Abstract
The sequencing of the genome and transcriptome of Schistosoma haematobium, a highly prevalent blood fluke and human parasite with a proven link to malignant bladder cancer, marks the 160th anniversary of its discovery as the first schistosome known to infect humans. Comparative genomic analyses of S. haematobium and the more prevalent human-schistosomiasis pathogens (Schistosoma mansoni and Schistosoma japonicum) identified both shared and distinct genomic features.
In 1851, Theodor Bilharz discovered Distomum haematobium (now S. haematobium), the first schistosome identified as a cause of human schistosomiasis1. An estimated 200 million individuals across 75 countries are infected with schistosomiasis2, a neglected tropical disease responsible for 300,000 deaths annually. In 1911, urogenital schistosomiasis was linked to bladder cancer3. A century later, Robin Gasser and his colleagues report, on page 221 of this issue4, the genome and transcriptome of the cancer-inducing schistosome, S. haematobium. This study provides molecular insights, such as stageand sex-enriched biological pathways, that suggest new approaches to prevention and strategies for control.
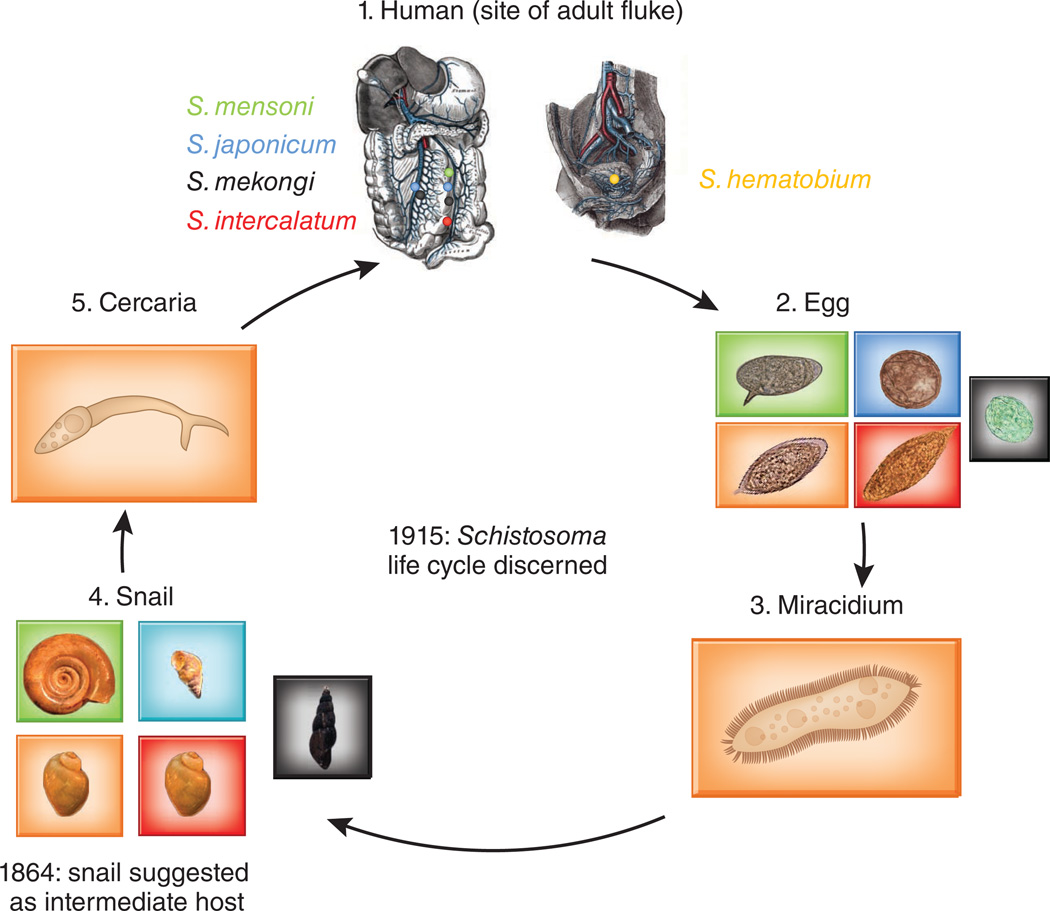
Schistosomiasis occurs predominantly in the tropics and subtropics, with 85% of cases occurring in Africa. Of the three major species, S. mansoni and S. haematobium are endemic to Africa, the Middle East and/or South America, and S. japonicum is confined to Asia. There are two major forms of schistosomiasis: intestinal (caused by S. mansoni and S. japonicum) and urogenital (caused by S. haematobium), reflecting the site of egg deposition by adult worms (Fig. 1). Because schistosomiasis symptoms are caused by eggs rather than adult worms, these two forms of the disease are clinically and biologically distinct.
