancer screening by selected diabetes risk behaviors and risk factors, adults aged 50–75 years with diabetes and no diagnosis of colorectal cancer [22]
| Overall | Age | Sex | Race/Ethnicity | |||||
|---|---|---|---|---|---|---|---|---|
| 50–64 | 65–75 | Male | Female | NH white | NH black | Hispanic | ||
| wt.% (95 % CI)" for each column as in Table 2 | ||||||||
| Totala | 61.0 (58.0, 63.9) | 56.0 (52.0, 59.9) | 69.1 (64.7, 73.2) | 63.3 (58.8, 67.5) | 58.4 (54.3, 62.3) | 63.1 (59.2, 66.7) | 66.6 (60.1, 72.6) | 51.1 (44.0, 58.1) |
| Aerobic activityb | –* | –* | ||||||
| Adequate | 64.1 (58.4, 69.4) | 56.9 (49.6, 64.0) | 77.6 (69.8, 83.8) | 67.0 (59.7, 73.5) | 59.1 (49.9, 67.6) | 65.4 (58.4, 71.8) | 66.9 (53.7, 77.9) | 64.4 (49.7, 76.9) |
| Inadaquate | 58.8 (55.1, 62.3) | 53.8 (49.0, 58.7) | 66.5 (60.9, 71.6) | 60.5 (54.9, 65.9) | 57.0 (52.3, 61.6) | 61.3 (56.4, 66.0) | 66.2 (59.0, 72.8) | 45.6 (38.2, 53.3) |
| Resistance trainingc | –* | –g | –g | |||||
| Adequate | 65.6 (55.7, 74.2) | 55.6 (42.1, 68.3) | 81.6 (68.4, 90.1) | 66.4 (54.2, 76.7) | 64.0 (48.0, 77.4) | 64.7 (52.7, 75.0) | –g | –g |
| Inadequate | 59.9 (56.7, 63.1) | 54.9 (50.7, 59.1) | 68.2 (63.2, 72.8) | 62.6 (57.6, 67.3) | 57.0 (52.7, 61.3) | 62.4 (58.1, 66.4) | 66.7 (59.9, 72.8) | 46.8 (39.8, 53.8) |
| Smokingd | –* | |||||||
| Nonsmoker | 62.2 (59.1, 65.3) | 57.5 (53.1, 61.8) | 69.0 (64.1, 73.5) | 64.7 (59.9, 69.1) | 59.4 (55.2, 63.6) | 65.3 (61.2, 69.2) | 67.1 (59.7, 73.8) | 51.3 (43.9, 58.6) |
| Current Smoker | 54.9 (47.4, 62.2) | 50.7 (42.1, 59.3) | 69.5 (55.9, 80.3) | 56.1 (44.9, 66.7) | 53.4 (43.1, 63.4) | 52.9 (43.5, 62.1) | 66.4 (48.9, 80.2) | 48.7 (30.1, 67.7) |
| Aspirin usee | –g | |||||||
| Regular user | 63.7 (57.0, 69.9) | 55.0 (45.6, 64.0) | 75.9 (66.0, 83.5) | 67.7 (59.2, 75.2) | 56.4 (45.6, 66.5) | 61.2 (53.1, 68.7) | 72.4 (57.2, 83.8) | 62.0 (42.6, 78.2) |
| Does not use regularly | 58.3 (49.5, 66.7) | 56.0 (43.0, 68.2) | 61.3 (48.2, 72.9) | 56.8 (43.1, 69.6) | 59.7 (47.8, 70.6) | 62.3 (51.3, 72.2) | 57.8 (37.8, 75.5) | –g |
| BMI (kg/m2f ) | –* | –* | –** | |||||
| <25 | 52.2 (44.5, 59.8) | 44.3 (34.6, 54.4) | 62.8 (50.7, 73.5) | 63.4 (52.3, 73.2) | 39.5 (30.0, 49.9) | 60.3 (49.1, 70.5) | 49.8 (33.8, 65.9) | 39.5 (24.2, 57.3) |
| Overweight | 65.5 (60.0, 70.7) | 60.7 (52.9, 68.0) | 72.1 (64.5, 78.5) | 69.8 (62.9, 76.0) | 57.0 (48.4, 65.2) | 68.7 (61.8, 74.8) | 73.1 (64.2, 80.4) | 51.7 (38.6, 64.5) |
| Obese | 60.5 (56.4, 64.4) | 55.8 (50.8, 60.8) | 69.1 (62.2, 75.2) | 59.2 (52.9, 65.1) | 61.7 (56.8, 66.5) | 61.0 (55.7, 66.0) | 66.0 (56.2, 74.6) | 52.8 (43.5, 62.0) |
C confidence interval, CRC colorectal cancer, CVD cardiovascular disease, BMI body mass index
All percentages are weighted to the US civilian non-institutionalized population
*p < 0.05; **p < 0.01; ***p < 0.001 for tests of independence of being up-to-date with CRC screening and adherence to diabetes care recommendations
aSample size is 1,587 individuals with data on CRC screening
