Abstract
Background & Aims
Fluoroquinolone-induced liver injury is rare; no prospective studies of well-characterized case series have been published. We studied patients with fluoroquinolone-induced hepatoxicity, using data from the Drug-Induced Liver Injury Network (DILIN) to characterize injury patterns, outcomes, and associated features.
Methods
We identified subjects with fluoroquinolone hepatotoxicity who enrolled in the DILIN from September 2004 to January 2010. Demographic, clinical, and laboratory data were analyzed by descriptive statistical methods.
Results
Of the 679 registrants in the DILIN prospective study, 12 had hepatoxicity from fluoroquinolones (6 ciprofloxacin, 4 moxifloxacin, 1 levofloxacin, and 1 gatifloxacin). Seven were women; the median age was 57 years (range 23–80 years), and the median time from the start of fluoroquinolone therapy to symptoms was only 4 days (range 1–39 days). Nine cases developed symptoms on medication (2, 8, and 32 days after they stopped the medication, 3 patients each). Cases were equally distributed among hepatocellular injury (predominantly increased levels of alanine aminotransferase), cholestatic injury (predominantly increased levels of alkaline phosphatase [AP]), and both. Seven cases had immunoallergic features. Patients with mixed hepatocellular and cholestatic injury had mild disease without jaundice—all recovered. In contrast, 2 of 4 patients with hepatocellular injury and jaundice died, 1 of acute liver failure. One patient with cholestatic injury developed vanishing bile duct syndrome and required liver transplantation; another had a persistently increased serum level of AP.
Conclusions
Fluoroquinolone liver injury is rapid in onset and often has immunoallergic features, indicating a hypersensitivity reaction. The pattern of injury is can be hepatocellular, cholestatic, or mixed—mixed cases are the least severe. Acute and chronic liver failure can occur.
Keywords: ALT, drug toxicity, side effect, antibiotics, adverse reaction
INTRODUCTION
Fluoroquinolones are among the most widely prescribed antibiotics. They are popular because of their high oral bioavailability, ease of dosing and broad antimicrobial coverage.(1–5) They have been recommended as empiric antibiotic therapy in national guidelines.(6, 7) Severe side effects from the fluoroquinolones are uncommon, but include tendon rupture, hemolytic uremic syndome, Stevens-Johnson syndrome, interstitial nephritis, arrhythmias, and drug-induced liver injury (DILI).(8–12) In a recent publication of DILI from the United States, the fluoroquinolones were among the most common causes of idiosyncratic acute liver injury.(9) Fatal instances of hepatic injury from fluoroquinolones have been described. Although there have been multiple case reports of hepatotoxicity from the fluoroquinolones, there have been no larger reports even from large referral centers.(13–31) An exception is trovafloxacin, a third generation fluoroquinolone, which was the subject of an FDA public health advisory (32) and was subsequently withdrawn from the market as a result of numerous cases of liver injury.(33–35)
In 2003, the NIDDK established the Drug-Induced Liver Injury Network (DILIN) (36) to gather and characterize cases of DILI both prospectively (all herbals and medications except acetaminophen) and retrospectively for selected medications. The DILIN registry continues to enroll across 8 geographically dispersed U.S. centers. Subjects are interviewed for clinical information, and blood samples are collected with the aim of identifying genetic and other biochemical markers that may increase our understanding, diagnosis, and treatment of drug-induced liver injury. The study design and methods have been reported (37) as have results of the first 300 cases.(9) In this report, we describe our prospectively enrolled subjects with fluoroquinolone hepatoxicity to report pattern of injury, associated features, follow-up and outcome.
METHODS
Overall Design
The study design of the DILIN prospective study has been described.(9, 37) Briefly, patients aged 2 or older were enrolled on the basis of clinical suspicion of liver injury due to a medication or herbal product within 6 months of clinical onset. Inclusion criteria included aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels > 5 times the upper limit of normal (ULN) (or pretreatment baseline if abnormal) on 2 consecutive occasions, or alkaline phosphatase (AP) levels > twice the ULN (or pretreatment baseline if abnormal) on 2 consecutive occasions, or total serum bilirubin > 2.5 mg/dL (with elevated AST, ALT, or AP), or international normalized ratio (INR) > 1.5 (with elevated AST, ALT, or AP). A variety of tests are obtained at enrollment including serologies for acute hepatitis A, B, CMV and EBV. Hepatitis C antibody tests are also obtained with confirmatory HCV RNA as necessary. Autoimmune markers (ANA, ASMA, AMA) and abdominal imaging (ultrasound, CT or MRI) are also required. Patients with suspected acetaminophen hepatotoxicity were excluded as were those with a liver or bone marrow transplant. Patients with chronic hepatitis B or C or with nonalcoholic fatty liver disease were eligible for enrollment, but those with other chronic liver diseases (such as alcoholic, autoimmune or genetic liver diseases) were excluded.
Enrolled patients were seen for a baseline study visit at which time a detailed history was obtained by a DILIN investigator, and clinical, laboratory, and imaging results were extracted from the chart. These enrollment visits often took place days or weeks (up to 24 weeks) after initial clinical presentation. Further laboratory testing to exclude other causes of liver injury were obtained and serum, plasma, urine and DNA specimens were collected for future mechanistic studies. Attempts were made to follow all subjects for at least 6 months after enrollment and those with persistent liver-related abnormalities were asked to return at 12 and 24 months.
Causality
The method for assigning causality has been described in detail.(38) Each case was evaluated by 3 hepatologists including the site investigator who enrolled the case. Each evaluator independently assigned a subjective score representing percentage likelihood of attribution in which 1 = definite or > 95% likelihood, 2 = very likely or 75–95%, 3 = probable or 50–74%, 4 = possible or 25–49%, and 5 = unlikely or < 25%. When there were discrepancies, a consensus score was achieved after e-mail or conference call discussions. Cases which were still in disagreement were voted upon by one member from each center with the final score assigned by majority vote.(37, 39–41)
Participants
This study was based upon all subjects that had a fluoroquinolone suspected of causing DILI prospectively enrolled and adjudicated by February 2010. The analysis was limited to cases that were considered definite or highly likely. Cases considered probable were included only if no other agent was implicated or suspected (i.e. single-agent case).
Data and Outcomes
Demographic, clinical history and laboratory results entered into the database were analyzed with special attention to time course of DILI, latency, severity, type of reaction, associated symptoms, resolution, chronic enzyme elevation, need for transplant and death. The pattern of hepatic injury was categorized as hepatocellular, cholestatic, or mixed based upon the R-ratio of serum ALT and AP elevation (39): R-ratio = [ALT value/ALT upper limit of normal]/[AP value/AP upper limit of normal]. R-ratios of > 5 were considered hepatocellular, < 2 cholestatic, and 2–5 mixed.(39) Standard descriptive statistics were applied to continuous variables.
Liver biopsy was not required for enrollment in DILIN. Biopsies done for clinical management were obtained and evaluated in a standardized fashion by the DILIN liver histopathologist (DEK). Instructive histology from this cohort were chosen for this report.