Figure 1.
An overview of the schistosome life cycle. Schistosoma haematobium adults live in the veins of the vesical plexus around the bladder and along the ureters, whereas the adult intestinal schistosomes live in inferior and/or superior mesenteric veins around the walls of the large bowel, small intestine or rectum (1). Schistosome eggs (2) are transmitted in the urine (for urogenital schistosomiasis) or stool (for intestinal schistosomiasis) of infected individuals and hatch into miracidia (3), which infect snails (an intermediate host) (4). In the snail, the miracidia transform into sporocysts, which give rise to cercariae (5). The cercariae leave the snail, swim freely in the freshwater and infect humans by penetrating the skin.
Schistosome genomes
The genomes of S. mansoni and S. japonicum have previously been sequenced5,6 and have provided insights into animals’ organ development as well as schistosome infection and survival within a host. A recent comparative analysis of these two genomes7 identified homology synteny blocks (HSBs) containing ~100 Mb and ~4,400 genes. The boundaries of the adjacent HSBs tend to flank putative evolutionary breakpoint regions (EBRs), which are gene rich and therefore likely to be composed of open chromatin. The HSBs are derived from ancestral sequences, and the encoded products can be good candidates for pan-species drug and vaccine targets; the EBR gene products (140 for S. mansoni and 854 for S. japonicum) could be good lineage-specific markers.
The study by Young et al. provides an important comparative genomics resource, as S. haematobium is the first genome sequence for a species that causes urogenital schistosomiasis. The size and features of the S. haematobium genome are very similar to those of the other two schistosomes. This similarity includes ~40% repeat content, ~45% GC content and ~13,000 inferred protein-encoding genes. Although there are few predicted proteomic differences between S. haematobium and the intestinal schistosomes (such as the 73 genes unique to S. haematobium, 17 of which are supported by RNA-Seq data), such differences could be related to S. haematobium’s environment, host-pathogen interaction or association to bladder cancer. For example, Young et al. identified 55 homologs to proteins that have already been characterized, in other helminths, as being involved in modulating the host’s immune system. Twenty of these are putative excretory-secretory proteins, some of which are likely to be important for the eggs to attach to the bladder wall so that they are not washed away. Interestingly, some of the proteins specific to S. haematobium stimulate properties conducive to tumorigenesis, such as wound healing, mitosis and cell migration.
The authors also report RNA-Seq characterization of the S. haematobium transcriptome. This provides a broad picture of gene expression and stage-specific roles in the adult male, adult female and egg stages. Specifically, adult females show enriched gene expression in pathways linked to blood feeding, whereas males show enrichment in pathways related to musculature. The authors’ careful comparison of data for S. haematobium with existing microarray-based expression data from both S. mansoni8 and S. japonicum9 identified genes and biological pathways that vary among different stages and sexes of S. haematobium. This will also be a useful complement to a recent RNA-Seq data set for the adult male S. mansoni10, enabling the comparison of gene expression between males of these closely related species.
