Abstract
We postulated that quantitative monitoring of Epstein–Barr virus (EBV) shedding after transplantation could distinguish EBV-associated illnesses and predict clinical outcome. EBV DNA was measured in solid organ (SOT) and hematopoietic cell transplants (HCT) using our own real-time TaqMan EBV PCR. The proportion of patients who had EBV DNAemia post-transplant was significantly lower in HCT vs. SOT (p < 0.001). Over a 7.5-yr period, post-transplant lymphoproliferative disorder (PTLD) occurred in 66 (5.8%) of 1131 patients who met adequate monitoring criteria. SOT recipients developed PTLD significantly later than HCT recipients (median, 2.8 yr vs. 121 d; p < 0.001). PTLD was documented in 53 (14%) of 376 patients who had EBV in ≥1 whole blood sample vs. 13 (2%) of 755 patients who had at least three EBV-negative blood samples and were never positive. PTLD risk in viremic patients increased with the peak quantity of EBV DNAemia (p < 0.001). PTLD occurred in 37/333 (11%) of patients with peak blood levels 103–105 copies/mL vs. 16/43 (37%) of patients with levels >105 (p < 0.001). EBV PCR was predictive in 29 (78%) of 37 patients tested within three wk prior to tissue diagnosis of PTLD, and thus, we conclude that EBV PCR with careful attention paid to changes in EBV DNAemia could lead to earlier diagnosis and treatment of PTLD.
Keywords: Epstein–Barr virus DNAemia, Epstein–Barr virus, hematopoietic cell transplantation, post-transplant lymphoproliferative disorder, primary Epstein–Barr virus infection, quantitative Epstein–Barr virus PCR, solid organ transplantation
Monitoring of Epstein–Barr virus (EBV) DNA in peripheral blood is routinely performed in transplant centers because these patients are at risk for serious EBV-associated diseases including the potentially life-threatening post-transplant lymphoproliferative disorder (PTLD). The clinical utility of quantitative EBV monitoring in the transplant setting has been the subject of numerous articles, which typically have focused on a particular type of transplant, such as hematopoietic cells (1-6), kidney (7-10), heart (11), or liver (12-15). Several reports have included different types of solid organ transplants, but the number of patients studied was relatively small (16, 17). We report our experience over a seven-yr period, which is unique both in terms of the large number of subjects and the inclusion of multiple types of solid organ (SOT) and hematopoietic cell transplant (HCT) patients. We postulated that quantitative monitoring of EBV shedding after transplantation could distinguish EBV-associated illnesses and predict clinical outcome.
Materials and methods
A quantitative EBV PCR assay was developed in-house, as previously described (18). The target is a 71-bp portion of the EBNA-1 gene. The limit of detection of this assay is four copies per reaction, which corresponds to 103 copies/mL of sample. We reviewed quantitative EBV tests performed between 1 October 2003 and 1 April 2011 in the Clinical Virology Laboratory at the University of Minnesota Medical Center – Fairview (Minneapolis, MN, USA) on 1131 patients (700 SOT patients and 431 HCT patients). Because >95% of the body fluids tested were whole blood, only data from blood samples were statistically analyzed. DNA-emia was defined as having at least 1000 copies EBV/mL whole blood. Patients considered not to have DNAemia were never positive for EBV and had at least three or more negative blood samples post-transplant. Patients with fewer than three negative tests were excluded from the analysis because their virology data were considered to be too sparse. Pediatric patients were defined as those <18 yr old at the time of transplantation. PTLD was defined using the criteria from the 2008 WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues (19). Patients who were treated with rituximab because of clinical suspicion of PTLD in the absence of a tissue diagnosis were excluded from the PTLD group, because their diagnoses were not pathologically confirmed. This study was approved by the University of Minnesota Institutional Review Board, study number 0707E13223.