Role of Funding Source and Institutional Board Review (IRB)
The DILIN Network is structured as a U01 cooperative agreement with funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Separate IRB approvals were obtained at each participating DILIN center.
RESULTS
Subjects
Among 679 cases enrolled in the DILIN database which had undergone causality assessment by February 2010, fluoroquinolones were listed as a potential cause in 30. In 15 cases the fluoroquinolone was the only implicated drug, of which 3 were considered definite, 4 very likely, 3 probable, and 5 were considered only possible or unlikely. Only the 10 single drug cases that were considered definite, very likely or probable were included in this study. Among the other 15 cases in which fluoroquinolones were one of several implicated agents, none were considered definite and 2 were considered very likely with the other competing drugs scoring only possible (metronidazole) or unlikely (amoxicillin/clavulanate). Only these two cases that were considered very likely due to the fluoroquinolone were included in the study bringing the total to 12. Thus, 12 of the 30 cases met all inclusion criteria, with 6 due to ciprofloxacin, 4 moxifloxacin, 1 levofloxacin, and 1 gatifloxicin.
Clinical Characteristics and Onset of Injury
Characteristics of the 12 cases are shown in Table 1. All were adults, and all except one were over 30 years of age. The median age was 57 years, and 7 were women. Jaundice, nausea and abdominal pain were frequent presenting symptoms. Overall, the time to liver injury was short and the onset abrupt. Median time from starting the medication to earliest sign or symptom of DILI was 4 days (range 1 to 39 days) and median time to documented onset (abnormal liver tests) was 8.5 days (range 1 to 41 days). Median time to either was only 2.5 days. Nine of the patients developed symptoms while still taking the antibiotic; the remaining 3 became symptomatic 2, 8, and 32 days after stopping the fluoroquinolone. Two patients had pre-existing nonalcoholic fatty liver disease (NAFLD), but no patient had chronic hepatitis B or C. Diabetes and heart disease were common, 5/12 (42%) and 4/12 (33%) respectively.
Table 1.
Clinical Characteristics of Fluoroquinolone Liver Injury Cases (n = 12)
| Age in yrs., median (min., max.) | 57 (23.6, 80.9) |
| Gender | 7/12 women (58%) |
| Race (self-report) | |
| White | 6/12 (50%) |
| Black | 3/12 (25%) |
| Other | 2/12 (17%) |
| Unknown | 1/12 (8%) |
| Latino | 1/12 (8%) |
| Concurrent alcohol | 4/12 (33%) |
| Body Mass Index, kg/m2, mean (std. dev.) | 26.8 (3.67) |
| Latencies, median days (min, max) | |
| Drug start to symptoms | 4.0 (1, 39) |
| Drug start to DILI onset* | 8.5 (1, 41) |
| Drug start to symptoms or DILI onset* | 2.5 (1, 39) |
| Signs and symptoms | |
| Jaundice | 5/12 (42%) |
| Nausea | 7/12 (58%) |
| Fever | 5/12 (42%) |
| Abdominal pain | 7/12 (58%) |
| Rash^ | 6/12 (50%) |
| Pruritus | 7/12 (58%) |
ALT > 5x ULN or AP > 2x ULN on two consecutive testings
one case of Stevens Johnson
Patterns of Injury
Patterns of injury by enrollment R-ratio and peak enzymes and bilirubin are shown in Table 2. All patients were symptomatic, 7 developed jaundice (defined as total serum bilirubin > 2.5 mg/dL), 8 were hospitalized for the DILI, 3 developed symptoms or signs of hepatic or other organ failure, one ultimately required liver transplantation, and one died of liver failure. The patterns of enzyme elevations were evenly distributed among cholestatic (n=4), hepatocellular (n=4) and mixed (n=4) categories. The pattern of injury by calculated R-ratio tended to remain constant during the acute course and the peak of illness, although a few transitioned becoming more cholestatic during follow up. Mixed cases tended to have less severe injury with lower bilirubin, ALT and AP levels. Cases with predominantly hepatocellular injury were often severe; 2 of the 4 cases resulted in death within 6 months (one known to be due to acute liver failure and the second due to death at home, not fully explained by acute liver failure). The 4 cholestatic cases did not result in death, but two developed chronic injury, one with histologically verified vanishing bile duct syndrome requiring liver transplantation 1 year after presentation. The other had persistent elevations in serum AP (200 U/L) without jaundice or pruritus 17 months after the injury. One other patient with a cholestatic pattern of injury recovered to normal liver enzymes by 206 days. The fourth case had falling liver enzymes (peak AP 837 down to 538 U/L, peak ALT 823 to 67 U/L) by 40 days post-injury, but thereafter he was lost to follow-up. In contrast, patients with a mixed pattern of serum enzyme elevations tended to have less severe injury with lower peak bilirubin (none were jaundiced), AP and ALT levels and shorter time from enzyme elevation to return to normal (37 to 81 days).
Table 2.
Clinical Course and Liver Injury Pattern (n = 12)
| Case | Drug | Age yr. | Sex | Expert Opinion Score* | Pattern^ | R-ratios |
Peak values |
Hospitalized | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| onset | range | ALT (U/L) | AP (U/L) | bilirubin (mg/dL) | INR | ||||||||
| 1 | Ciprofloxacin | 36 | F | 1 | C | 0.22 | 0.1–0.3 | 151 | 1931 | 16.5 | 1.2 | no | chronic** |
| 2 | Ciprofloxacin | 62 | F | 1 | C | 1.57 | 0.5–1.6 | 413 | 891 | 1.2 | 0.9 | no | recovered |
| 3 | Moxifloxacin | 46 | F | 2 | C | 1.70 | 0.5–2.3 | 395 | 603 | 33.4 | 5.3 | yes | chronic**/transplanted |
| 4 | Moxifloxacin | 64 | M | 3 | C | 1.96 | 0.3–2.6 | 823 | 837 | 1.5 | NA | yes | lost to follow-up |
| 5 | Gatifloxicin | 59 | M | 2 | M | 2.19 | 1.6–3.8 | 577 | 333 | 1.2 | 1.1 | yes | recovered |
| 6 | Moxifloxacin | 71 | M | 3 | M | 2.42 | 1.4–2.4 | 220 | 253 | 2.3 | 1.0 | yes□ | recovered |
| 7 | Levofloxacin | 23 | F | 2 | M | 2.84 | 2.2–2.8 | 199 | 170 | 0.8 | 1.0 | yes | recovered |
| 8 | Ciprofloxacin | 45 | F | 3 | M | 4.77 | 1.5–4.8 | 420 | 275 | 1.5 | 1.0 | no | recovered |
| 9 | Ciprofloxacin | 55 | M | 2 | HC | 5.17 | 0.6–10.3# | 1632 | 771 | 17.1 | 1.4 | yes | died at home# |
| 10 | Moxifloxacin | 45 | M | 1 | HC | 11.41 | 5.6–11.4 | 1311 | 379 | 3.6 | 0.9 | yes | recovered |
| 11 | Ciprofloxacin | 70 | F | 2 | HC | 12.29 | 12.3–42.2 | 1950 | 159 | 24.6 | 1.3 | yes | lost to follow-up |
| 12 | Ciprofloxacin | 80 | F | 2 | HC | 13.27 | 13.3–33.5 | 1684 | 136 | 21.1 | 8.2 | yes | died: liver failure |
1 = Definite, > 95% likelihood; 2 = Very likely, 75–95%; 3 = Probable, 50–75%
C = cholestatic, R < 2; M = mixed, 2 < R < 5; HC = hepatocellular, R > 5
developed peritonitis and cholestasis while on peritoneal dialysis; cause of death unknown.