Comparative genomics
As the S. haematobium genome is the first to be sequenced among the urogenital schistosomes, its sequence may help to clarify the differences among schistosomes in the intermediate host snail species and the parasite’s targeted location within the host. The snail was suggested as an intermediate host of Schistosoma in 1864 (ref. 1). Since then, several different snail species (Fig. 1) have been identified as schistosome carriers (including Bulinus, Biomphalaria and Oncomelania for S. haematobium, S. mansoni and S. japonicum, respectively). S. haematobium has a different life cycle from the intestinal schistosomes: the females lay eggs in the bladder and the infection is transmitted via urine and an intermediate snail host. S. mansoni and S. japonicum are spread through fecal matter in a similar manner. However, the ureter and bladder, where the urogenital flukes mate and lay eggs, are considered sterile environments11, whereas the intestine and fecal matter have entire microbiomes associated with them (see, for example, ref. 12).
Using genome-wide, intragenus comparative studies, the authors found significantly higher synteny between S. haematobium and S. mansoni than between S. haematobium and S. japonicum or S. mansoni and S. japonicum. This suggests, as have earlier reports based on more limited sequence data13, that S. japonicum diverged early in the evolution of the phylum Platyhelminthes. Using S. mansoni as a reference, Young et al. also identified four times as many intrachromosomal rearrangements in S. japonicum (7.4%) as in S. haematobium (1.7%), providing further support for this suggested evolutionary distance. Furthermore, intrachromosomal rearrangements in S. haematobium were very rare, and the 11 identified inversions of syntenic blocks contained 28 genes encoding enzymes, transcription factors, structural molecules and chaperones. Although by improving the assemblies used for the performed analysis one could identify additional events, this analysis has highlighted conserved regions between the basal and derived species and has identified putative orthologous segments that may be explored as antischistosomal targets.
Young et al. identified few differences among the predicted proteomes of the three species. The majority of the predicted proteins (10,880) were shared among them. A small fraction were shared between S. haematobium and only one of the two intestinal schistosomes (1,333 with S. mansoni and 235 with S. japonicum). Given that S. haematobium is more prevalent than S. mansoni in Africa and that the two are often co-endemic14, this analysis provides a valuable resource for suggesting single drug targets for both diseases.
The S. haematobium genome and transcriptome have filled an important gap in schistosome genomics, contributing to comparative genomic approaches and providing a basis for functional studies. Now that these three schistosome reference genomes are available, there will also be interest in characterizing clinical isolates (to provide a map of natural schistosome genetic variation) as well as the genomes of more geographically localized species such as Schistosoma mekongi and Schistosoma intercalatum. By facilitating the study of the molecular mechanisms involved in pathogen nutrition and metabolism, host-dependent development and maturation, immune invasion, drug resistance and evolution, these three reference genomes will make a lasting contribution to helminth research.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Sandbach FR. Med. Hist. 1976;20:259–275. doi: 10.1017/s0025727300022663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawton SP, Hirai H, Ironside JE, Johnston DA, Rollinson D. Parasite Vector. 2011;4:131. doi: 10.1186/1756-3305-4-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson AR. J. Pathol. Bacteriol. 1911;16:76–94. [Google Scholar]
- 4.Young ND, et al. Nat. Genet. 2012;44:221–225. doi: 10.1038/ng.1065. [DOI] [PubMed] [Google Scholar]
- 5.Berriman M, et al. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium. Nature. 2009;460:345–351. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swain MT, et al. Trends Parasitol. 2011;27:555–564. doi: 10.1016/j.pt.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzpatrick JM, et al. PLoS Negl. Trop. Dis. 2009;3:e543. doi: 10.1371/journal.pntd.0000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gobert GN, Moertel L, Brindley PJ, McManus DP. BMC Genomics. 2009;10:128. doi: 10.1186/1471-2164-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almeida GT, et al. Exp. Parasitol. 2011 published online. [Google Scholar]
- 11.Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Proc. Natl. Acad. Sci. USA. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin J, et al. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu W, et al. Nat. Genet. 2003;35:139–147. doi: 10.1038/ng1236. [DOI] [PubMed] [Google Scholar]
- 14.Rollinson D. Parasitology. 2009;136:1593–1610. doi: 10.1017/S0031182009990552. [DOI] [PubMed] [Google Scholar]