bSample size is 1,495 individuals capable of light/moderate leisure-time physical activity and with data on both CRC screening and leisure-time physical activity
cSample size is 1,502 individuals capable of muscle strengthening and with data on CRC screening and muscle strengthening activities
dSample size is 1,583 individuals with data on CRC screening and smoking
eSample size is 444 individuals with CVD with data on CRC screening and aspirin use
fSample size is 1,545 individuals with data on CRC screening and BMI
gEstimates in which the relative standard error is >50 % or in which denominator is < 30 are considered unstable and have been suppressed. p values are not presented for comparisons using these estimates. Estimates in which the relative standard error is >30 % but <50 % or in which the denominator is <50 but >30 are set in italics. Caution should be taken when interpreting these results
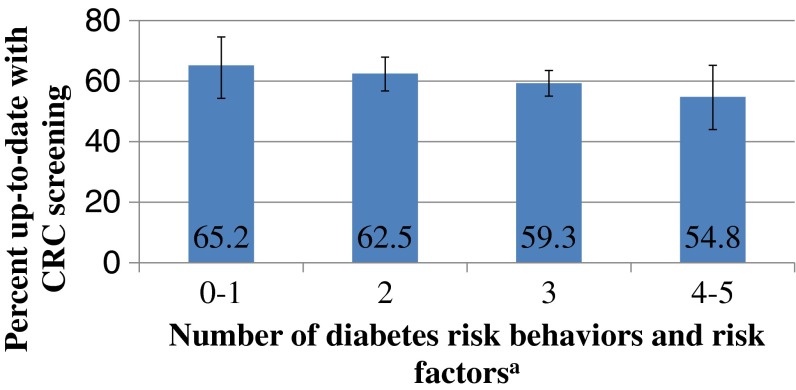
Fig 1.
Percentage up-to-date with CRC screening by number of diabetes risk behaviors and risk factors, adults aged 50–75 years with diabetes and no diagnosis of CRC, n = 1,425 [22]. Test for independence, p = 0.415; test for linear trend, p = 0.097. Note: All percentages are weighted to the US civilian non-institutionalized population. The diabetes risk behaviors and risk factors included are inadequate aerobic activity, inadequate resistance training, smoking, excessive alcohol use, and being overweight or obese. Those with zero or one diabetes risk behaviors or risk factors were combined into one category due to the very small number of individuals with no diabetes risk behaviors or risk factors. Those with four or five diabetes risk behaviors or risk factors were combined into one category due to the small number of individuals with five diabetes risk behaviors or risk factors
Confidence intervals were calculated using the logit transformation. The chi-square test of independence was used to test for differences in CRC screening and diabetes risk behaviors and risk factors by demographic characteristics as well as differences in CRC screening among those meeting and not meeting the selected diabetes care recommendations. Tests for linear trend used the Cochran-Mantel-Haenszel trend test [32]. Differences were considered significant at p-value less than 0.05. Because this study is intended to examine the extent to which adults with diabetes who are not up-to-date with CRC screening may also not be meeting recommended diabetes self-management behaviors, estimates and associations are not adjusted for other factors or variables.
RESULTS
Our sample represented approximately 13 million adults with diabetes, accounting for over 16 % of American adults aged 50–75 years who had not been diagnosed with CRC. The mean reported number of years since diabetes diagnosis was 11.6 (95 % confidence interval (CI), 11.0–12.3). Table 1 displays the characteristics of our sample; nearly 90 % used diabetes medications, primarily oral hypoglycemic agents.