hospitalized for non-liver reason
chronic DILI with persistently elevated AP and/or bilirubin
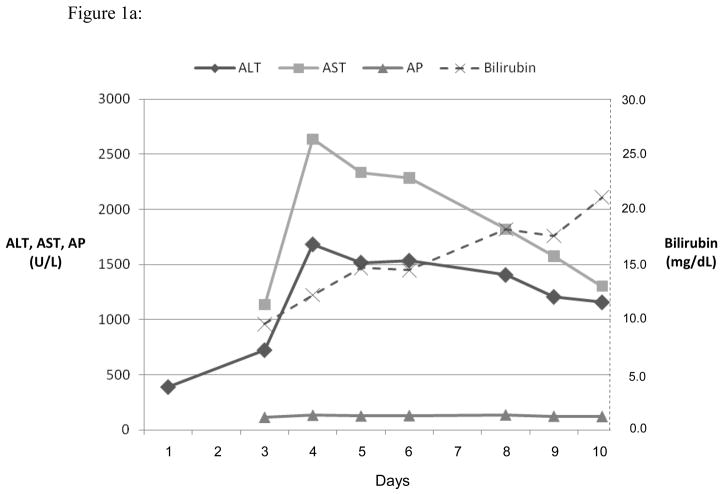
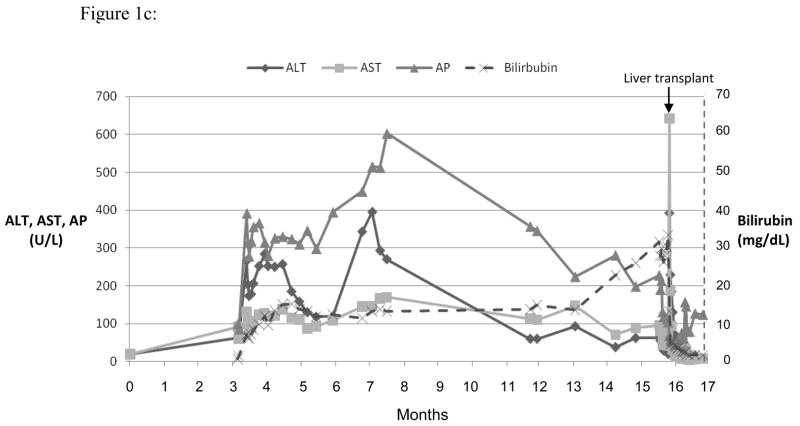
Three representative cases are shown in the Figures 1, a-c. Figure 1a shows the course of serum enzyme and bilirubin levels in a patient with a hepatocellular pattern of injury (Case 12 in Table 2) occurring 18 days after starting ciprofloxacin. She developed acute liver failure but was not a suitable transplant candidate at age 80 with multiple co-morbidities. She was transferred to hospice care and died 4 weeks after initial elevation of ALT. Figure 1b shows a patient with a mixed pattern of enzyme elevation and self-limiting course (Case 7 in Table 2), arising 7 days after starting a course of levofloxacin with near complete and complete resolution at 4 and 8 weeks, respectively. Figure 1c shows a patient with a cholestatic pattern of serum enzyme elevations and prolonged, severe cholestasis arising 8 days after starting a course of moxifloxacin (Case 3 in Table 2). She developed prolonged jaundice (1.3 years) ultimately leading to hepatic failure and liver transplantation with histology of the explanted liver showing vanishing bile duct syndrome (Figure 2d).
Figure 1.
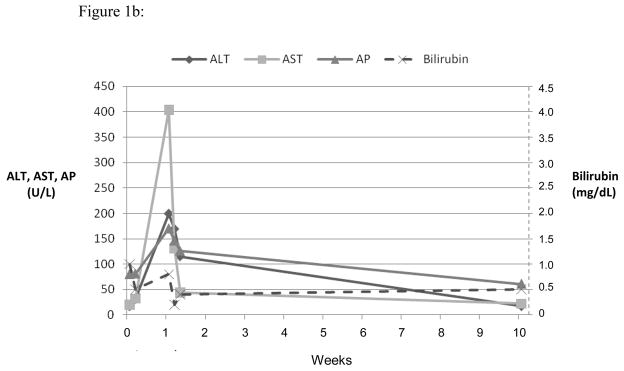
Serum ALT, AST, AP and bilirubin over time after 3 different fluoroquinolone induced liver injuries. Expert opinion causality scores for all three cases were 2, or very likely. (a) Ciprofloxacin induced hepatocellular injury causing acute liver failure and death. (b) Levofloxacin induced mixed hepatocellular-cholestatic injury with recovery. (c) Moxifloxicin induced cholestatic liver injury leading to prolonged cholestasis, ductopenia and liver failure requiring transplant.
Figure 2.