Statistical analysis
The number of subjects with and without EBV DNAemia was compared in the combined SOT group vs. those who received HCT using a Fisher’s exact test. The time to develop PTLD was compared between the SOT group and the HCT group using a Mann–Whitney test. Differences in the proportion of patients developing PTLD among the various types of organs in the SOT group were of interest. However, as numbers were small, the proportions developing PTLD in each transplant type were visually examined in an exploratory fashion. The proportion of PTLD in any organ transplant type that appeared to be substantively different from the rest was compared with all other solid organ transplant types using a Fisher’s exact test. Only large differences were tested statistically to preserve an honest type 1 error rate. The association between PTLD and the quantity of EBV in blood was evaluated by grouping the quantities of EBV into log10 categories of 3–4, 4–5, 5–6, and >6 copies/mL and using a chi-squared test for trend. All levels of significance were taken as 0.05, 2-sided. Statistical analyses were performed using GraphPad InStat version 3.06 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Proportion of patients with EBV DNAemia post-transplant by transplant type
EBV DNAemia after transplantation occurred in 376 (33%) of 1131 patients tested between 1 October 2003 and 1 April 2011 (Table 1). A total of 6762 quantitative EBV tests were performed on these 376 patients (mean, 18 tests/patient; median, 12; range, 1–107), and 2796 tests (41%) were positive. Among the 755 transplant patients who were never documented to have EBV viremia, 5254 tests were performed (mean, 7 tests/patient; median, 5; range, 3–58). Kidney was the most frequently transplanted solid organ alone or in combination with another organ (414/700 = 59%), followed by liver (135/700 = 19%). A significantly higher proportion of SOT recipients had DNAemia post-transplant than HCT patients (p < 0.001, Fisher exact test, 2-sided). A significantly higher proportion of pediatric kidney transplant patients were viremic than adult kidney transplant patients (p < 0.001, Fisher’s exact test, 2-sided).
Table 1.
Proportion of adults and children with Epstein–Barr virus (EBV) DNAemia by type of transplant
| Transplant type | No. of patients | No. EBV positive (%) | No. of adults | No. EBV positive (%) | No. of children | No. EBV positive (%) |
|---|---|---|---|---|---|---|
| Solid organ | 700 | 272 (39)a | 426 | 154 (36) | 274 | 118 (43) |
| Kidney | 331 | 127 (38) | 185 | 56 (30)a | 146 | 71 (49)a |
| Liver | 120 | 46 (38) | 40 | 19 (48) | 80 | 27 (34) |
| Heart | 64 | 26 (41) | 20 | 8 (40) | 44 | 18 (41) |
| Lung | 58 | 30 (52) | 58 | 30 (52) | 0 | 0 |
| Pancreas | 33 | 9 (27) | 33 | 9 (27) | 0 | 0 |
| Kidney/pancreas | 72 | 25 (35) | 72 | 25 (35) | 0 | 0 |
| Other combinations | 22 | 9 (41) | 18 | 7 (39) | 4 | 2 (50) |
| Hematopoietic cells | 431 | 104 (24)a | 242 | 58 (24) | 189 | 46 (24) |
| All patients | 1131 | 376 (33) | 680 | 212 (31) | 451 | 164 (36) |
Significantly different (p < 0.001, Fisher’s exact test, 2-sided).
Quantity of EBV DNAemia by transplant type in the study population
Positive EBV tests ranged in copy number from 103.0 to 107.3 copies/mL of whole blood. The distribution of patients by type of transplant and viral loads is illustrated in Fig. 1. Most (89%) viremic transplant patients had peak viral loads between 103.0 and 105.0 copies/mL. The median, mean, and range of viral loads were similar for all transplant types (data not shown).
Fig. 1.