Sociodemographic characteristics and CRC screening and diabetes self-management behaviors
As shown in Table 2, 61 % of our sample was up-to-date with CRC screening. Those without health insurance coverage, Hispanics, those of other race/ethnicity, those with less than a high school education, and those aged 50–64 years were less likely to be up-to-date with screening. Less than one third of our sample met aerobic activity recommendations based on their LTPA while only 10 % reported resistance training consistent with recommendations for adults with diabetes. Women and those with lower education were more likely to report inadequate amounts of physical activity based on their LTPA than were men and those with greater education, respectively. Only 2.3 % of the population reported alcohol consumption in excess of recommendations, with significantly more men and those aged 50–64 reporting excess use. Sixteen percent of the population reported current smoking. Those in the 50–64 age group were more likely to smoke than were those ages 65–75. Hispanics were less likely to smoke than were blacks and whites, and those with more education were less likely to smoke than those with less education. Among those with a personal history of CVD, 31.1 % did not use aspirin regularly. Women were less likely to report taking aspirin regularly than were men, and those with no more than a high school education were less likely to report taking aspirin regularly than were those with a college education.
BMI/weight status
Eighty-seven percent of our sample had a BMI over 25, and 58.4 % were obese. Those of other race/ethnicity were less likely to be obese than were whites, blacks, or Hispanics, and those taking both oral hypoglycemic agents and insulin were more likely to be obese than were those taking neither medication. Obesity was also higher among those aged 50–64 years, women, and those with some college education.
Adherence to diabetes self-management recommendations
We examined the subset of adults who were capable of light to moderate physical activity and muscle strengthening activities and who had data on CRC screening and all diabetes care recommendations examined (n = 1,425), excluding aspirin use because it was restricted to those with a personal history of CVD. We found that less than 1 % met all six of the recommendations and guidelines examined (CRC screening, aerobic activity, resistance training, not smoking, limited alcohol consumption, and not overweight or obese). Ninety-three percent (95 % CI, 91.4–94.6 %) failed to meet multiple recommendations and guidelines, with nearly three quarters (95 % CI, 70.6–76.6 %) reporting at least three risk factors or behaviors that were inconsistent with recommendations and guidelines, including CRC screening. Moreover, 39.1 % (95 % CI, 36.0–42.3 %) were simultaneously not up-to-date with CRC screening and not meeting at least one diabetes care recommendation (excluding aspirin use); nearly all of those who were not up-to-date with CRC screening (98.5 % (95 % CI, 96.3–99.4 %)) had at least one diabetes risk behavior or risk factor (not shown). CRC screening did not differ by the number of diabetes risk behaviors and risk factors reported (see Fig. 1).
Table 3 presents estimates of CRC screening by each of the diabetes risk behaviors and risk factors examined in this study. For the overall sample and most demographic subgroups, any differences in CRC screening by aerobic activity levels were not statistically significant. However, among Hispanics and those aged 65–75 years, those engaged in adequate aerobic activity were significantly more likely to be up-to-date with CRC screening compared with those reporting inadequate levels of activity. A similar result was obtained for resistance training among the older age group.
Although current smokers did not differ significantly from nonsmokers in CRC screening overall, among non-Hispanic whites, a significantly smaller proportion of current smokers were up-to-date with CRC screening compared with current nonsmokers. Overweight adults with diabetes were more likely to be up-to-date with CRC screening than were those whose reported BMI was under 25. When stratifying by demographic groups, the differences in screening by BMI were significant among women and adults aged 50–64 years.
Results for CRC screening by alcohol consumption are not presented in Table 3 because of the small numbers obtained when stratifying by demographic characteristics. For the overall sample, no significant difference was detected in the percentage of individuals who were up-to-date with CRC screening when comparing those who met vs. exceeded alcohol intake recommendations (61.0 % (95 % CI, 58.0–64.0 %) vs. 66.0 % (95 % CI, 46.9–81.0 %)). Likewise, results are not presented for non-Hispanics of other race because of small numbers for many of the analyses. However, no statistically significant differences were observed for these analyses where sample sizes were large enough to support comparisons.
DISCUSSION
Given the increased risk for CRC among people with diabetes, screening according to guidelines is important for CRC prevention. Additionally, adherence to some diabetes self-management recommendations may reduce risk for CRC. We used data from a national study to identify opportunities for improving CRC prevention and screening use among adults with diabetes.