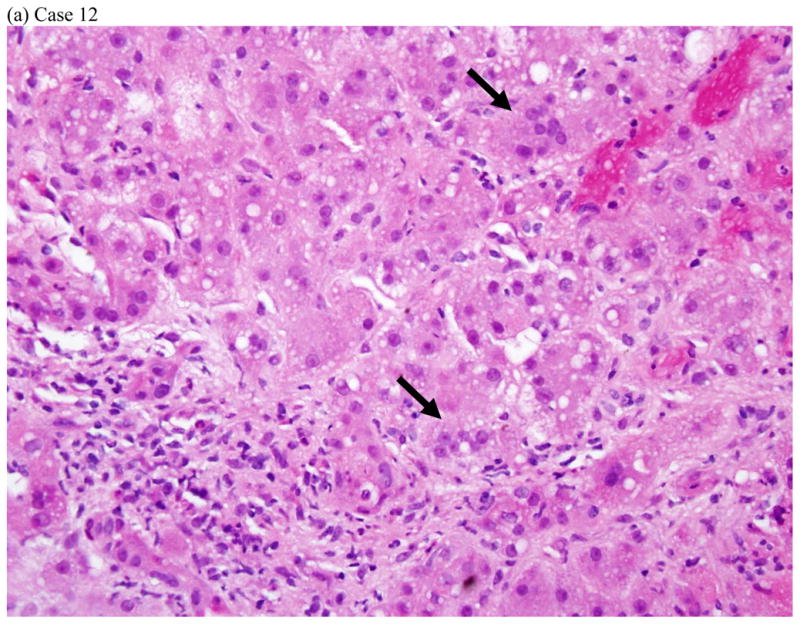
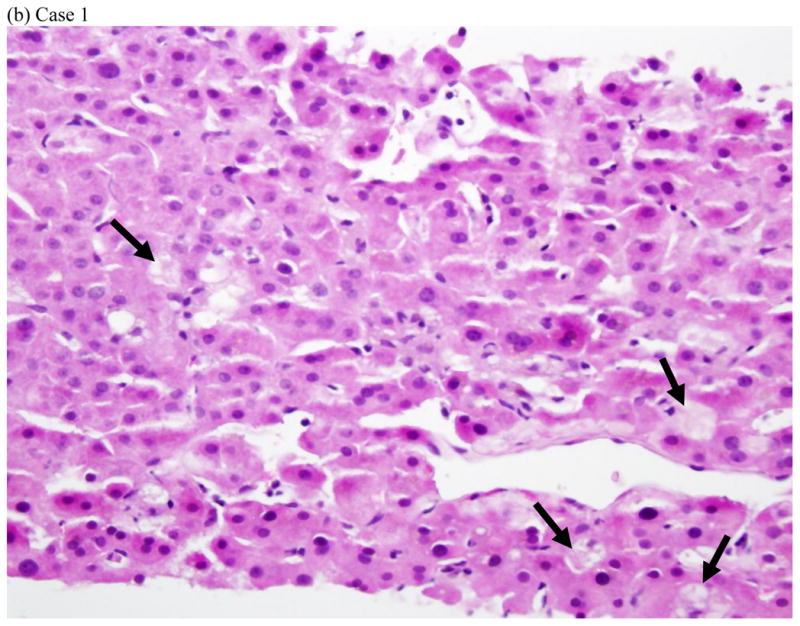
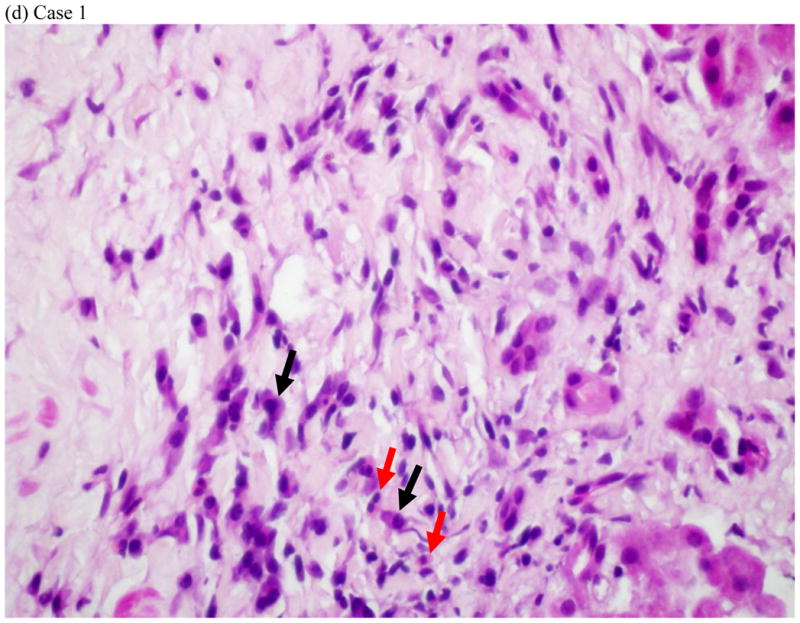
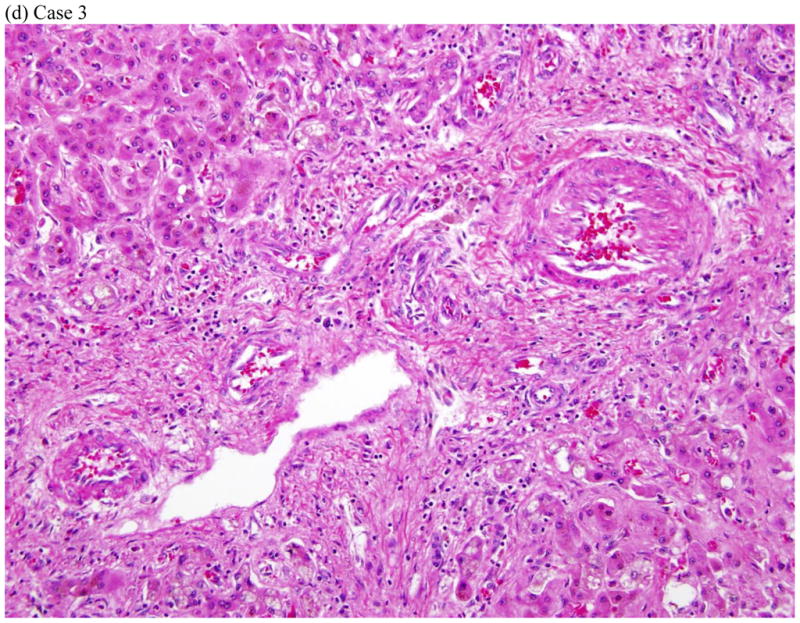
Represent histopathology of three cases of fluoroquinolone induced liver injuries. (a) acute hepatitis with giant cell transformation (arrows) during hepatocellular injury, (b) cholestatic hepatitis showing mild zone 3 cholestasis (arrows), (c) mild portal inflammation with plasma cells (black arrows) and scattered eosinophils (red arrows) during cholestatic injury, and (d) vanishing bile duct syndrome after cholestatic injury (All hematoxylin and eosin stains, 400x, 200x, 600x and 400x magnification respectively).
Immunoallergic Features and Other Drug Allergies (Table 3)
Table 3.
Immunoallergic Features and Drug Allergies (n = 12)
| # | Drug | Latency* | Rash | Fever | Eosinophilia^ | ANA or ASMA# | Other Signs/Symptoms | Other Drug Allergies |
|---|---|---|---|---|---|---|---|---|
| 1 | Ciprofloxacin | 9 | + | + | + | − | − | none |
| 2 | Ciprofloxacin | 12 | − | + | − | − | + hypersensitivity pneumonitis | macrodantin, sulfonamides |
| 3 | Moxifloxacin | 1 | + | + | + | − | − | valacyclovir |
| 4 | Moxifloxacin | 2 | − | − | − | − | − | none |
| 5 | Gatifloxicin | 1 | − | + | − | − | + leucopenia/neutropenia | none |
| 6 | Moxifloxacin | 1 | − | − | − | − | − | none |
| 7 | Levofloxacin | 1 | − | − | − | + | − | none |
| 8 | Ciprofloxacin | 39 | − | − | − | + | − | levofloxacin |
| 9 | Ciprofloxacin | 30 | + | + | − | − | − | penicillin |
| 10 | Moxifloxacin | 7 | + | − | − | − | + Stevens Johnson Syndrome | amoxicillin, erythromycin, tramadol |
| 11 | Ciprofloxacin | 4 | − | − | − | + | − | amoxicillin |
| 12 | Ciprofloxacin | 18 | + | − | + | + | − | penicillin |
days from drug start to any symptoms of DILI
absolute eosinophil count > 500/uL
ANA = anti-nuclear antibody; ASMA = anti-smooth muscle antibody
Seven of the 12 had fever, rash and/or eosinophilia. Three patients had a positive ANA or ASMA without other immunoallergic features. Since patients were enrolled up to 24 weeks after the event, transient eosinophilia at the time of onset may have been missed. Fever was reported more often in cholestatic pattern of injury. Rash occurred in patients with both high and low R-ratios, but not in cases with a mixed pattern of enzyme elevations. Hypersenstivity pneumonitis, Stevens-Johnson Syndrome and bone marrow suppression were also seen. Seven of 12 patients had a history of allergy to other medications including one patient who had separate hepatoxicity events after taking two different fluoroquinolones.