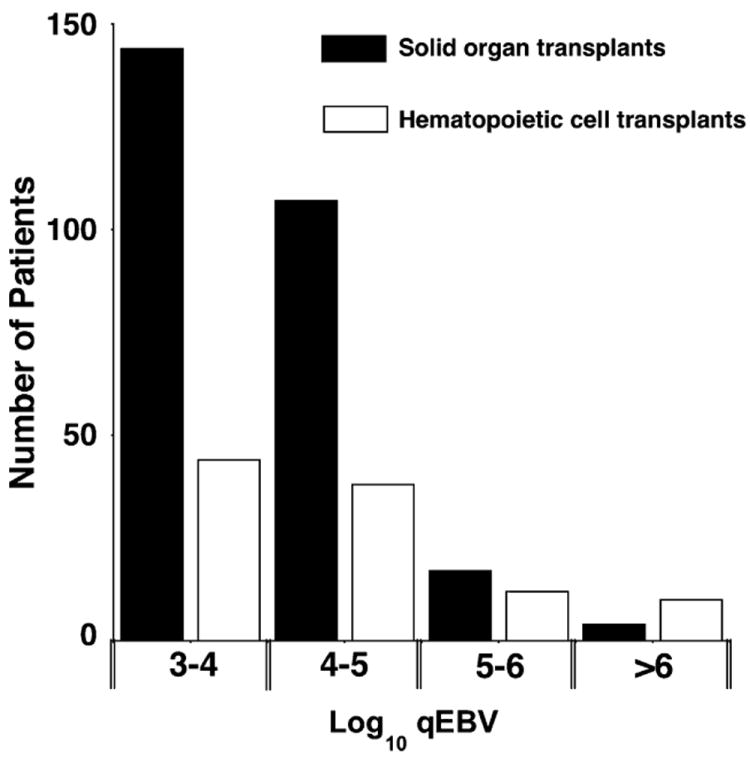
Distribution of transplant patients by peak log10 whole blood Epstein–Barr virus (EBV) viral load.
Correlation of EBV DNAemia with PTLD in transplant patients
PTLD occurred in 66 (5.8%) of the 1131 patients included in our study. It occurred more often in SOT patients as compared with HCT patients, but this difference was not statistically significant. It was diagnosed in 53 (14%) of 376 transplant patients with EBV DNAemia, and in 13 (2%) of 755 patients with three or more negative tests for DNAemia. Of the 53 viremic patients, 35 were SOT patients and 18 were HCT patients. The maximum viral loads were somewhat higher in HCT patients (median, 105.1 copies/mL; range 103.9–107.3) as compared with SOT patients (median, 104.4 copies/mL; range, 103.1–107.2).
As depicted in Fig. 2, the occurrence of PTLD was significantly related to the quantity of EBV (p < 0.001, chi-square test for trend). Thirty-seven (11%) of 333 patients with peak EBV levels 103.0–105.0 copies/mL whole blood developed PTLD as compared with 16 (37%) of 43 patients whose peak levels were >105.0 copies/mL (p < 0.001, Fisher exact test, 2-sided).
Fig. 2.
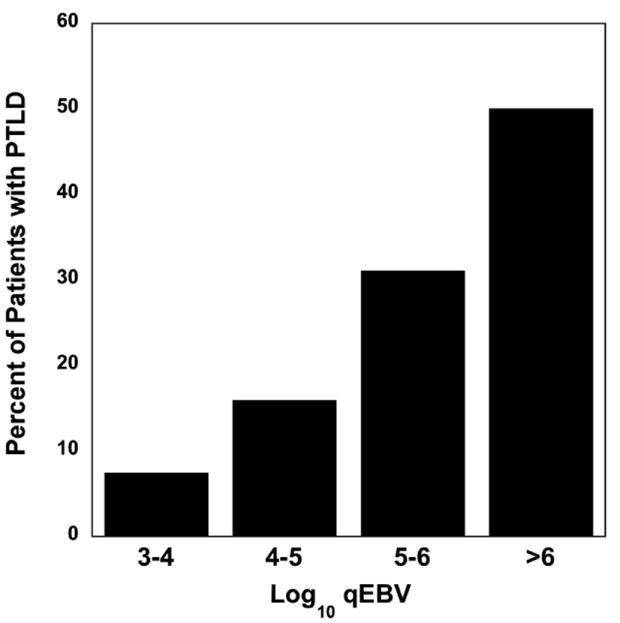
Proportion of patients with post-transplant lymphoproliferative disorder (PTLD) by peak log10 copies Epstein–Barr virus (EBV)/mL whole blood. The occurrence of PTLD was significantly related to the quantity of EBV (p < 0.001, chi-squared test for trend).