Prevalence of CRC screening and diabetes self-management behaviors
We found that 39 % of adults with diabetes were not up-to-date with CRC screening. This compares to about 41 % of adults in the general population [23, 24]. Moreover, large numbers of adults with diabetes in our sample did not meet recommendations for diabetes self-care that could reduce the risk for CRC. Other national studies similarly reported that only between 25 and 42 % of adults with diabetes met physical activity recommendations [16, 33, 34], that over 16 % of adults with diabetes smoked [35], and that the vast majority were overweight or obese [33].
CRC screening by risk behaviors and risk factors
Several studies have suggested that people who are not physically active or who smoke tend to be screened less frequently for CRC [21, 36–38]. In our study, those with these risk behaviors were no less likely to be up-to-date with CRC screening overall. However, among certain subgroups such as Hispanics and those aged 65–75 years, those who reported adequate physical activity were more likely to be up-to-date with CRC screening. Additionally, among whites, those who smoked were less likely to be up-to-date with CRC screening. Thus, for some segments of the population, there may be common barriers or facilitators for certain diabetes self-care behaviors and for receiving recommended CRC screening, such as self-efficacy, health literacy, and socioeconomic factors [39, 40] .
Our analysis also indicated that overweight or obese adults with diabetes were more likely to be up-to-date with CRC screening than those who were not overweight or obese. In previous literature, mixed findings have been reported on the association between CRC screening and BMI in the general population [36, 41]. Some studies have reported that the relationship between CRC screening and BMI is modified by race or sex [42, 43]. For example, Cohen et al. [44] and Leone et al. [43] found that for blacks, CRC screening was greater among obese vs. nonobese individuals, but among whites CRC screening was not associated or was inversely associated with obesity. Messina et al. [45] found that overweight and obese women were less likely to report a recent CRC screening than were normal weight women; however, there was no association among men. In our sample, the vast majority of respondents had a BMI greater than 25. Though there was no difference in CRC screening by BMI among men, overweight and obese women with diabetes were more likely to be up-to-date with CRC screening than were women with a BMI under 25. While the percentage screened varied by race/ethnicity, we found no differences in the association between BMI and screening by race/ethnicity, although numbers were small.
Number of risk behaviors and risk factors by CRC screening
In a study among individuals aged 50 years and older in the general population using the 2000 NHIS, Coups et al. observed that those who did not meet CRC screening guidelines had more CRC behavioral risk factors on average than those who met screening guidelines [36]. In our study, CRC screening was not significantly associated with the number of selected diabetes risk behaviors and risk factors, but the results similarly suggest a pattern of lower CRC screening among those with more diabetes risk behaviors and risk factors. However, it is important to consider that not all risk behaviors and risk factors may be of equal importance.
Limitations
There are some limitations to this analysis. Persons who did not respond may have different patterns of screening or diabetes risk behaviors and risk factors. Moreover, because the data are self-reported, there may be recall bias or over-reporting of health behaviors [46]. The analyses were largely descriptive and because of the limited sample sizes in some stratified analyses, some comparisons may have been underpowered to detect differences that may exist between subgroups or differences in screening by diabetes risk behaviors and risk factors. The data are also cross-sectional, and therefore the health behaviors and demographic information reported may not reflect those at the time of screening. We were unable to distinguish between diabetes types which may differentially affect behaviors; however, approximately 95 % of Americans with diabetes have type 2 diabetes, and much of the research linking diabetes and cancer has focused on type 2 diabetes [4]. Although nutrition therapy is an important part of diabetes management, and some aspects of diet and nutrition have also been linked to CRC risk [12], dietary behaviors were not examined in this study because the ADA states that the best mix of macronutrients for those with diabetes varies by individual circumstances [10, 47]. Additionally, the USPSTF CRC screening guidelines do not apply to individuals with increased risk for CRC, such as those with inflammatory bowel disease or inherited syndromes such as Lynch syndrome and familial adenomatous polyposis [9]. Inadequate information is available in NHIS to reliably ascertain whether individuals in our sample belonged to such groups. Screening for such groups involves earlier initiation and shorter screening intervals [48]. To the extent that respondents in our sample may have been in one of these higher risk groups, our findings may underestimate recommended screening use.