Histology
Liver tissue was available for central review in 5 cases: four needle biopsies obtained during the DILI episode and one explant. Three of the needle biopsies were from patients with a predominantly hepatocellular biochemical injury. Two of these showed acute hepatitis with lobular disarray and severe portal and lobular inflammation (Fig. 2a). One of these two had striking hepatocyte giant cell transformation. The third case showed a mixed pattern of injury with zone 3 coagulative necrosis and a distribution of inflammation reminiscent of chronic hepatitis. In this case there was less lobular inflammation and no disarray, but dense portal inflammation and interface hepatitis were present in most portal areas. Although all three of these patients had jaundice, there was little-to-no cholestasis or duct injury on biopsy. Two of the cases, including a needle biopsy and the explant, were from patients with cholestatic presentations (Fig 2b–d). The needle biopsy showed a cholestatic hepatitis with mild intrahepatic cholestasis and mild to moderate portal and lobular inflammation (Fig. 2b–c). As noted above, the explant showed severe ductopenia and only mild inflammation (Fig. 2d). By the time of transplant most of the hepatocyte parenchyma had been replaced by a ductular reaction embedded in dense fibrosis. Although these cases showed diverse patterns of injury histologically, all four of the needle biopsies showed increased numbers of eosinophils. Two (one hepatocellular and one cholestatic) showed increased numbers of plasma cells in the infiltrate.
DISCUSSION
Hepatotoxicity from fluoroquinolones has been described in multiple case reports and summarized in several reviews. However there are no well-characterized and prospectively followed groups of cases published on hepatic injury from this important class of antibiotics. The 12 cases presented here confirm that hepatic injury from the fluoroquinolones has a “class effect” and the clinical presentation and phenotype of injury is similar with the different agents. The predominant feature of the hepatic injury was the short latency (median 2 to 9 days) and abrupt onset of injury (Table 1). Nine of our 12 cases developed symptoms while still taking the antibiotic. Immunoallergic features (rash, fever and eosinophilia) were common (Table 3), but rarely as prominent as occurs with hepatotoxicity from sulfonamides, macrolides, or aromatic anticonvulsants. Only one of the 12 patients had “DRESS syndrome” (drug rash with eosinophilia and systemic symptoms), a 45 year old patient who developed Stevens-Johnson syndrome 7 days after starting a 10 day course of ciprofloxacin. Six other patients had fever or rash, but eosinophilia was uncommon, perhaps because the pattern of referral of patients to DILIN often resulted in a delay of a few weeks before enrollment (by which time the eosinophil count might have normalized). Immunoallergic features have been described in several case reports of fluoroquinolone hepatotoxicity.(15, 21, 27, 30, 31)
The pattern of enzyme elevations described here varied greatly. Indeed, the full range of clinical patterns was seen from very cholestatic cases with high AP (603 U/L) and high bilirubin (33.4 mg/dL) that led to prolonged jaundice and vanishing bile duct syndrome (Case 3, Table 2 and Figure 2d;), to obvious hepatocellular injury with high ALT (1684 U/L) that led rapidly to hepatic failure and death (Case 12, Table 2 and Figure 2a). In between these extremes were 6 cases without jaundice, 4 of which had a “mixed” pattern of serum enzyme elevations and a self-limited, benign course (Cases 5–8, Table 2).
The pathophysiology of fluoroquinolone hepatotoxicity is not known. The short latency, frequent immunoallergic features, heightened injury that has been described upon re-exposure, and the lack of a common pattern of metabolism of the various fluoroquinolones argue for a hypersensitivity reaction.(42) All four of our subjects with available liver histology showed increased eosinophils and two had increased plasma cells. It has been postulated that the trifluorinated quiniolones with their 1-(2,4)-difluorophenyl group may carry an even greater risk for severe immune-mediated toxicities (e.g. temafloxacin syndrome and trovafloxacin hepatotoxicity).(8) Interestingly, one half of our cases had a history of allergies to non-fluoroquinolone medications suggesting increased susceptibility in such individuals (Table 3). The immunoallergic phenotype makes it advisable for patients with hepatotoxicity from one fluorquiniolone to avoid this class of antibiotic altogether.(43) Indeed, one of our cases (Case 8, Table 3), had repeated reactions to two different fluoroquinolones prescribed for recurrent diverticulitis. She was enrolled during a reaction to ciprofloxacin. On follow-up, she had yet another reaction when she was given levofloxacin (ALT up 1680 IU/L).
While our study is small, the cases were prospectively enrolled, and followed under protocol. Therefore the quality of data on our subjects is likely higher than the average case reports in the literature. In fact, our 12 cases contained the vast majority (>95%) of the 19 “Minimal Elements” suggested for DILI case reporting (Supplement Table 1).(44) Only 5 of the 12 had had no liver tests checked prior to initiation of the fluoroquinolone and 6 did not require a liver biopsy.
Attribution was made by expert opinion process, the gold standard for assigning DILI causality. While we set out to enroll cases of at least probable attribution, the majority of our qualifying cases actually had scored better than probable. In the DILIN, definite cases have no other remotely possible causes and a pattern of injury that is stereotypic for the agent based on previously described reports or papers in the literature. Thus the bar for being considered definite (>95% likelihood) is high. Very likely cases also have high attribution, but do not quite meet definite criteria. Therefore, 9 of 12 (75%) cases were highly attributable to the fluoroquinolone scoring definite or very likely. Cases 5 and 12 were the only two with competing agents and both were considered to be very likely due to the fluoroquinolone. In case 5, amoxicillin/clavulanate was taken for just one day starting the day of the reaction and was therefore deemed an unlikely culprit. Metronidazole was a competing agent in case 12 and was deemed only possible based on its low risk for DILI. The three probable cases had no other competing agents.
Of the 7 fluoroquinolones that were available in the United States during the study period, 4 (ciprofloxacin, levofloxacin, moxifloxicin, and gatifloxacin) were represented in our study. Gatifloxacin has since been withdrawn from the market due to problems with glucose homeostasis. While our study did not include ofloxacin and norfloxacin, each has been reported to cause hepatoxicity with similar injury patterns to those described here. (27, 45–48) There are no cases of gemifloxicin hepatotoxicity reported, but it is relatively new and animal studies suggest its potential to cause liver injury as well.(49) In addition, there are several other fluoroquinolones available outside the US (Supplement Table 2), but little is known about their risk for hepatotoxicity.