Thirty-five (80%) of 44 patients who had a test performed within a six-wk window of their PTLD diagnosis were viremic (Table 2). Of the nine patients who did not have EBV DNAemia, seven had monomorphic PTLD, EBER-positive, while two had monomorphic PTLD, EBER-negative. The quantitative EBV PCR was predictive (positive) in 29 (78%) of 37 patients tested within three wk prior to PTLD diagnosis. EBV DNA was detected in the blood of three patients at the time of PTLD diagnosis (days −2, 0, and 0) even though their biopsy tissues were negative for EBV early RNA expression by in situ hybridization (EBER ISH). However, the level of EBV DNA-emia was low in these patients (103.5, 103.6, 103.7).
Table 2.
Epstein–Barr virus (EBV) PCR results near time of post-transplant lymphoproliferative disorder (PTLD) diagnosis
| Characteristic | No. of patients | Comments |
|---|---|---|
| PTLD | 66 | EBER positive tissue diagnosis: 51/59 |
| Samples collected ± 3 wk of PTLD tissue diagnosis | 44 | Reason for qEBV not being tested within ± 3 wk of PTLD diagnosis: test not available yet, 13; test available but not requested, 9 |
| DNAemia ± 3 wk of tissue diagnosis | 35 | Assay positive in 35 (80%) of 44 patients with samples tested within a 6-wk window of PTLD |
| DNAemia before PTLD | 29 | Assay predictive in 29 (78%) of 37 patients with samples tested 3 wk before PTLD |
Of 30 patients who were viremic within a six-wk window of their PTLD diagnosis and who had >1 EBV test performed, 19 (63%) had their peak EBV levels within a six-wk window of their PTLD diagnosis. Of 28 patients who were viremic within a six-wk window of their PTLD diagnosis and who had follow-up testing, 16 cleared their blood of EBV DNA with treatment, seven cleared their blood of EBV DNA with treatment but had subsequent recurrence of detectable EBV DNA viremia, and five never cleared their blood of EBV DNA. The five patients who never cleared their blood of EBV DNA all died within one month of PTLD diagnosis. These were all adult patients (3 HCT, 2 SOT). For these five patients, the median peak viral load was 105.9 copies/mL (mean, 106.2; range, 104.5–106.7) with median duration of viremia of 24 d (mean, 150 d; range, 14 d–1.8 yr). These patients were managed in a variety of ways, including rituximab alone (two patients), rituximab plus conventional chemotherapy (one patient), reduced immunosuppression (one patient), and ganciclovir and rituximab followed by discontinuation of immunosuppression (one patient).
The proportion of patients who developed PTLD differed according to type of transplant and whether they were children or adults at the time of transplant (Table 3), but none of these differences were statistically significant. The highest proportion of PTLD was among lung transplants (12.1%) and the lowest among pancreas transplant recipients (3.0%). The proportion of pediatric renal transplant patients who developed PTLD was higher than that of their adult counterparts (6.2% vs. 2.2%), but this difference was not statistically significant.
Table 3.