In our analysis, as in previous research [25], only LTPA was measured, though other forms of physical activity (e.g., transportation, activities of daily living) may satisfy recommendations, and the LTPA questions in the NHIS combine light and moderate activity. Therefore, our estimates may not accurately reflect the proportion meeting recommendations. In addition, physicians or diabetes educators may not necessarily advise patients in accordance with the ADA or general population guidelines used in this analysis [49–51]. Furthermore, there may be contraindications to performing some diabetes care recommendations. For example, gastrointestinal bleeding is a contraindication to aspirin therapy.
Strengths
Strengths of this analysis are that this study is the first to our knowledge to evaluate multiple diabetes risk behaviors and risk factors in relation to CRC screening among adults with diabetes aged 50–75 years. It uses a nationally representative survey to make population estimates and examines several demographic and diabetes-related characteristics to identify populations that may require more outreach to promote guideline-concordant behavior. Given that nearly all adults who were not up-to-date with CRC screening also had one or more diabetes risk behaviors or risk factors (inadequate aerobic activity, inadequate resistance training, excessive alcohol consumption, smoking, overweight/obese), opportunities may exist to not only promote CRC screening in this at-risk population, but also to promote diabetes self-management. This, in turn, may reduce CRC risk. With the large percentage of adults with diabetes who do not meet guidelines for both CRC screening and one or more recommendations for diabetes self-care, many in this population may benefit from such interventions.
Future directions
In this nationally representative sample, the vast majority of older adults with diabetes did not meet multiple behavioral recommendations. The National Institutes of Health, Society of Behavioral Medicine, and others have recognized a need for health interventions addressing multiple behaviors simultaneously [52, 53], and there is growing interest in and demand for multiple behavioral interventions for outcomes such as CRC, including interventions combining screening promotion and behaviors that reduce CRC risk [36, 54, 55]. Recent investigations into multiple health behavior interventions suggest that they may be effective in changing behaviors related to CRC and other health outcomes [12, 53, 54, 56]. Much of the work on the implementation of interventions across not just multiple risk factors but multiple medical conditions appears to have focused on clinical settings [3, 57]. Given the growing population of adults with multiple chronic conditions [58] and the potential for cost savings in interventions addressing multiple behaviors or conditions [59], public health approaches to address multiple health behaviors and multiple chronic conditions are of increasing interest. A trial reported by Campbell et al. may provide an example of potential ways to intervene in this area. In this study, two theory-driven interventions targeting nutrition, physical activity, and CRC screening among rural African Americans were evaluated. Those receiving the intervention using tailored print newsletters and videotapes had increased fruit and vegetable consumption and physical activity levels, and an increase in receipt of FOBT in the past year that was of borderline significance [54]. Such interventions may improve outcomes for both CRC and diabetes, and further assessment of their effectiveness in this population is needed. Evidence-based interventions to promote cancer screening, diabetes care, and other health behaviors are available from the Guide to Community Preventive Services (http://www.thecommunityguide.org) [60].
Our analysis demonstrates that many older adults with diabetes are simultaneously not meeting guidelines for CRC screening and recommendations for diabetes self-management that influence risk factors for CRC. Given the increasing prevalence of diabetes in the USA and evidence indicating an increased risk of CRC among adults with diabetes [3, 5], opportunities to jointly promote CRC screening and prevention and diabetes self-management among this population should be explored. Such efforts should identify and address barriers to guideline-concordant behavior and ensure that they are reaching populations who are less likely to meet guidelines for CRC screening and several recommendations for diabetes self-care. Given recent efforts by DHHS and other organizations [3, 58, 61, 62] to promote programs addressing multiple chronic conditions and collaboration across chronic diseases, expanded attention to the design, effectiveness, and cost-effectiveness of interventions targeting multiple conditions (such as CRC and diabetes) is warranted.
Acknowledgments
This work was done while the primary author was an ASPH/CDC Public Health Fellow supported by Cooperative Agreement Number U36/CCU300430 from the CDC to the Association of Schools of Public Health. The NHIS and the preparation of the manuscript were entirely funded by the US government. The authors would like to acknowledge Zahava Berkowitz of the Division of Cancer Prevention and Control, CDC for statistical assistance and Charlotte Schoenborn of the NCHS, CDC for assistance with physical activity recodes. The authors would also like to acknowledge Henry Kahn and Gloria Beckles of the Division of Diabetes Translation, CDC for reviewing and commenting on an earlier version of this manuscript.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Association of Schools of Public Health.