Estimates of hepatotoxicity incidence are hampered by biases and poor quality of reporting. Package insert information suggests asymptomatic, mild and reversible elevations in liver enzymes may occur in 2–3% of patients taking fluoroquinolones, (50) while a population-based study from England suggested that the incidence of illness due to hepatotoxicity is 0.54 per 10,000 persons taking ciprofloxacin.(51) Estimate of “acute liver injury” from all fluoroquinolones in Sweden is 0.7 per 100,000 users.(4) The predominance of ciprofloxacin in our study probably reflected market share more than increased risk, but moxifloxacin (incriminated in 4 cases) was probably over-represented. From 1996 to 2001, the number of prescriptions written in the US was 66 million for ciprofloxacin, 24 million for levofloxacin, 3 million for gatifloxacin and 1 million for moxifloxacin.(50)
Acute liver failure rates are also difficult to ascertain, but estimates are quite low. From 2008 pharmacy data in the United States, levofloxacin, moxifloxacin and gatifloxacin have similar rates of acute liver failure: 2.1, 6.6 and 6.1 cases per 10 million prescriptions, respectively.(4) In contrast, trovafloxacin, which was withdrawn from use in the United States in 1999 for hepatotoxicity, had a higher estimated rate of 58 per 10 million prescriptions. By comparison, amoxicillin-clavulanate has a rate of 10 per 10 million.(4)
While rare, it is important to stress that liver injury from the fluoroquinolones can be severe causing prolonged jaundice, morbidity and acute and chronic liver failure resulting in death or transplant. Patients with fluoroquinolone-induced liver injury should be cautioned to avoid re-exposure to other fluoroquinolones in the future and be informed that some brand names do not intuitively imply the fluoroqinolone class (e.g. Avelox, Factive, Cravit). Since there are no established means of preventing hepatotoxicity from the fluoroquinolones, it is important to prescribe them for clear clinical indications only and to take a careful history of drug allergies. Therapies for DILI are limited. Patients with prominent features of hypersensitivity may improve with use of corticosteroids, but these agents should not be used in cases without these features. N-acetylcysteine has been shown to improve “transplant-free survival” in patients with non-acetominophen acute liver failure, particularly those with drug-induced liver injury with early hepatic encephalopathy.(52) Finally, all cases of drug-induced liver injury should be reported to national registries whenever possible. In the United States, cases are reported to the FDA through MedWatch (http://www.fda.gov/Safety/MedWatch/default.htm).
Thus, fluoroquinolones are highly effective and widely used antibiotics that rarely cause clinical hepatotoxicity and even more rarely cause life-threatening liver injury. The clinical phenotype of hepatotoxicity appears to be shared by all of the fluoroquinolones and is characterized usually by a short period of latency and abrupt onset with features of hypersensitivity. Some fluoroquinolones may carry a higher risk than others. Most patients with fluoroquinolone-associated hepatotoxicity recover, but the period of illness can be prolonged, can result in hepatic failure and death or need for liver transplantation. Ultimately, prevention and management of fluoroquinolone hepatotoxicity will require a better understanding of its pathogenesis which provides the rationale for collection of well-characterized cases of this rare condition for metabolic, immunologic and genetic study: the primary aim of the DILIN network.
Supplementary Material
Acknowledgments
Funding: The DILIN Network is structured as a U01 cooperative agreement with funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under grants: 2U01-DK065176-06 (Duke), 2U01-DK065201-06 (UNC), 2U01-DK065184-06 (Michigan), 2U01-DK065211-06 (Indiana), 5U01DK065193-04 (UConn), 5U01-DK065238-08 (UCSF/CPMC), 1U01-DK083023-01 (UTSW), 1U01-DK083027-01 (TJH/UPenn), 1U01-DK082992-01 (Mayo), 1U01-DK083020-01 (USC). Additional funding is provided by CTSA grants: UL1 RR025761 (Indiana), UL1 RR025747 (UNC), UL1 RR024134 (UPenn), UL1 RR024986 (Michigan), UL1 RR024982 (UTSW), UL1 RR024150 (Mayo) and in part by the Intramural Research Program of the NIH, National Cancer Institute.
A full list of DILIN investigators, co-investigators and staff is shown in Appendix 1 of the supporting information. The authors thank our referring providers and patients for participation in this study. We also thank Jay H. Hoofnagle and Robert J. Fontana for their advice and help in preparing this article.
Abbreviations
- DILI
Drug-induced Liver Injury
- DILIN
Drug-induced Liver Injury Network
- APAP
Acetaminophen
- RUCAM
Roussel UCLAF Causality Assessment Method
- NIH
National Institutes of Health
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- AP
Alkaline phosphatase
- ANA
Anti-nuclear antibody
- AMA
Anti-mitochondrial antibody
- ASMA
anti-smooth muscle antibody
- LFTs
Liver Function Tests (AST, ALT, AP, bilirubin)
Appendix 1
Clinical Centers
Indiana University, Indianapolis, IN
Naga Chalasani, MD (PI), Raj Vuppalanchi, MD (co-I), Jean Molleston, MD (co-I), Lawrence Lumeng, MD (co-I), Audrey Corne (research coordinator), Angie Plummer (research coordinator)
University of Connecticut
Herbert Bonkovsky, MD (PI), Petr Protiva, MD (co-I), James Freston, MD, PhD (co-I), Robert Rosson, MD (co-I), Robert A. Levine, MD (Satellite site investigator), Benedict Maliakkal, MD (Satellite site investigator), Paul Appleton, MD (Research coordinator), Mariola Smialek, RN (research coordinator)
University of Michigan, Ann Arbor, MI
Robert J. Fontana, MD (PI), Hari Conjeevaram, MD (co-I), Rich Moseley, MD, (co-I), Stuart Gordon, MD (Satellite site investigator), Suzanne Welch (Research coordinator), Jessica Worley (Research coordinator), Jordan Kridler (Research coordinator), Sonal Trivedi (Research coordinator), Sweta Kochlar (Research coordinator).
University of North Carolina, Chapel Hill, NC
Paul Watkins, MD (PI), Paul H. Hayashi, MD (co-I), Mark Russo, MD (co-I), Late Harry Guess, MD, PhD (co-investigator), Kimberly Beaver, MD (Satellite site investigator), Alastair Smith, MD (Satellite site investigator), James Lewis, MD (Satellite site investigator), Susan Pusek (Research coordinator), Tracy Russell (Research coordinator), Lorraine Mehltretter, (Administrative assistant).
California Pacific Medical Center, San Francisco, CA
Tim Davern, MD (PI), Maurizo Bonacini, MD (co-I), Kristine Partovi (research coordinator), Katharine Fajardo (Research coordinator), Seaton Tai (Research coordinator)
University of Texas Southwestern Medical Center, Dallas, TX
William Lee, MD (PI), Don Rockey, MD (co-I), Anne Larson (co-I), LaFaine Grant, MD (co-I), Kenni Landgraf, RN (Research Coordinator).