Proportion of post-transplant lymphoproliferative disorder (PTLD) by type of transplant
| Transplant type | No. of subjects | Adult PTLD (%) | Pediatric PTLD |
|---|---|---|---|
| Kidney | 331 | 4/185 (2.2) | 9/146 (6.2) |
| Liver | 120 | 4/40 (10) | 6/80 (7.5) |
| Heart | 64 | 2/20 (10) | 4/44 (9.1) |
| Lung | 58 | 7 (12.1) | 0 |
| Pancreas | 33 | 1 (3.0) | 0 |
| Multi-organ | 94 | 9/90 (10.0) | 1/4 (25) |
| All solid organs | 700 | 26/426 (6.1) | 20/274 (7.3) |
| Hematopoietic cells | 431 | 7/242 (2.9) | 13/189 (6.9) |
| All patients | 1131 | 33/680 (4.9) | 33/451 (7.3) |
Pretransplant EBV antibody status and PTLD
Pretransplant antibody status was available on 57 PTLD patients. Of these, four were patients less than one yr of age who were EBV-antibody positive. As their antibody likely represented maternal antibody, they were excluded from the analysis. Of the remaining 53 patients, 25 (47%) were EBV-naïve and 28 (53%) were EBV-antibody positive. If the probability of PTLD were the same in the EBV-naïve group and the antibody-positive group, and as 85% of our transplant recipients are EBV antibody positive pretransplant, we would have expected eight cases of PTLD because of primary EBV infection and 45 because of reactivation or superinfection. The observed number with PTLD associated with primary EBV infection was significantly higher (p < 0.001, Fisher exact test, 2-sided), suggesting that primary EBV infection is a risk factor for PTLD. Five (20%) of 25 patients with PTLD because of primary EBV infection died as compared with 10 (36%) of 28 patients whose PTLD resulted from reactivation or superinfection with EBV. This difference was not statistically significant.
Time from transplant to diagnosis of PTLD
SOT patients developed PTLD significantly later than HCT recipients (p < 0.001, Mann–Whitney test). SOT patients were diagnosed with PTLD a median of 2.8 yr post-transplant (mean, 5.1 yr; range, 82 d–25.5 yr) as compared with a median of 121 d (mean, 135 d; range, 38–296 d) for HCT recipients. Within the SOT PTLD group, pediatric patients were diagnosed with PTLD a median of 2.7 yr post-transplant (mean, 6.3 yr; range, 175 d–25.5 yr) as compared with a median of 3.2 yr (mean, 4.2 yr; range, 82 d–13.4 yr) for adult patients. There was no statistical difference in time from transplant to diagnosis of PTLD between SOT pediatric and adult patients. Within the HCT PTLD group, pediatric patients were diagnosed with PTLD a median of 108 d (mean, 131 d, range, 46–296 d) as compared with a median of 126 d (mean, 143 d, range, 38–247 d) for adult patients. There was no statistical difference in time from transplant to diagnosis of PTLD between HCT pediatric and adult patients.
Within the SOT PTLD group, there were 34 patients greater than one yr of age whose EBV antibody status was known at the time of transplant. EBV-naïve SOT patients were diagnosed with PTLD a median of 2.8 yr post-transplant (mean, 5.1 yr; range, 114 d–25.5 yr) as compared with a median of 3.5 yr (mean, 4.7 yr; range, 175 d–11.8 yr) for EBV-antibody-positive SOT recipients. Within the HCT PTLD group, there were 18 patients greater than one yr of age whose EBV antibody status was known at the time of transplant. The four EBV-naïve HCT patients were diagnosed with PTLD a median of 77 d post-transplant (mean, 115 d; range, 38–269 d) as compared with a median of 130 d (mean, 150 d; range, 56–296 d) for the 14 EBV-antibody-positive HCT recipients. There was no statistical difference in time from transplant to diagnosis of PTLD between EBV-naïve and EBV-antibody-positive SOT or HCT recipients.
Discussion
The frequency of EBV DNAemia post-transplant varied with transplant type and was significantly higher in SOT recipients as compared with HCT recipients (Table 1). Also, pediatric renal transplant patients had a significantly higher rate of EBV DNAemia post-transplant than their adult counterparts. Among SOT patients, the highest rate of EBV viremia post-transplant was in lung allografts (30/58; 52%) and the lowest was in pancreas transplants (9/33; 27%).
PTLD developed in 66 (5.8%) of 1131 transplant patients in this study. Half of them (33 patients) were children between six months and 17.9 yr of age at the time of transplant. Significantly more patients who were naïve to EBV at transplant developed PTLD than expected confirming previous data that primary EBV infection is a risk factor for PTLD (20).
The incidence of PTLD in all patients studied increased significantly with increasing viral load (Fig. 2; p < 0.0001, chi-square test for trend). The correlation between high-level EBV DNAemia and increased risk of PTLD has also previously been demonstrated in other studies (1, 21, 22). Although we cannot provide an exact quantitative threshold for tissue-invasive EBV disease, following the levels of EBV DNAemia provides guidance with regard to risk of PTLD.