Implications
Practice: Adults with diabetes in the 50- to 75-year age group who are not meeting recommended behaviors for diabetes self-management should be screened for failure to meet other recommended diabetes self-care behaviors and counseled about colorectal cancer screening.
Policy: Resources should be directed toward encouraging the coordination of programs aimed at the prevention and management of chronic conditions with shared risk factors or co-morbidity.
Research: Future research efforts should aim to identify effective interventions that optimize the use of recommended CRC screening and promote good patient outcomes in adults with diabetes.
References
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2008 Incidence and Mortality Web-based Report. Available from www.cdc.gov/uscs.
- 2.National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 3.Eyre H, Kahn R, Robertson RM, et al. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation. 2004;109:3244–3255. doi: 10.1161/01.CIR.0000133321.00456.00. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 5.Yuhara H, Steinmaus C, Cohen SE, et al. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol. 2011;106:1911–1921. doi: 10.1038/ajg.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Surveillance of demographic characteristics and health behaviors among adult cancer survivors—behavioral risk factor surveillance system, United States, 2009. MMWR. 2012;61:1–23. [PubMed] [Google Scholar]
- 7.Dehal AN, Newton CC, Jacobs EJ, et al. Impact of diabetes mellitus and insulin use on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30:53–59. doi: 10.1200/JCO.2011.38.0303. [DOI] [PubMed] [Google Scholar]
- 8.Richardson LC, Pollack LA. Therapy insight: influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Rev Clin Oncol. 2005;2:48–53. doi: 10.1038/ncponc0062. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Preventive Services Task Force Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association Standards of medical care in diabetes—2009. Diabetes Care. 2009;32(Suppl 1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Albeniz X, Chan AT. Aspirin for the prevention of colorectal cancer. Best Pract Res Clin Gastroenterol. 2011;25:461–472. doi: 10.1016/j.bpg.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshu CE, Parmigiani G, Colditz GA, Platz EA. Opportunities for the primary prevention of colorectal cancer in the United States. Cancer Prev Res (Phila) 2012;5:138–145. doi: 10.1158/1940-6207.CAPR-11-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Colorectal cancer prevention (PDQ®). Available from http://cancer.gov/cancertopics/pdq/prevention/colorectal/HealthProfessional/page2. Accessed 2 March 2012
- 14.Colberg SR, Albright AL, Blissmer BJ, et al. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2010;42:2282–2303. doi: 10.1249/MSS.0b013e3181eeb61c. [DOI] [PubMed] [Google Scholar]
- 15.Heinrich KM, Maddock J. Multiple health behaviors in an ethnically diverse sample of adults with risk factors for cardiovascular disease. Perm J. 2011;15:12–18. doi: 10.7812/tpp/10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30:203–209. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 17.Bell RA, Shelton BJ, Paskett ED. Colorectal cancer screening in North Carolina: associations with diabetes mellitus and demographic and health characteristics. Prev Med. 2001;32:163–167. doi: 10.1006/pmed.2000.0785. [DOI] [PubMed] [Google Scholar]
- 18.Lewis JD, Capra AM, Achacoso NS, et al. Medical therapy for diabetes is associated with increased use of lower endoscopy. Pharmacoepidemiol Drug Saf. 2007;16:1195–1202. doi: 10.1002/pds.1441. [DOI] [PubMed] [Google Scholar]
- 19.Owens MD, Beckles GLA, Ho KK-Y, et al. Women with diagnosed diabetes across the life stages: underuse of recommended preventive care services. J Women’s Health. 2008;17:1415–1423. doi: 10.1089/jwh.2008.1125. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson JE, Culpepper L. Associations between colorectal cancer screening and glycemic control in people with diabetes, Boston, Massachusetts, 2005–2010. Prev Chronic Dis. 2011;8:A82. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao G, Ford ES, Ahluwalia IB, Li C, Mokdad AH. Prevalence and trends of receipt of cancer screenings among US women with diagnosed diabetes. J Gen Intern Med. 2008;24:270–275. doi: 10.1007/s11606-008-0858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics. 2010 National Health Interview Survey (NHIS) Public Use Data Release: NHIS Survey Description. Available from ftp://ftp.cdc.gov/pub/health_statistics/nchs/dataset_documentation/nhis/2010/srvydesc.pdf. Accessed 6 December 2011
- 23.Shapiro JA, Klabunde C, Thompson TD, et al. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21:895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention Cancer screening—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:41–45. [PubMed] [Google Scholar]
- 25.Schoenborn CA, Stommel M. Adherence to the 2008 adult physical activity guidelines and mortality risk. Am J Prev Med. 2011;40:514–521. doi: 10.1016/j.amepre.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Atlanta, GA, 2008.