University of Southern California, Los Angeles, CA
Andrew Stoltz, MD (PI), Neil Kaplowitz, MD (co-I), Susan Milstein, RN (Research coordinator)
Mayo Clinic, Rochester, MN
Jayant Talwalker, MD (PI), Stephanie Johnson, RN (Research coordinator)
Jefferson University & University of Pennsylvania, Philadelphia, PA
Victor Navarro, MD (PI), Rajender Reddy, MD (Co-I), Maricruz Vega, MPH, CHES (Research coordinator), Amina Wirjosemito (Research coordinator), Kristina Evans, MPH (Research coordinator)
Data Coordinating Center
Duke Clinical Research Institute
James Rochon, MD (PI), John McHutchison, MD (co-I), Hans Tilllmann, MD (co-I), Mary Maggio (project manager), Hongqiu Yang, PhD (biostatistician), Kathy Galan, RN (Project Lead), Elaina Cosslin (PLA), Lesley Sunas, (PLA), Morgan Collini (CRA), Tanya Rose (CTA), Michelle Crowder, (Program Manager), Carmel Scharenbroich (Data Manager), Hoss Rostami (Data Manager), Sherry Jiezhun (Statistician), Tarka Monroe (CDA 4)
NIH/NIDDK
Jose Serrano, MD (Project officer), Leonard Seeff, MD, Jay Hoofnagle, MD, David Toke, PhD, Dana Witt, Heather Higgins.
NIH/NCI
David Kleiner, MD
FDA/DHHS
Mark Avigan, MD and John Senior, MD, employees of the U.S. Food and Drug Administration have participated in selected aspects of the DILIN activities.
Footnotes
Disclosures: The authors have no financial, professional or personal disclosures.
Author Contributions: ES Orman, MD: Study design, literature searches, analysis and interpretation of data, drafting of manuscript.
HS Conjeevaram, MD: Analysis and interpretation of data, drafting of manuscript.
R Vuppalanchi, MD: Analysis and interpretation of data, drafting of manuscript
JW Freston, MD: Analysis and interpretation of data, drafting of manuscript
J Rochon, PhD: Acquisition, analysis and interpretation of data
DE Kleiner, MD, PhD: Acquisition, analysis and interpretation of histologic data, drafting of manuscript
PH Hayashi MD, MPH: Study design, literature searches, analysis and interpretation of data, drafting of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolfson JS, Hooper DC. Overview of fluoroquinolone safety. Am J Med. 1991;91(6):153S–161S. doi: 10.1016/0002-9343(91)90330-z. [DOI] [PubMed] [Google Scholar]
- 2.McCaig LF, Besser RE, Hughes JM. Antimicrobial drug prescription in ambulatory care settings, United States, 1992–2000. Emerging Infect Dis. 2003;9(4):432–7. doi: 10.3201/eid0904.020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertino J, Fish D. The safety profile of the fluoroquinolones. Clin Ther. 2000;22(7):798–817. doi: 10.1016/S0149-2918(00)80053-3. discussion 797; 798–817; discussion 797. [DOI] [PubMed] [Google Scholar]
- 4.Van Bambeke F, Tulkens PM. Safety profile of the respiratory fluoroquinolone moxifloxacin: comparison with other fluoroquinolones and other antibacterial classes. Drug Saf. 2009;32(5):359–78. doi: 10.2165/00002018-200932050-00001. [DOI] [PubMed] [Google Scholar]
- 5.Kahn JB. Latest industry information on the safety profile of levofloxacin in the US. Chemotherapy. 2001;47(Suppl 3):32–37. doi: 10.1159/000057842. discussion 44–48; 32–37; discussion 44–48. [DOI] [PubMed] [Google Scholar]
- 6.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community- acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren JW, Abrutyn E, Hebel JR, et al. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA) Clin Infect Dis. 1999;29(4):745–58. doi: 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- 8.Owens RC, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(Suppl 2):S144–157. S144–157. doi: 10.1086/428055. [DOI] [PubMed] [Google Scholar]
- 9.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924, 1934, 1934.e1–4. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abboud G, Kaplowitz N. Drug-induced liver injury. Drug Saf. 2007;30(4):277–94. doi: 10.2165/00002018-200730040-00001. [DOI] [PubMed] [Google Scholar]
- 11.Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354(7):731–9. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]
- 12.Watkins PB, Seeff LB. Drug-induced liver injury: summary of a single topic clinical research conference. Hepatology. 2006;43(3):618–31. doi: 10.1002/hep.21095. [DOI] [PubMed] [Google Scholar]
- 13.Bhagirath KM. A case report of highly suspected ciprofloxacin-induced hepatotoxicity. Turk J Gastroenterol. 2008;19(3):204–6. [PubMed] [Google Scholar]
- 14.Coban S, Ceydilek B, Ekiz F, et al. Levofloxacin-induced acute fulminant hepatic failure in a patient with chronic hepatitis B infection. Ann Pharmacother. 2005;39(10):1737–40. doi: 10.1345/aph.1G111. [DOI] [PubMed] [Google Scholar]
- 15.Nori S, Nebesio C, Brashear R, Travers JB. Moxifloxacin-associated drug hypersensitivity syndrome with toxic epidermal necrolysis and fulminant hepatic failure. Arch Dermatol. 2004;140(12):1537–8. doi: 10.1001/archderm.140.12.1537. [DOI] [PubMed] [Google Scholar]
- 16.Zimpfer A, Propst A, Mikuz G, et al. Ciprofloxacin-induced acute liver injury: case report and review of literature. Virchows Arch. 2004;444(1):87–9. doi: 10.1007/s00428-003-0917-9. [DOI] [PubMed] [Google Scholar]
- 17.Goetz M, Galle PR, Schwarting A. Non-fatal acute liver injury possibly related to high-dose ciprofloxacin. Eur J Clin Microbiol Infect Dis. 2003;22(5):294–6. doi: 10.1007/s10096-003-0914-6. [DOI] [PubMed] [Google Scholar]
- 18.Schwalm J, Lee CH. Acute hepatitis associated with oral levofloxacin therapy in a hemodialysis patient. CMAJ. 2003;168(7):847–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Bataille L, Rahier J, Geubel A. Delayed and prolonged cholestatic hepatitis with ductopenia after long-term ciprofloxacin therapy for Crohn’s disease. J Hepatol. 2002;37(5):696–9. doi: 10.1016/s0168-8278(02)00268-4. [DOI] [PubMed] [Google Scholar]
- 20.Soto S, Lopez-Roses L, Avila S, et al. Moxifloxacin-induced acute liver injury. Am J Gastroenterol. 2002;97(7):1853–4. doi: 10.1111/j.1572-0241.2002.05873.x. [DOI] [PubMed] [Google Scholar]