The incidence of PTLD was higher in lung allograft recipients as compared with other solid organ transplant recipients. Because the frequency of quantitative EBV testing and other diagnostic procedures was not controlled in our study, a bias could have been introduced if some transplant teams pursued quantitative EBV testing more frequently than others. However, our lung transplant patients, who had the highest proportion of PTLD, were tested for quantitative EBV less frequently (mean, 5.4 samples; median, 4.0) than the other solid organ transplant recipients (mean, 12.2 samples; median, 5.0).
We have shown that quantitative EBV testing is a sensitive method for detecting PTLD, although not specific. Quantitative EBV tests were performed within three wk of a PTLD diagnosis in 44 patients, and 35 (80%) of these were positive. In addition, of 37 patients who had quantitative EBV tests within the three wk prior to a PTLD diagnosis, 29 (78%) were positive. Our results are in agreement with a study of pediatric heart transplant recipients, which showed that chronic high EBV viremia (defined as >16 000 genome copies/mL whole blood on ≥50% of samples over at least six months) predicted de novo or recurrent PTLD (11).
Interlaboratory variability is an important consideration when using quantitative viral assays to monitor patients who change location and/or health care providers. The amount of EBV in a particular sample varies substantially among laboratories (23, 24). Thus, if the laboratory used to monitor a patient is changed, the quantitative values need to be calibrated ideally by testing several samples in both laboratories to establish an appropriate conversion factor.
Another consideration is which is the best clinical sample to use for monitoring EBV infection and disease post-transplant? Matrices tested include unfractionated whole blood, peripheral blood mononuclear cells, peripheral blood leukocytes, and plasma (25). Whole blood is our matrix of choice rather than plasma because, as stated by Stevens et al., “cell-associated loads can persist at high levels without accompanying EBV DNA levels in plasma and EBV loads in peripheral blood mononuclear cells or whole blood are always higher than those found in plasma” (25). At this point, the ideal matrix for quantitative EBV monitoring is still an open question.
Quantitative EBV PCR is a sensitive method for identifying patients at risk for PTLD as well as for monitoring response to therapy. Although the positive predictive value of the quantitative EBV PCR for PTLD is not very high (approximately [53*18]/2796 = 34% in this study), this does not negate the value of this test. We have shown in a large study that PTLD risk in viremic patients increases with the peak quantity of EBV DNA-emia. This test is relatively inexpensive ($140 cost to the patient in our hospital), and PTLD is a serious complication that can benefit from early intervention. This study was not designed to answer the question of “what is the optimal way to use the EBV level post-transplant” or “how often should the EBV level be monitored post-transplant.” Rather, our study adds support to what is already in the literature for the routine monitoring of transplant patients for primary infection or reactivation of EBV in the blood (26). If EBV is present, the level of viremia should be used in combination with clinical findings to determine the appropriate course of action. Transplant patients at highest risk of PTLD (for example, lung transplant patients) may benefit from more frequent testing. The ideal duration of testing may also vary between transplant types. In our study, SOT recipients developed PTLD significantly later than HCT recipients (median, 2.8 yr vs. 121 d). Hopefully, our data together with the established literature will lead to a prospective clinical trial evaluating different approaches to quantitative monitoring of EBV post-transplant.
EBV DNA monitoring coupled with tapering the immunosuppression when EBV viral loads exceeded a certain threshold resulted in a decreased incidence of PTLD in one study of pediatric liver transplant recipients (14). Although the best type of clinical intervention for EBV DNAemia is still not clear (rituximab, EBV-specific T-cells, decreased immunosuppression, and/or antiviral agents), utilization of EBV PCR post-transplant with careful attention paid to changes in the quantitative level of EBV will likely lead to earlier diagnosis and treatment of potentially life-threatening PTLD.
Acknowledgments
Supported in part by grants from the NIH (2PO1 DK 13083), the University of Minnesota International Center for Antiviral Research and Epidemiology, and the Minnesota Medical Foundation.