- 27.Boyle T, Bull F, Fritschi L, Heyworth J. Resistance training and the risk of colon and rectal cancers. Cancer Causes Control. 2012;23:1091–1097. doi: 10.1007/s10552-012-9978-x. [DOI] [PubMed] [Google Scholar]
- 28.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. Washington, DC, 2008.
- 29.World Cancer Research Fund, American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC, 2007.
- 30.National Center for Health Statistics. NHIS—Adult Physical Activity Information: Leisure-time Physical Activity Recodes. Available from http://www.cdc.gov/nchs/nhis/physical_activity/pa_recodes.htm. Accessed 17 November 2011
- 31.Health, United States, 2010: With Special Feature on Death and Dying. Hyattsville, MD: US Department of Health and Human Services, CDC, National Center for Health Statistics; 2010. [PubMed] [Google Scholar]
- 32.SUDAAN Language Manual, Release 10.0. Research Triangle Park: Research Triangle Institute; 2008. [Google Scholar]
- 33.Nwasuruba C, Khan M, Egede LE. Racial/ethnic differences in multiple self-care behaviors in adults with diabetes. J Gen Intern Med. 2007;22:115–120. doi: 10.1007/s11606-007-0120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao G, Ford ES, Li C, Balluz LS. Physical activity in U.S. older adults with diabetes mellitus: prevalence and correlates of meeting physical activity recommendations. J Am Geriatr Soc. 2011;59:132–137. doi: 10.1111/j.1532-5415.2010.03236.x. [DOI] [PubMed] [Google Scholar]
- 35.Carter-Pokras OD, Johnson TM, Bethune LA, et al. Lost opportunities for smoking cessation among adults with diabetes in Florida (2007) and Maryland (2006) Prev Chronic Dis. 2011;8:A51. [PMC free article] [PubMed] [Google Scholar]
- 36.Coups EJ, Manne SL, Meropol NJ, Weinberg DS. Multiple behavioral risk factors for colorectal cancer and colorectal cancer screening status. Cancer Epidemiol Biomarkers Prev. 2007;16:510–516. doi: 10.1158/1055-9965.EPI-06-0143. [DOI] [PubMed] [Google Scholar]
- 37.Meissner HI, Yabroff KR, Dodd KW, et al. Are patterns of health behavior associated with cancer screening? Am J Health Promot. 2009;23:168–175. doi: 10.4278/ajhp.07082085. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro JA, Seeff LC, Nadel MR. Colorectal cancer-screening tests and associated health behaviors. Am J Prev Med. 2001;21:132–137. doi: 10.1016/S0749-3797(01)00329-4. [DOI] [PubMed] [Google Scholar]
- 39.Bayliss EA, Ellis JL, Steiner JF. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Ann Fam Med. 2007;5:395–402. doi: 10.1370/afm.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control. 2008;19:339–359. doi: 10.1007/s10552-007-9100-y. [DOI] [PubMed] [Google Scholar]
- 41.Cohen SS, Palmieri RT, Nyante SJ, et al. Obesity and screening for breast, cervical, and colorectal cancer in women: a review. Cancer. 2008;112:1892–1904. doi: 10.1002/cncr.23408. [DOI] [PubMed] [Google Scholar]
- 42.Fagan HB, Wender R, Myers RE, Petrelli N. Obesity and cancer screening according to race and gender. J Obes. 2011;2011:218250. doi: 10.1155/2011/218250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leone LA, Campbell MK, Satia JA, Bowling JM, Pignone MP. Race moderates the relationship between obesity and colorectal cancer screening in women. Cancer Causes Control. 2010;21:373–385. doi: 10.1007/s10552-009-9469-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen SS, Murff HJ, Signorello LB, Blot WJ. Obesity and colorectal cancer screening among black and white adults. Cancer Causes Control. 2012;23:709–716. doi: 10.1007/s10552-012-9940-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Messina CR, Lane DS, Anderson JC. Body mass index and screening for colorectal cancer: gender and attitudinal factors. Cancer Epidemiol. 2012;36:400–408. doi: 10.1016/j.canep.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vernon SW, Tiro JA, Vojvodic RW, et al. Reliability and validity of a questionnaire to measure colorectal cancer screening behaviors: does mode of survey administration matter? Cancer Epidemiol Biomarkers Prev. 2008;17:758–767. doi: 10.1158/1055-9965.EPI-07-2855. [DOI] [PubMed] [Google Scholar]