- 21.Contreras MA, Luna R, Mulero J, et al. Severe ciprofloxacin-induced acute hepatitis. Eur J Clin Microbiol Infect Dis. 2001;20(6):434–5. doi: 10.1007/s100960100513. [DOI] [PubMed] [Google Scholar]
- 22.Karim A, Ahmed S, Rossoff LJ, et al. Possible levofloxacin-induced acute hepatocellular injury in a patient with chronic obstructive lung disease. Clin Infect Dis. 2001;33(12):2088–90. doi: 10.1086/338156. [DOI] [PubMed] [Google Scholar]
- 23.Spahr L, Rubbia-Brandt L, Marinescu O, et al. Acute fatal hepatitis related to levofloxacin. J Hepatol. 2001;35(2):308–9. doi: 10.1016/s0168-8278(01)00082-4. [DOI] [PubMed] [Google Scholar]
- 24.Labowitz JK, Silverman WB. Cholestatic jaundice induced by ciprofloxacin. Dig Dis Sci. 1997;42(1):192–4. doi: 10.1023/a:1018870029216. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal A, Gurka J. Probable ciprofloxacin induced cholestasis. Aust N Z J Med. 1995;25(5):541–2. doi: 10.1111/j.1445-5994.1995.tb01506.x. [DOI] [PubMed] [Google Scholar]
- 26.Alcalde M, Donoso MS, Carcfa-Diaz M, et al. Liver disfunction due to ciprofloxacin. Acta Gastroenterol Belg. 1995;58(5–6):475–6. [PubMed] [Google Scholar]
- 27.Hautekeete ML, Kockx MM, Naegels S, et al. Cholestatic hepatitis related to quinolones: a report of two cases. J Hepatol. 1995;23(6):759–60. doi: 10.1016/0168-8278(95)80045-x. [DOI] [PubMed] [Google Scholar]
- 28.Villeneuve JP, Davies C, Cote J. Suspected ciprofloxacin-induced hepatotoxicity. Ann Pharmacother. 1995;29(3):257–9. doi: 10.1177/106002809502900305. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs S, Simon Z, Brezis M. Fatal hepatic failure associated with ciprofloxacin. Lancet. 1994;343(8899):738–9. doi: 10.1016/s0140-6736(94)91624-1. [DOI] [PubMed] [Google Scholar]
- 30.Sherman O, Beizer JL. Possible ciprofloxacin-induced acute cholestatic jaundice. Ann Pharmacother. 1994;28(10):1162–4. doi: 10.1177/106002809402801005. [DOI] [PubMed] [Google Scholar]
- 31.Grassmick BK, Lehr VT, Sundareson AS. Fulminant hepatic failure possibly related to ciprofloxacin. Ann Pharmacother. 1992;26(5):636–9. doi: 10.1177/106002809202600504. [DOI] [PubMed] [Google Scholar]
- 32.Lumpkin MM. Trovan (Trovafloxacin/Alatrofloxacin Mesylate). 1999. Jun, 2010. Food and Drug Administration 09. [Google Scholar]
- 33.Lazarczyk DA, Goldstein NS, Gordon SC. Trovafloxacin hepatotoxicity. Dig Dis Sci. 2001;46(4):925–6. doi: 10.1023/a:1010741510046. [DOI] [PubMed] [Google Scholar]
- 34.Chen HJ, Bloch KJ, Maclean JA. Acute eosinophilic hepatitis from trovafloxacin. N Engl J Med. 2000;342(5):359–60. doi: 10.1056/NEJM200002033420517. [DOI] [PubMed] [Google Scholar]
- 35.Lucena MI, Andrade RJ, Rodrigo L, et al. Trovafloxacin-induced acute hepatitis. Clin Infect Dis. 2000;30(2):400–1. doi: 10.1086/313680. [DOI] [PubMed] [Google Scholar]
- 36.Hoofnagle JH. Drug-induced liver injury network (DILIN) Hepatology. 2004;40(4):773. doi: 10.1002/hep.20445. [DOI] [PubMed] [Google Scholar]
- 37.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32(1):55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rockey DC, Seeff LB, Rochon J, et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51(6):2117–26. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs--II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46(11):1331–6. doi: 10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- 40.Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46(11):1323–30. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 41.Benichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11(2):272–6. doi: 10.1016/0168-8278(90)90124-a. [DOI] [PubMed] [Google Scholar]
- 42.Schmid DA, Campi P, Pichler WJ. Hypersensitivity reactions to quinolones. Curr Pharm Des. 2006;12(26):3313–26. doi: 10.2174/138161206778194033. [DOI] [PubMed] [Google Scholar]
- 43.Scherer K, Bircher AJ. Hypersensitivity reactions to fluoroquinolones. Curr Allergy Asthma Rep. 2005;5(1):15–21. doi: 10.1007/s11882-005-0049-1. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal VK, McHutchison JG, Hoofnagle JH. Drug-Induced Liver Injury Network. Important elements for the diagnosis of drug-induced liver injury. Clin Gastroenterol Hepatol. 2010;8(5):463–70. doi: 10.1016/j.cgh.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjornsson E, Olsson R, Remotti H. Norfloxacin-induced eosinophilic necrotizing granulomatous hepatitis. Am J Gastroenterol. 2000;95(12):3662–4. doi: 10.1111/j.1572-0241.2000.03404.x. [DOI] [PubMed] [Google Scholar]
- 46.Davoren P, Mainstone K. Norfloxacin-induced hepatitis. Med J Aust. 1993;159(6):423–426. doi: 10.5694/j.1326-5377.1993.tb137931.x. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Navidad A, Domingo P, Cadafalch J, et al. Norfloxacin-induced hepatotoxicity. J Hepatol. 1990;11(2):277–8. doi: 10.1016/0168-8278(90)90125-b. [DOI] [PubMed] [Google Scholar]
- 48.Romero-Gomez M, Suarez Garcia E, Fernandez MC. Norfloxacin-induced acute cholestatic hepatitis in a patient with alcoholic liver cirrhosis. Am J Gastroenterol. 1999;94(8):2324–5. doi: 10.1111/j.1572-0241.1999.02324.x. [DOI] [PubMed] [Google Scholar]
- 49.Bikash R, Kanti SA, Pinaki S, et al. Twenty eight days repeated oral dose toxicity study of gemifloxacin in Wistar albino rats. Regul Toxicol Pharmacol. 2010 doi: 10.1016/j.yrtph.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Iannini PB. The safety profile of moxifloxacin and other fluoroquinolones in special patient populations. Curr Med Res Opin. 2007;23(6):1403–13. doi: 10.1185/030079907X188099. [DOI] [PubMed] [Google Scholar]
- 51.Jick SS, Jick H, Dean AD. A follow-up safety study of ciprofloxacin users. Pharmacotherapy. 1993;13(5):461–4. [PubMed] [Google Scholar]
- 52.Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856,64, 864.e1. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stahlmann R. Clinical toxicological aspects of fluoroquinolones. Toxicol Lett. 2002;127(1– 3):269–77. doi: 10.1016/s0378-4274(01)00509-4. [DOI] [PubMed] [Google Scholar]
- 54. [Accessed June 6, 2010];eMedExpert. http://www.emedexpert.com/lists/antibiotics.shtml#5.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.