Footnotes
Conflict of interest: None.
References
- 1.Aalto SM, Juvonen E, Tarkkanen J, et al. Epstein-Barr viral load and disease prediction in a large cohort of allogeneic stem cell transplant recipients. Clin Infect Dis. 2007;45:1305. doi: 10.1086/522531. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad I, Cau NV, Kwan J, et al. Preemptive management of Epstein–Barr virus reactivation after hematopoietic stem-cell transplantation. Transplantation. 2009;87:1240. doi: 10.1097/TP.0b013e31819f1c49. [DOI] [PubMed] [Google Scholar]
- 3.Greenfield HM, Gharib MI, Turner AJ, et al. The impact of monitoring Epstein–Barr virus PCR in paediatric bone marrow transplant patients: can it successfully predict outcome and guide intervention? Pediatr Blood Cancer. 2006;47:200. doi: 10.1002/pbc.20604. [DOI] [PubMed] [Google Scholar]
- 4.Kinch A, Oberg G, Arvidson J, Falk KI, Linde A, Pauksens K. Post-transplant lymphoproliferative disease and other Epstein–Barr virus diseases in allogeneic haematopoietic stem cell transplantation after introduction of monitoring of viral load by polymerase chain reaction. Scand J Infect Dis. 2007;39:235. doi: 10.1080/00365540600978906. [DOI] [PubMed] [Google Scholar]
- 5.Meerbach A, Wutzler P, Hafer R, Zintl F, Gruhn B. Monitoring of Epstein–Barr virus load after hematopoietic stem cell transplantation for early intervention in post-transplant lymphoproliferative disease. J Med Virol. 2008;80:441. doi: 10.1002/jmv.21096. [DOI] [PubMed] [Google Scholar]
- 6.Weinstock DM, Ambrossi GG, Brennan C, Kiehn TE, Jakubowski A. Preemptive diagnosis and treatment of Epstein–Barr virus-associated post transplant lymphoproliferative disorder after hematopoietic stem cell transplant: an approach in development. Bone Marrow Transplant. 2006;37:539. doi: 10.1038/sj.bmt.1705289. [DOI] [PubMed] [Google Scholar]
- 7.Sato T, Fujieda M, Tanaka E, et al. Monitoring of Epstein–Barr virus load and antibody in pediatric renal transplant patients. Pediatr Int. 2008;50:454. doi: 10.1111/j.1442-200X.2008.02579.x. [DOI] [PubMed] [Google Scholar]
- 8.Holmes MV, Caplin B, Atkinson C, et al. Prospective monitoring of Epstein–Barr virus DNA in adult renal transplant recipients during the early posttransplant period: role of mycophenolate mofetil. Transplantation. 2009;87:852. doi: 10.1097/TP.0b013e318199f983. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T, Ikezumi Y, Okubo S, et al. Epstein–Barr virus DNA load and seroconversion in pediatric renal transplantation with tacrolimus immunosuppression. Pediatr Transplant. 2007;11:749. doi: 10.1111/j.1399-3046.2007.00738.x. [DOI] [PubMed] [Google Scholar]
- 10.Toyoda M, Moudgil A, Warady BA, Kiehn TE, Jakubowski A. Clinical significance of peripheral blood Epstein-Barr viral load monitoring using polymerase chain reaction in renal transplant recipients. Pediatr Transplant. 2008;12:778. doi: 10.1111/j.1399-3046.2008.00904.x. [DOI] [PubMed] [Google Scholar]
- 11.Bingler MA, Feingold B, Miller SA, et al. Chronic high Epstein-Barr viral load state and risk for late-onset post-transplant lymphoproliferative disease/lymphoma in children. Am J Transplant. 2008;8:442. doi: 10.1111/j.1600-6143.2007.02080.x. [DOI] [PubMed] [Google Scholar]