- 47.Bantle JP, Wylie-Rosett J, Albright AL, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl 1):S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 48.American Cancer Society (2011). Colorectal cancer. Available from http://www.cancer.org/acs/groups/cid/documents/webcontent/003096-pdf.pdf. Accessed 2 December 2011
- 49.Barnes PM, Schoenborn CA. Trends in adults receiving a recommendation for exercise or other physical activity from a physician or other health professional. NCHS Data Brief, no. 86. Hyattsville: National Center for Health Statistics; 2012. [PubMed] [Google Scholar]
- 50.McNabb WL. Adherence in diabetes: can we define it and can we measure it? Diabetes Care. 1997;20:215–218. doi: 10.2337/diacare.20.10.1518. [DOI] [PubMed] [Google Scholar]
- 51.Wilson W, Ary DV, Biglan A, et al. Psychosocial predictors of self-care behaviors (compliance) and glycemic control in non-insulin-dependent diabetes mellitus. Diabetes Care. 1986;9:614–622. doi: 10.2337/diacare.9.6.614. [DOI] [PubMed] [Google Scholar]
- 52.National Institutes of Health. Meeting Summary. Bethesda: NIH Science of Behavior Change. 2009.
- 53.Prochaska JJ, Spring B, Nigg CR. Multiple health behavior change research: an introduction and overview. Prev Med. 2008;46:181–188. doi: 10.1016/j.ypmed.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell MK, James A, Hudson MA, et al. Improving multiple behaviors for colorectal cancer prevention among african american church members. Health Psychol. 2004;23:492–502. doi: 10.1037/0278-6133.23.5.492. [DOI] [PubMed] [Google Scholar]
- 55.McNeill LH, Coeling M, Puleo E, et al. Colorectal cancer prevention for low-income, sociodemographically-diverse adults in public housing: baseline findings of a randomized controlled trial. BMC Public Health. 2009;9:353. doi: 10.1186/1471-2458-9-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Emmons KM, Stoddard AM, Fletcher R, et al. Cancer prevention among working class, multiethnic adults: results of the healthy directions-health centers study. Am J Public Health. 2005;95:1200–1205. doi: 10.2105/AJPH.2004.038695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen D, DiCicco-Bloom B, Strickland PO, et al. Opportunistic approaches for delivering preventive care in illness visits. Prev Med. 2004;38:565–573. doi: 10.1016/j.ypmed.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 58.U.S. Department of Health and Human Services. Multiple chronic conditions—a strategic framework: optimum health and quality of life for individuals with multiple chronic conditions. Washington, DC, 2010.
- 59.Prochaska JJ, Nigg CR, Spring B, Velicer WF, Prochaska JO. The benefits and challenges of multiple health behavior change in research and in practice. Prev Med. 2010;50:26–29. doi: 10.1016/j.ypmed.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sabatino SA, Lawrence B, Elder R, et al. Community Preventive Services Task Force. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for The Guide to Community Preventive Services. Am J Prev Med. 2012;43(1):765–786. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention. Coordinated Chronic Disease Program Launched. Available from http://www.cdc.gov/Features/ChronicDiseaseProgram/. Accessed 28 March 2012
- 62.U.S. Department of Health and Human Services. Behavioral Interventions to Address Multiple Chronic Health Conditions in Primary Care (R01). Available from http://grants.nih.gov/grants/guide/pa-files/PA-12-024.html. Accessed 28 March 2012