- 12.Jang JY, Kim KM, Lee YJ, Lee SG, Chi HS. Quantitative Epstein–Barr virus viral load monitoring in pediatric liver transplantation. Transplant Proc. 2008;40:2546. doi: 10.1016/j.transproceed.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Kullberg-Lindh C, Ascher H, Saalman R, Olausson M, Lindh M. Epstein-Barr viremia levels after pediatric liver transplantation as measured by real-time polymerase chain reaction. Pediatr Transplant. 2006;10:83. doi: 10.1111/j.1399-3046.2005.00404.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee TC, Savoldo B, Rooney CM, et al. Quantitative EBV viral loads and immunosuppression alterations can decrease PTLD incidence in pediatric liver transplant recipients. Am J Transplant. 2005;5:2222. doi: 10.1111/j.1600-6143.2005.01002.x. [DOI] [PubMed] [Google Scholar]
- 15.Loginov R, Aalto S, Piiparinen H, et al. Monitoring of EBV-DNAemia by quantitative real-time PCR after adult liver transplantation. J Clin Virol. 2006;37:104. doi: 10.1016/j.jcv.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Baldanti F, Grossi P, Furione M, et al. High levels of Epstein–Barr virus DNA in blood of solid-organ transplant recipients and their value in predicting posttransplant lymphoproliferative disorders. J Clin Microbiol. 2000;38:613. doi: 10.1128/jcm.38.2.613-619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung E, Shenton BK, GREEN K, et al. Dynamic EBV gene loads in renal, hepatic, and cardiothoracic transplant recipients as determined by real-time PCR light cycler. Transpl Infect Dis. 2004;6:156. doi: 10.1111/j.1399-3062.2004.00073.x. [DOI] [PubMed] [Google Scholar]
- 18.Balfour HH, Jr, Holman CJ, Hokanson KM, et al. A prospective clinical study of Epstein–Barr virus and host interactions during acute infectious mononucleosis. J Infect Dis. 2005;192:1505. doi: 10.1086/491740. [DOI] [PubMed] [Google Scholar]
- 19.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. International Agency for Research on Cancer (IARC); Lyon, France: 2008. [Google Scholar]
- 20.Ho M, Miller G, Atchison RW, et al. Epstein–Barr virus infections and DNA hybridization studies in post-transplantation lymphoma and lymphoproliferative lesions: the role of primary infection. J Infect Dis. 1985;152:876. doi: 10.1093/infdis/152.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens SJ, Verschuuren EA, Pronk I, et al. Frequent monitoring of Epstein–Barr virus DNA load in unfractionated whole blood is essential for early detection of posttransplant lymphoproliferative disease in high-risk patients. Blood. 2001;97:1165. doi: 10.1182/blood.v97.5.1165. [DOI] [PubMed] [Google Scholar]
- 22.Van Esser JW, Van der Holt B, Meijer E, et al. Epstein–Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell–depleted SCT. Blood. 2001;98:972. doi: 10.1182/blood.v98.4.972. [DOI] [PubMed] [Google Scholar]
- 23.Hayden RT, Hokanson KM, Pounds SB, et al. Multi-center comparison of different real-time PCR assays for quantitative detection of Epstein–Barr virus. J Clin Microbiol. 2008;46:157. doi: 10.1128/JCM.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preiksaitis JK, Pang XL, Fox JD, Fenton JM, Caliendo AM, Miller GG. Interlaboratory comparison of Epstein–Barr virus viral load assays. Am J Transplant. 2009;9:269. doi: 10.1111/j.1600-6143.2008.02514.x. [DOI] [PubMed] [Google Scholar]
- 25.Stevens SJ, Verschuuren EA, Verkuujlen SA, Van den Brule AJ, Meijer CJ, Middeldorp JM. Role of Epstein–Barr virus DNA load monitoring in prevention and early detection of post-transplant lymphoproliferative disease. Leuk Lymphoma. 2002;43:831. doi: 10.1080/10428190290016971. [DOI] [PubMed] [Google Scholar]
- 26.Allen U, Preiksaitis J. Epstein–Barr virus and post-transplant lymphoproliferative disorder in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S87. doi: 10.1111/j.1600-6143.2009.02898.x. [DOI] [PubMed] [Google Scholar]


